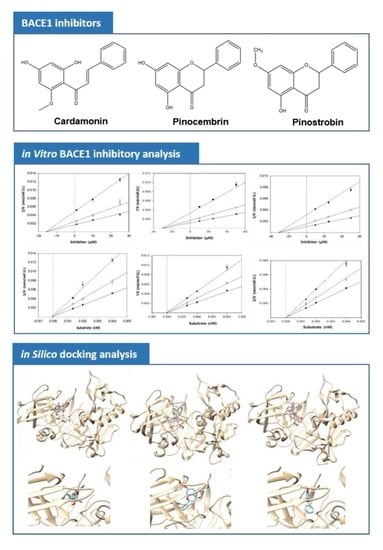
Biological Evaluation and Docking Analysis of Potent BACE1 Inhibitors from Boesenbergia rotunda
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. In Vitro Enzyme Inhibitory Assay for Biological Evaluation
2.3. BACE1 Inhibition Kinetics
2.4. In Silico Docking Studies
2.5. Statistics
3. Results
3.1. Anti-BACE1 Activity of Cardamonin, Pinocembrin, and Pinostrobin
3.2. BACE1 Kinetics of Cardamonin, Pinocembrin, and Pinostrobin
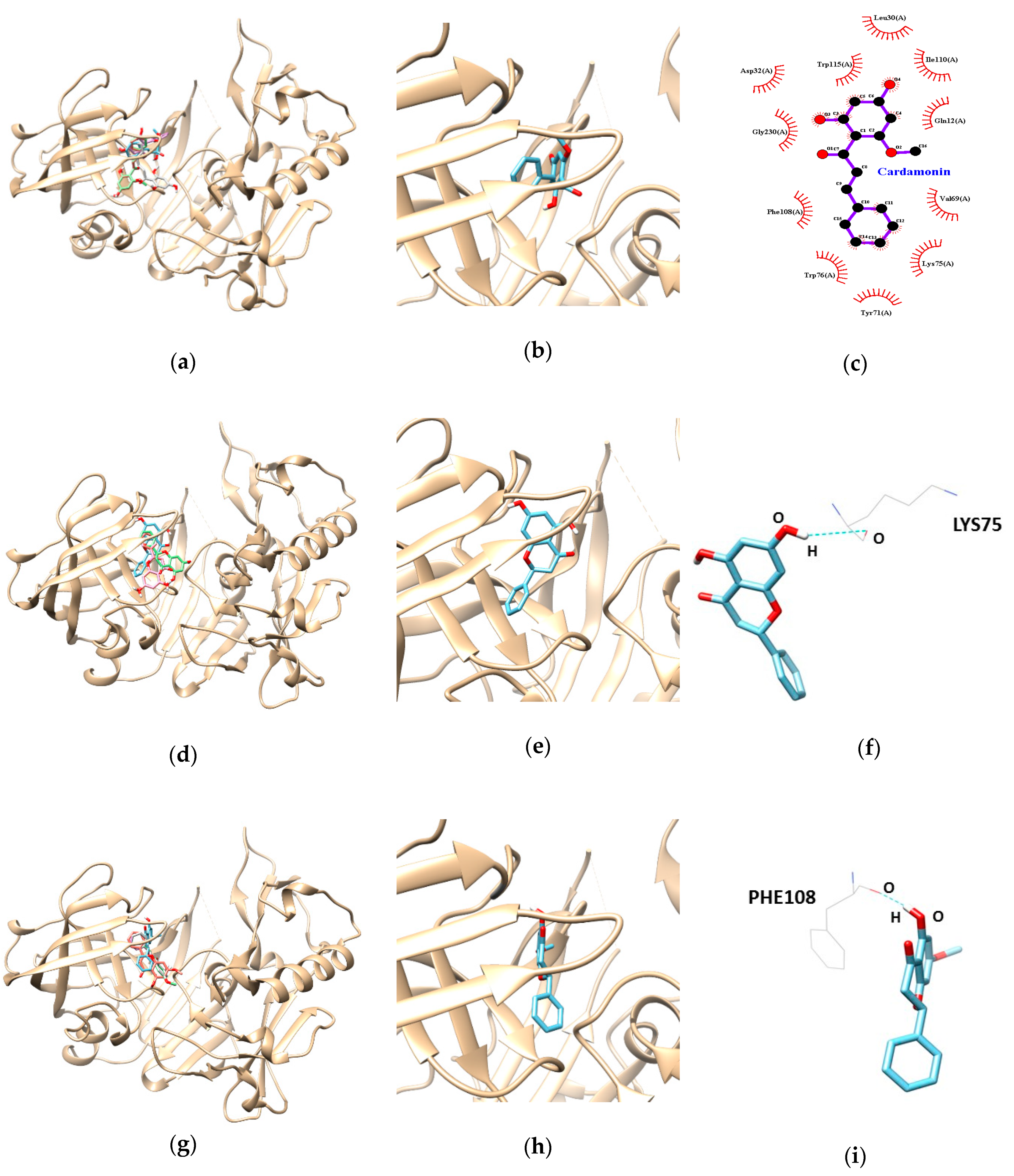
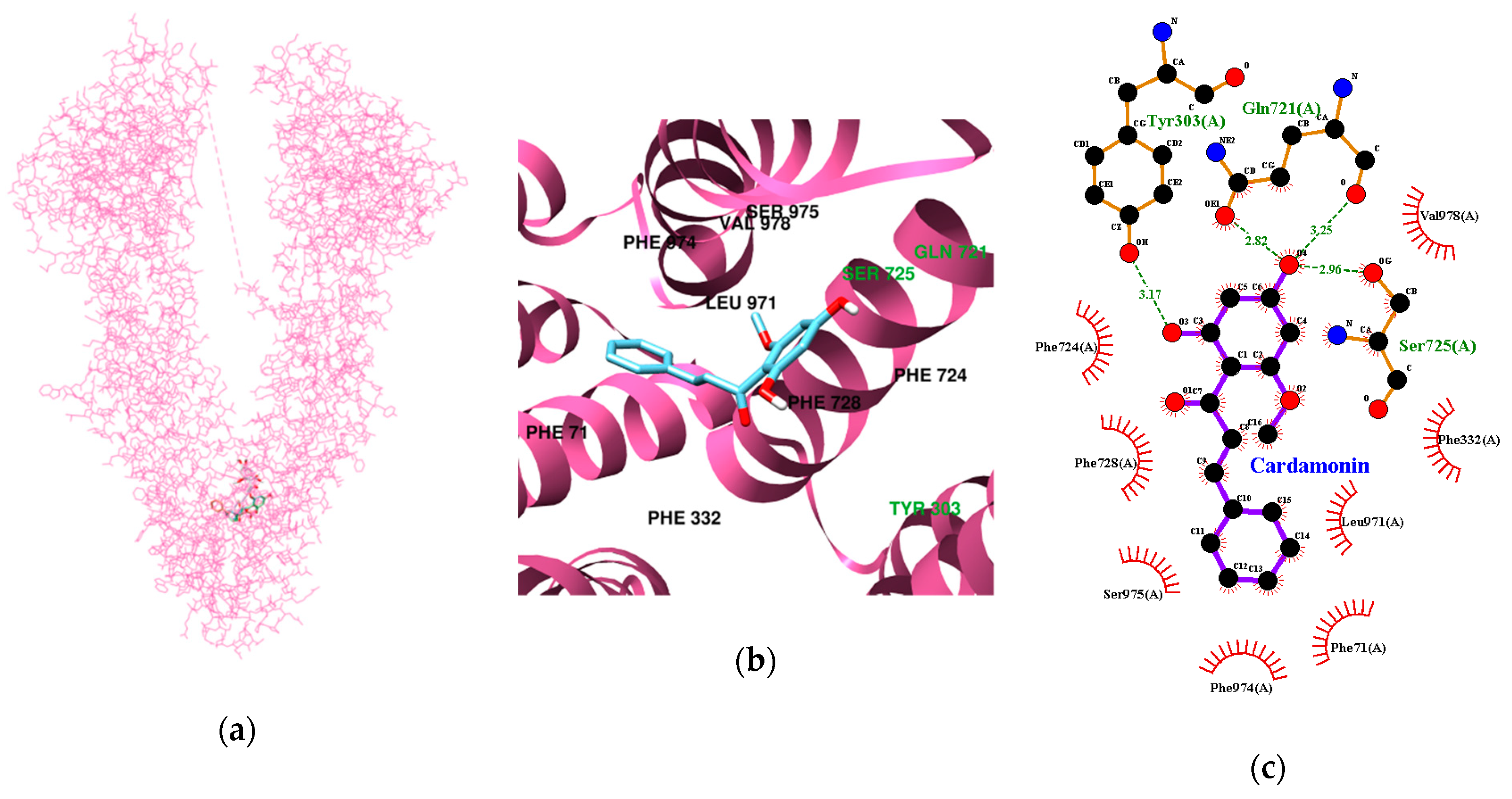
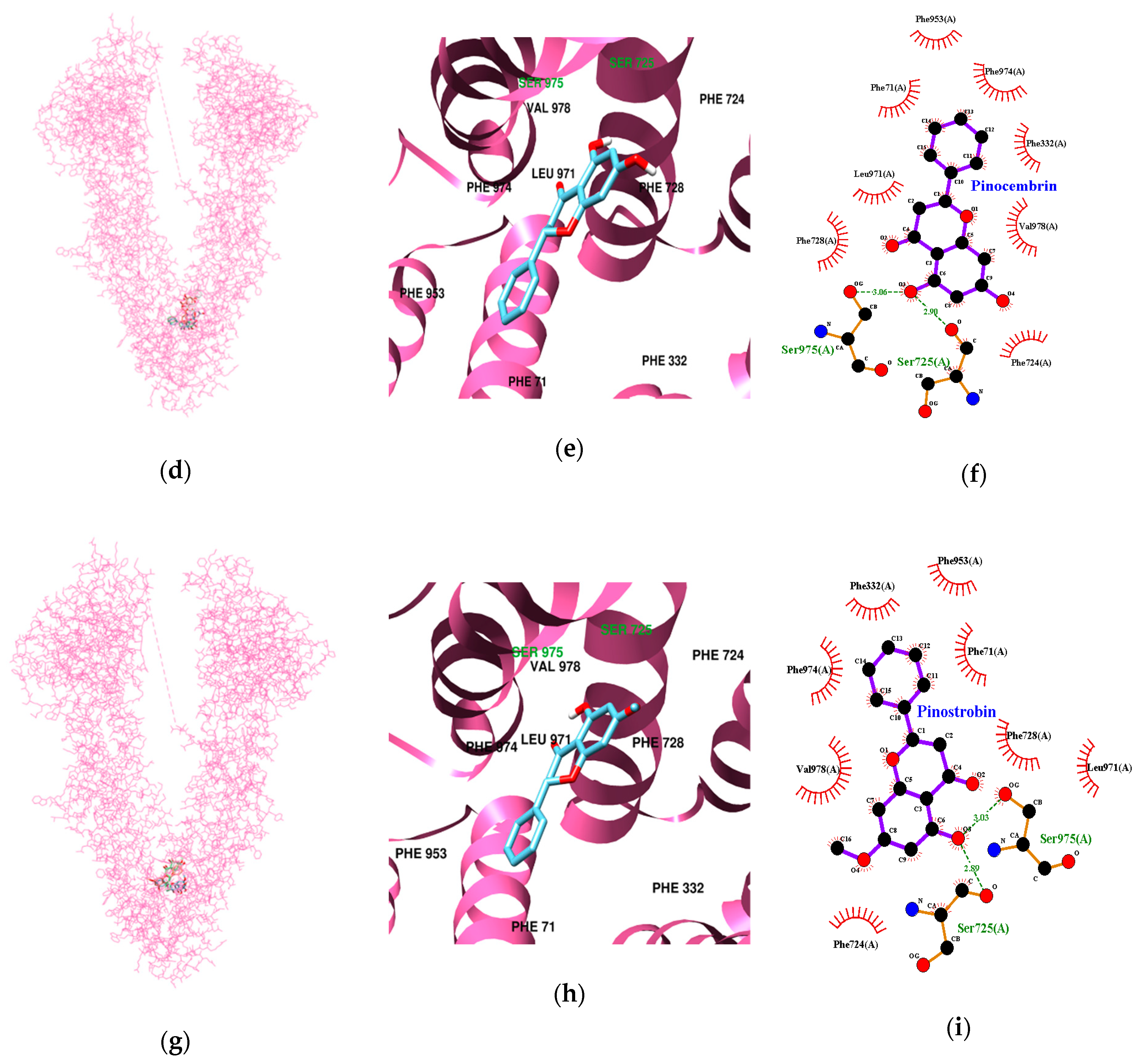
3.3. Molecular Docking Study of Cardamonin, Pinocembrin, and Pinostrobin with BACE1 and P-Glycoprotein (P-gp)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Selkoe, D. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J. Alzheimer’s Dis. 2001, 3, 75–80. [Google Scholar] [CrossRef]
- LaFerla, F. Pathways linking Abeta and tau pathologies. Biochem. Soc. Trans. 2010, 38, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, S.L.; Qian, L.; Jin, J.L.; Li, H.; Xu, Y.; Zhu, X.-L. Diammonium glycyrrhizinate attenuates Abeta(1–42)-induced neuroinflammation and regulates MAPK and NF-κB pathways in vitro and in vivo. CNS Neurosci. Ther. 2013, 19, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Botteri, G.; Salvadó, L.; Gumà, A.; Lee Hamilton, D.; Meakin, P.; Montagut, G.; Ashford, M.; Ceperuelo-Mallafré, V.; Fernández-Veledo, S.; Vendrell, J.; et al. The BACE1 product sAPPβ induces ER stress and inflammation and impairs insulin signaling. Metabolism 2018, 85, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Frisardi, V.; Imbimbo, B.; Capurso, C.; Logroscino, G.; Sancarlo, D.; Seripa, D.; Vendemiale, G.; Pilotto, A.; Solfrizzi, V. REVIEW: γ-Secretase inhibitors for the treatment of Alzheimer’s disease: The current state. CNS Neurosci. Ther. 2010, 16, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hicks, C.; He, W.; Wong, P.; Macklin, W.; Trapp, B.; Yan, R. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 2006, 9, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Savonenko, A.; Melnikova, T.; Laird, F.; Stewart, K.; Price, D.; Wong, P. Alteration of BACE1- dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc. Natl. Acad. Sci. USA 2008, 105, 5585–5590. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Brindisi, M.; Tang, J. Developing beta-secretase inhibitors for treatment of Alzheimer’s disease. J. Neurochem. 2012, 120, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Vassar, R. Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014, 13, 319–329. [Google Scholar] [CrossRef]
- Yan, R. Stepping closer to treating Alzheimer’s disease patients with BACE1 inhibitor drugs. Transl. Neurodegener. 2016, 5, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R. BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease. Alzheimers Res. Ther. 2014, 6, 89–102. [Google Scholar] [CrossRef]
- Eng-Chong, T.; Yean-Kee, L.; Chin-Fei, C.; Choon-Han, H.; Sher-Ming, W.; Thio Li-Ping, C.; Gen-Teck, F.; Khalid, N.; Rahman, N.; Karsani, S.; et al. Boesenbergia rotunda: From Ethnomedicine to Drug Discovery. Evid. Based Complement. Alternat. Med. 2012, 1–25. [Google Scholar] [CrossRef]
- Kirana, C.; Jones, G.; Record, I.; McIntosh, G. Anticancer properties of panduratin A isolated from Boesenbergia pandurata (Zingiberaceae). J. Nat. Med. 2007, 61, 131–137. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Medicinal Plants in the Management of Cancer: A Review. Int. J. Complement. Alt. Med. 2017, 9, 009291. [Google Scholar]
- Xian, Y.; Ip, S.; Lin, Z.; Mao, Q.; Su, Z.; Lai, X. Protective effects of pinostrobin on β-amyloid-induced neurotoxicity in PC12 cells. Cell Mol. Neurobiol. 2012, 32, 1223–1230. [Google Scholar] [CrossRef]
- Chow, Y.; Lee, K.; Vidyadaran, S.; Lajis, N.; Akhtar, M.; Israf, D.; Syahida, A. Cardamonin from Alpinia rafflesiana inhibits inflammatory responses in IFN-γ/LPS-stimulated BV2 microglia via NF-κB signalling pathway. Int. Immunopharmacol. 2012, 12, 657–665. [Google Scholar] [CrossRef]
- Peng, S.; Hou, Y.; Yao, J.; Fang, J. Activation of Nrf2-driven antioxidant enzymes by cardamonin confers neuroprotection of PC12 cells against oxidative damage. Food Funct. 2017, 8, 997–1007. [Google Scholar] [CrossRef]
- Liu, R.; Gao, M.; Yang, Z.H.; Du, G.H. Pinocembrin protects rat brain against oxidation and apoptosis induced by ischemia-reperfusion both in vivo and in vitro. Brain Res. 2008, 1216, 104–115. [Google Scholar] [CrossRef]
- Jin, X.; Liu, Q.; Jia, L.; Li, M.; Wang, X. Pinocembrin attenuates 6-OHDA-induced neuronal cell death through Nrf2/ARE pathway in SH-SY5Y cells. Cell Mol. Neurobiol. 2015, 35, 323–333. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Mir, A.; Cheng, L.; Wang, L.; Zhao, L.; Cui, Q.; Zhao, W.; Wang, H. Inhibition of beta-amyloid-induced neurotoxicity by pinocembrin through Nrf2/HO-1 pathway in SH-SY5Y cells. J. Neurol. Sci. 2016, 368, 223–230. [Google Scholar] [CrossRef]
- Saad, M.; Abdel Salam, R.; Kenawy, S.; Attia, A. Pinocembrin attenuates hippocampal inflammation, oxidative perturbations and apoptosis in a rat model of global cerebral ischemia reperfusion. Pharmacol. Rep. 2015, 67, 115–122. [Google Scholar] [CrossRef]
- Liu, R.; Wu, C.; Zhou, D.; Yang, F.; Tian, S.; Zhang, L.; Zhang, T.; Du, G. Pinocembrin protects against β-amyloid-induced toxicity in neurons through inhibiting receptor for advanced glycation end products (RAGE)-independent signaling pathways and regulating mitochondrion-mediated apoptosis. BMC Med. 2012, 10, 105–126. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Song, J.; Zhou, D.; Huang, C.; Bai, X.; Xie, T.; Zhang, X.; Li, Y.; Wu, C.; et al. Pinocembrin improves cognition and protects the neurovascular unit in Alzheimer related deficits. Neurobiol. Aging 2014, 35, 1275–1285. [Google Scholar] [CrossRef]
- Li, C.; Tang, B.; Feng, Y.; Tang, F.; Pui-Man Hoi, M.; Su, Z.; Ming-Yuen Lee, S. Pinostrobin Exerts Neuroprotective Actions in Neurotoxin-Induced Parkinson’s Disease Models through Nrf2 Induction. J. Agric. Food Chem. 2018, 66, 8307–8318. [Google Scholar] [CrossRef]
- Youn, K.; Park, J.H.; Lee, J.; Jeong, W.S.; Ho, C.T.; Jun, M. The Identification of Biochanin A as a Potent and Selective β-Site App-Cleaving Enzyme 1 (Bace1) Inhibitor. Nutrients 2016, 8, 637. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Cheng, Y.; Prusoff, W. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar]
- Mizutani, T.; Nakamura, T.; Morikawa, R.; Fukuda, M.; Mochizuki, W.; Yamauchi, Y.; Nozaki, K.; Yui, S.; Nemoto, Y.; Nagaishi, T.; et al. Real-time analysis of P-glycoprotein-mediated drug transport across primary intestinal epithelium three-dimensionally cultured in vitro. Biochem. Biophys. Res. Commun. 2012, 419, 238–243. [Google Scholar] [CrossRef]
- Youn, K.; Park, J.; Lee, S.; Lee, S.; Lee, J.; Yun, E.; Jeong, W.; Jun, M. BACE1 Inhibition by Genistein: Biological Evaluation, Kinetic Analysis, and Molecular Docking Simulation. J. Med. Food 2018, 21, 416–420. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S.; Puripattanavong, J.; Panphadung, T. HIV-1 protease inhibitory substances from the rhizomes of Boesenbergia pandurata Holtt. Songklanakarin J. Sci. Technol. 2003, 25, 6–11. [Google Scholar]
- De Castro, C.; Costa, P.; Laktin, G.; de Carvalho, P.; Geraldo, R.; de Moraes, J.; Pinto, P.; Couri, M.; Pinto Pde, F.; Da Silva Filho, A. Cardamonin, a schistosomicidal chalcone from Piper aduncum L. (Piperaceae) that inhibits Schistosoma mansoni ATP diphosphohydrolase. Phytomedicine 2015, 22, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M. Bioactive peptides and their potential use for the prevention of diseases associated with Alzheimer’s disease and mental health disorders. Ann. Psychiatry Ment. Health 2014, 2, 1017–1025. [Google Scholar]
- Pajouhesh, H.; Lenz, G. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef]
- Sasidharan, R.; Manju, S.; Uçar, G.; Baysal, I.; Mathew, B. Identification of Indole-Based Chalcones: Discovery of a Potent, Selective, and Reversible Class of MAO-B Inhibitors. Arch. Pharm. (Weinh.) 2016, 349, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, X.; Qi, Y.; Mei, C.; Sun, X.; Du, G. Uptake characteristics of pinocembrin and its effect on p-glycoprotein at the blood-brain barrier in in vitro cell experiments. J. Asian Nat. Prod. Res. 2012, 14, 14–21. [Google Scholar] [CrossRef]
- Goedert, M.; Crowther, R.; Spillantini, M. Tau mutations cause frontotemporal dementias. Nueron 1998, 21, 955–958. [Google Scholar] [CrossRef]
- Taniguchi, S.; Suzuki, N.; Masuda, M.; Hisanaga, S.; Iwatsubo, T.; Goedert, M.; Hasegawa, M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J. Biol. Chem. 2005, 280, 7614–7623. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Arendash, G.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.; Tan, J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Aparna, J.; Paul, A.; Lankadasari, M.; Mohammed, S.; Binu, V.; Santhoshkumar, T.; Reshmi, G.; Harikumar, K. Cardamonin inhibits colonic neoplasia through modulation of MicroRNA expression. Sci. Rep. 2017, 7, 13945. [Google Scholar] [CrossRef] [PubMed]
- Charoensin, S.; Punvittayagul, C.; Pompimon, W.; Mevatee, U.; Wongpoomchai, R. Toxicological and clastogenic evaluation of pinocembrin and pinostrobin isolated from Boesenbergia pandurata in Wistar rats. Thai J. Toxicol. 2010, 25, 29–40. [Google Scholar]
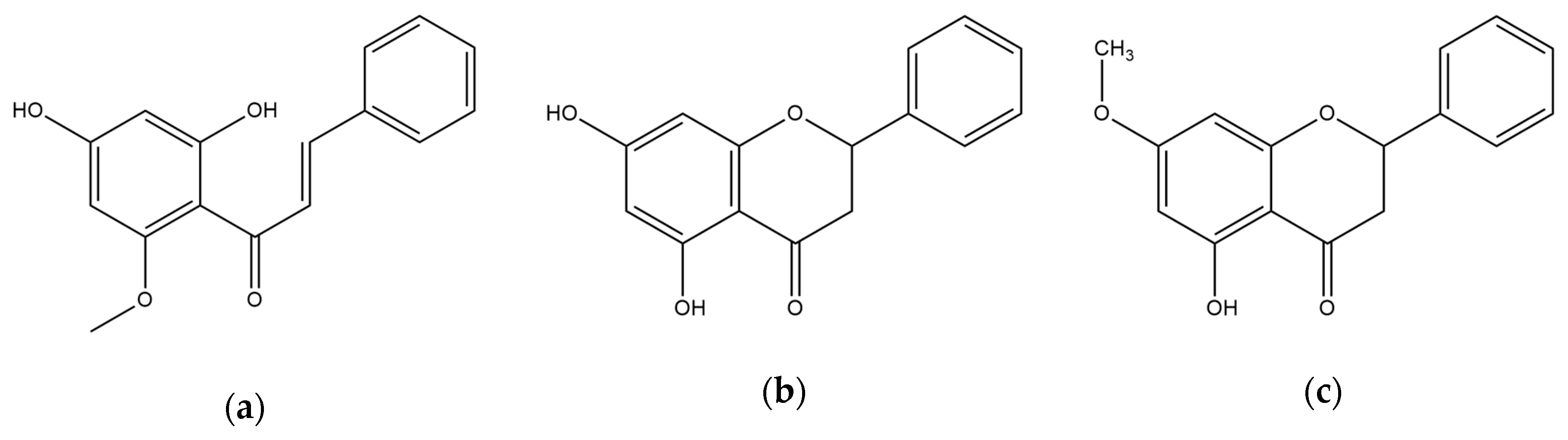
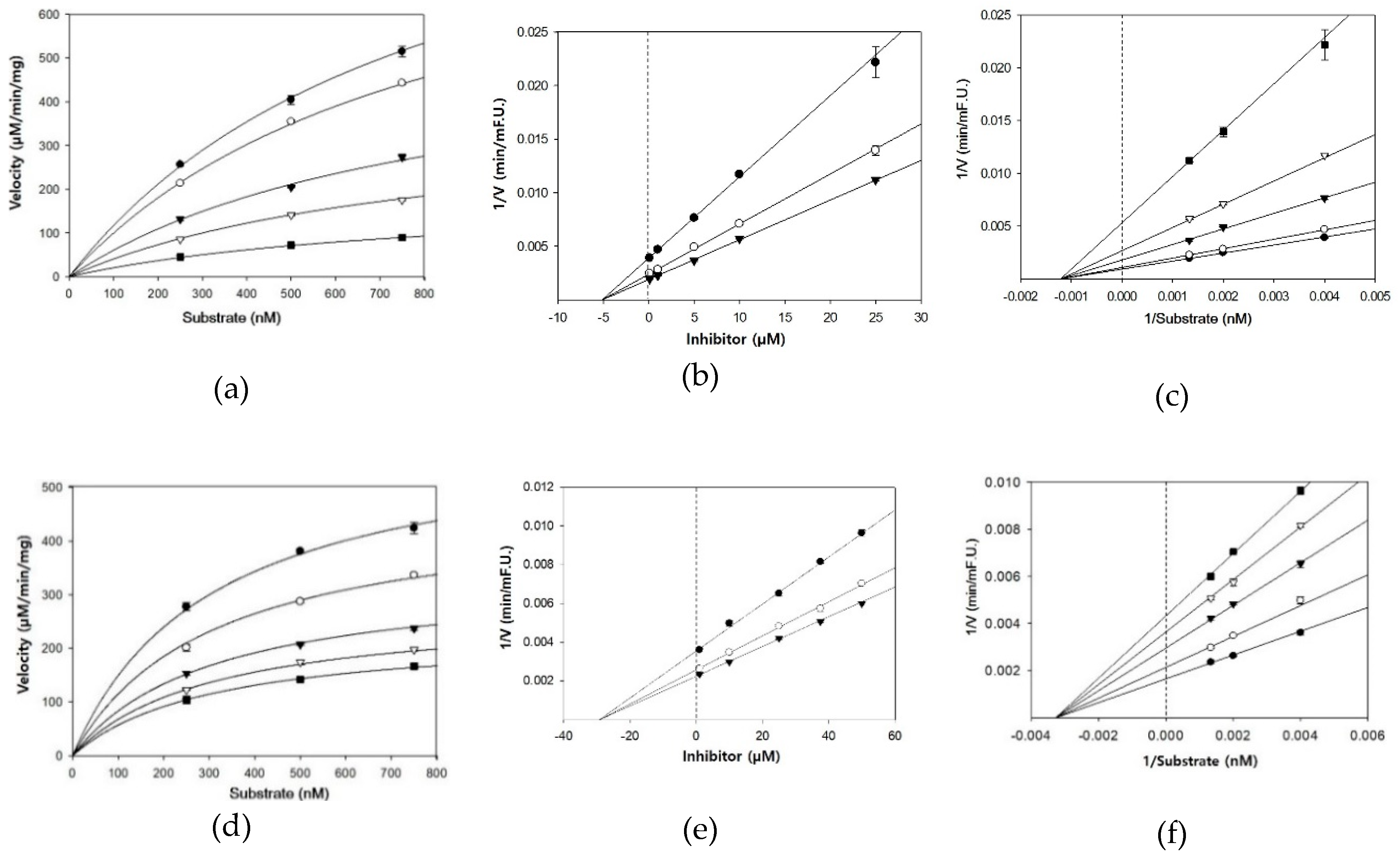
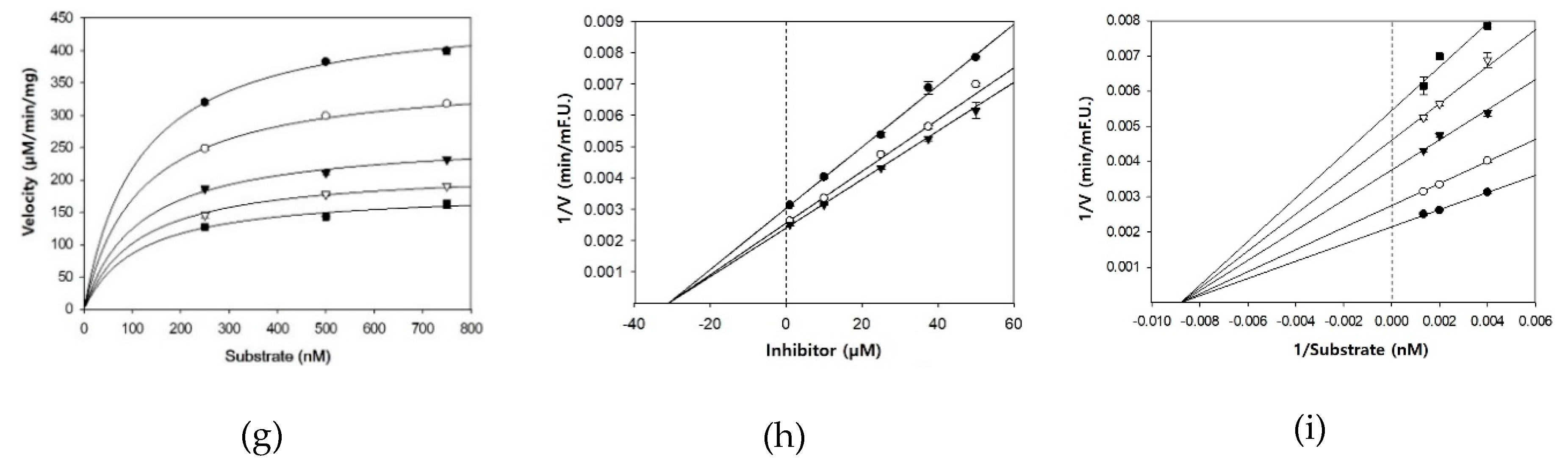
| Compounds | Concentration (µM) | IC50 (mean ± SD, µM) 1 | Km | Vmax | Ki 2 | Inhibition Mode |
|---|---|---|---|---|---|---|
| Cardamonin | 0.1 | 4.35 ± 0.38 | 833.3 | 1139 | 5.1 | Noncompetitive |
| 1 | 836 | |||||
| 5 | 543 | |||||
| 10 | 370 | |||||
| 25 | 190 | |||||
| Pinocembrin | 1 | 27.01 ± 2.12 | 312.5 | 507 | 29.3 | Noncompetitive |
| 10 | 471 | |||||
| 25 | 336 | |||||
| 37.5 | 270 | |||||
| 50 | 229 | |||||
| Pinostrobin | 1 | 28.44 ± 1.96 | 116.3 | 467 | 30.9 | Noncompetitive |
| 10 | 362 | |||||
| 25 | 268 | |||||
| 37.5 | 216 | |||||
| 50 | 181 | |||||
| Resveratrol 3 | 14.59 ± 0.79 |
| Sample (μM) | TACE | Trypsin | Chymotrypsin | Elastase |
|---|---|---|---|---|
| Cardamonin | ||||
| 50 | 1.38 ± 0.36 | 1.13 ± 0.18 | 6.20 ± 0.41 | 2.29 ± 0.41 |
| 100 | 2.69 ± 1.44 | 3.74 ± 0.53 | 4.23 ± 0.55 | 1.41 ± 0.19 |
| Pinocembrin | ||||
| 10 | 5.15 ± 0.98 | 4.35 ± 0.26 | 7.18 ± 1.87 | 3.24 ± 0.19 |
| 100 | 6.95 ± 1.52 | 4.96 ± 0.27 | 8.61 ± 1.09 | 2.56 ± 0.08 |
| Pinostrobin | ||||
| 10 | 8.53 ± 1.05 | 3.19 ± 0.16 | 3.29 ± 0.25 | 5.21 ± 0.41 |
| 100 | 9.19 ± 1.20 | 1.52 ± 0.05 | 5.41 ± 0.49 | 2.63 ± 0.37 |
| Ligands | Lowest Energy (kcal/mol) | No. of H-Bond | H-Bonds Interacting Residues | Bond Distance (Å) | van der Waals Interacting Residues |
|---|---|---|---|---|---|
| Cardamonin | −9.5 | Gln12, Leu30, Asp32, Val69, Tyr71, Lys75, Trp76, Phe108, Ile110, Trp115, Gly230 | |||
| Pinocembrin | −7.9 | 1 | LYS 75 | 3.09 | |
| Pinostrobin | −7.6 | 1 | PHE 108 | 1.99 |
| Ligands | Lowest Energy (kcal/mol) | No. of H-Bond | H-Bonds Interacting Residues | Bond distance (Å) | van der Waals Interacting Residues |
|---|---|---|---|---|---|
| Cardamonin | −9.78 | 4 | Tyr303, Gln721, Ser725 | 3.17 2.82 and 3.25 2.96 | Phe71, Phe332, Phe724, Phe728, Leu971, Phe974, Ser975, Val978 |
| Pinocembrin | −10.64 | 2 | Ser725, Ser975 | 2.90 3.06 | Phe71, Phe332, Phe724, Phe728, Phe953, Leu971, Phe974, Val978 |
| Pinostrobin | −10.37 | 2 | Ser725,Ser975 | 2.89 3.03 | Phe71, Phe332, Phe724, Phe728, Phe953, Leu971, Phe974, Val978 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youn, K.; Jun, M. Biological Evaluation and Docking Analysis of Potent BACE1 Inhibitors from Boesenbergia rotunda. Nutrients 2019, 11, 662. https://doi.org/10.3390/nu11030662
Youn K, Jun M. Biological Evaluation and Docking Analysis of Potent BACE1 Inhibitors from Boesenbergia rotunda. Nutrients. 2019; 11(3):662. https://doi.org/10.3390/nu11030662
Chicago/Turabian StyleYoun, Kumju, and Mira Jun. 2019. "Biological Evaluation and Docking Analysis of Potent BACE1 Inhibitors from Boesenbergia rotunda" Nutrients 11, no. 3: 662. https://doi.org/10.3390/nu11030662
APA StyleYoun, K., & Jun, M. (2019). Biological Evaluation and Docking Analysis of Potent BACE1 Inhibitors from Boesenbergia rotunda. Nutrients, 11(3), 662. https://doi.org/10.3390/nu11030662