Abstract
Cardio-oncology is a clinical/scientific discipline which aims to prevent and/or treat cardiovascular diseases in cancer patients. Although a large number of cancer treatments are known to cause cardiovascular toxicity, they are still widely used because they are highly effective. Unfortunately, therapeutic interventions to prevent and/or treat cancer treatment-induced cardiovascular toxicity have not been established yet. A major challenge for such interventions is to protect the cardiovascular system without compromising the therapeutic benefit of anticancer medications. Intriguingly, the polyphenolic natural compound resveratrol and its analogs have been shown in preclinical studies to protect against cancer treatment-induced cardiovascular toxicity. They have also been shown to possess significant anticancer properties on their own, and to enhance the anticancer effect of other cancer treatments. Thus, they hold significant promise to protect the cardiovascular system and fight the cancer at the same time. In this review, we will discuss the current knowledge regarding the cardio-protective and the anticancer properties of resveratrol and its analogs. Thereafter, we will discuss the challenges that face the clinical application of these agents. To conclude, we will highlight important gaps of knowledge and future research directions to accelerate the translation of these exciting preclinical findings to cancer patient care.
1. Introduction
Cardio-oncology has emerged as a novel clinical/scientific discipline with a goal to prevent and/or treat cardiovascular diseases in cancer patients, particularly those arising as adverse effects of cancer treatment [1]. While cardio-oncology has recently been acknowledged as a clinical subspecialty [2], the cardiovascular adverse effects of cancer treatment were recognized more than 40 years ago. For instance, the cardiotoxicity of anthracyclines was reported in cancer patients in the early 1970s [3,4]. Despite the known cardiotoxic effects of these anticancer agents, they are still commonly used to treat a wide variety of malignancies in pediatric and adult cancer patients, due to their effectiveness as anticancer agents. Although every effort has been exerted to design safer anticancer agents, it seems that the occurrence of cardiovascular adverse effects during cancer treatment is inevitable, since newer anticancer drugs have also demonstrated significant cardiovascular adverse effects. Trastuzumab has been shown to cause cardiac dysfunction in breast cancer patients which was exacerbated if used in combination with an anthracycline [5,6]. The new proteasome inhibitor carfilzomib has been reported to cause cardiovascular adverse events in more than 18% of patients [7]. Similarly, cardiovascular adverse effects have been reported in cancer patients receiving tyrosine kinase inhibitors e.g., sunitinib [8] and immune checkpoint inhibitors e.g., nivolumab and ipilimumab [9]. In addition to chemotherapy, radiation, particularly chest radiation, has been shown to be detrimental to the cardiovascular system [10]. Therefore, there is an urgent need to identify new therapeutic agents that can prevent and/or reverse cancer treatment-induced cardiovascular adverse effects without compromising their anticancer therapeutic benefits.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a polyphenolic compound that is naturally occurring in several plant based foods and beverages including red wine, grapes, berries, and peanuts [11]. In addition, one of the richest sources of resveratrol is Polygonum cuspidatum, a herb that plays an important role in Chinese traditional medicine [12]. Indeed, resveratrol is a phytoalexin produced by more than 70 species of plants in response to stressful situations such as infection [13]. Resveratrol has been a focus of scientific research over the past 25 years, primarily as an active ingredient in red wine that is responsible for the “French Paradox” [14]. The “French Paradox” describes the low incidence of cardiovascular diseases in the French population despite high consumption of saturated fats [15]. Nevertheless, the concentration of resveratrol in red wine and other plants is very low and research into resveratrol has mostly used high doses that can only be achieved by supplementary intake of resveratrol as a nutraceutical [16]. Additionally, orally administered resveratrol shows very low bioavailability despite almost complete absorption (reviewed in [17]). Resveratrol low bioavailability is due to its first-pass intestinal/hepatic metabolism to sulfate and glucuronide metabolites [18]. After an oral dose of 25 mg in healthy human volunteers, the plasma concentration of resveratrol was less than 10 µg/L (≈40 nM), while the total concentration of resveratrol and its metabolites was 400–500 µg/L (≈2 µM) [18,19]. Higher peak plasma concentrations of resveratrol (approximately 500–2000 µg/L ≈2–10 µM) were achieved with doses of 2000–5000 mg [20,21,22]. Accordingly, the findings of in vitro studies using resveratrol concentrations higher than 10 µM should be interpreted with caution.
Preclinical in vitro and in vivo studies have revealed that resveratrol possesses a plethora of beneficial effects in an array of diseases (comprehensively reviewed in [23]), including: cardiovascular diseases (reviewed in [24,25,26]), cancer (reviewed in [27,28]), obesity (reviewed in [29]), osteoporosis (reviewed in [30]), Alzheimer’s disease (reviewed in [31]), and diabetes (reviewed in [32]). Resveratrol has also gained considerable attention as an anti-aging molecule (reviewed in [33]). It may be surprising that a single molecule can have beneficial effects in such a wide variety of diseases. Indeed, this may be attributed to the multiple molecular targets of resveratrol (reviewed in [34]). Resveratrol has been shown to activate AMP-activated protein kinase (AMPK) [35], sirtuin 1 (SIRT1) [36,37], super oxide dismutase (SOD) [38], nuclear factor erythroid 2–related factor 2 (NRF2) [39], vascular endothelial growth factor (VEGF) [40], and endothelial nitric oxide synthase (eNOS) [37,41]. It has also been shown to inhibit cyclooxygenases [42], phosphodiesterases (PDEs) [43], nuclear factor kappa B (NF-κB) [44,45], aryl hydrocarbon receptor (AhR) [46], phosphoinositide 3-kinase (PI3K) [47], mammalian target of rapamycin (mTOR) [48], and ribosomal protein S6K [49].
Since resveratrol has been shown to possess both cardio-protective and anticancer properties (Figure 1), leveraging these properties in cardio-oncology seems very promising. Indeed, a considerable amount of preclinical literature demonstrates that resveratrol protects against the cardiovascular adverse effects of a number of cancer treatments. Similarly, a plethora of in vitro and in vivo studies demonstrate that resveratrol enhances the anticancer effects of these treatments. Therefore, the objective of this review is to summarize and critically evaluate the contemporary knowledge that describes the effects of resveratrol when combined with cancer treatments on the cardiovascular system as well as on the tumor/cancer cells, in a cardio-oncology perspective. We will first discuss the interaction of resveratrol with anthracyclines, since this group of chemotherapeutic agents is considered to be the most cardiotoxic. Thereafter, we will discuss the existent knowledge about the effect of resveratrol on cardiovascular toxicity and chemotherapeutic efficacy of other cancer treatments that are known to be cardiotoxic. Moreover, we will discuss the potential cardio-protective and anticancer effects of the most common resveratrol analogs. To conclude, we will highlight significant gaps in knowledge and discuss important future research directions in order to translate this preclinical knowledge to benefit cardio-oncology patients.
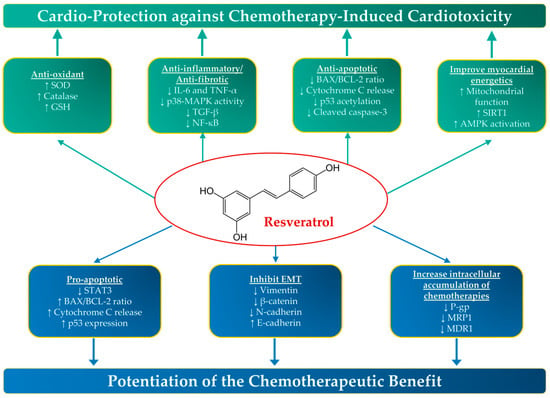
Figure 1.
The cardio-protective and anticancer properties of resveratrol. Resveratrol has been shown to possess both cardio-protective and anticancer properties in preclinical studies through a number of molecular mechanisms. AMPK, Adenosine Monophosphate-Activated Protein Kinase; Bax, BCL (B Cell Lymphoma)-Associated X; EMT, Epithelial Mesenchymal Transition; GSH, Glutathione; IL-6, Interleukin-6; MAPK, Mitogen-activated protein kinase; MDR1, Multidrug Resistance-1; MRP1, Multidrug resistance-associated protein-1; NF-κB, nuclear factor kappa B; P-gp, P-glycoprotein; STAT3, Signal transducer and activator of transcription 3; SIRT1, Sirtuin 1; SOD, Superoxide Dismutase; TNF-α, Tumor Necrosis Factor-alpha; ↑, increase; and ↓, decrease.
2. Anthracyclines
2.1. Resveratrol Protects against Anthracycline-Induced Cardiotoxicity
Anthracyclines (e.g., doxorubicin (DOX) and daunorubicin) are a group of chemotherapeutic agents used to treat a wide variety of human malignancies. However, the clinical use of these highly effective agents is limited by a significant anthracycline-induced cardiotoxicity which can progress to end-stage heart failure [50,51]. DOX has both acute and chronic toxic effects on the cardiovascular system. The acute effects occur in approximately 11% of patients during or soon after DOX administration and include various arrhythmias, hypotension, and acute heart failure [52,53]. On the other hand, chronic DOX cardiotoxicity is dose-dependent and results in irreversible cardiomyopathic changes that affect 1.7% of patients treated with DOX [54]. The exact mechanism of anthracycline-induced cardiotoxicity and its progression to heart failure has not been fully elucidated yet; however, several mechanisms have been proposed. These mechanisms include increased oxidative stress, mitochondrial dysfunction, apoptotic cell death, altered molecular signaling, and perturbed myocardial energy metabolism [52,55,56,57]. Interestingly, resveratrol has been shown to prevent all these detrimental changes, leading to significant protection against DOX-induced cardiotoxicity in preclinical studies in vitro (Table 1) and in vivo (Table 2).

Table 1.
In vitro studies demonstrating the cardio-protective effects of resveratrol against anthracycline-induced cardiotoxicity.

Table 2.
In vivo studies demonstrating the cardio-protective effect of resveratrol against anthracycline-induced cardiotoxicity.
DOX tends to generate reactive oxygen species (ROS) during its metabolism [84], largely because it is converted to an unstable semiquinone intermediate that favors ROS generation [85]. In addition, mitochondrial DNA damage induced directly by DOX or indirectly by DOX-generated ROS leads to respiratory chain failure and generation of more ROS [86]. DOX-induced oxidative stress is evident through increased levels of ROS, lipid peroxidation, and reductions in endogenous antioxidants and sulfhydryl group levels [87,88,89]. Resveratrol has been shown to abrogate DOX-induced oxidative stress by upregulating several antioxidant enzymes such as superoxide dismutase and catalase in vitro [58,60,62,66] and in vivo [68,70,79,81]. DOX-induced cardiotoxicity has also been associated with cardiomyocyte apoptosis [53]. DOX-generated ROS promotes the release of calcium from the sarcoplasmic reticulum, leading to an increase in intracellular calcium levels [90]. Mitochondria can capture a large quantity of the released calcium, eventually leading to the release of cytochrome c and apoptosis inducing factor [91,92]. In addition, DNA lesions induced directly by DOX or indirectly by DOX-generated ROS result in increased expression and activation of p53, which up-regulates genes such as the pro-apoptotic BAX [93]. Resveratrol has been shown to mitigate DOX-induced apoptosis to confer cardio-protection both in vitro [62,64] and in vivo [73,76,77].
Necrotic cell death has also been implicated in DOX-induced cardiotoxicity. Several studies have demonstrated that cardiac expression of pro-inflammatory cytokines, inflammatory cell infiltration, and necrotic cells are increased in the hearts of DOX-treated animals [53,94]. Mechanistically, DOX-generated ROS can lead to mitochondrial calcium overloading and opening of mitochondrial permeability transition pores which result in mitochondrial swelling, ATP depletion, and eventually necrotic cell death [95]. A few studies have shown that resveratrol protects against DOX-induced necrotic cell death [78,80]. Although a number of studies have shown that resveratrol protects against DOX-induced myocardial fibrosis [80,81,82], little is known about the effect of resveratrol on modulating the inflammatory response to DOX. Autophagy has also been shown to contribute to DOX-induced cell death [96]. However, the studies describing the effect of resveratrol on DOX-induced autophagy are inconsistent. On one hand, one study has shown that resveratrol prevents DOX-induced autophagic cell death [61]. On the other hand, a number of studies demonstrate that resveratrol induces autophagy to protect against DOX-induced apoptosis and cardiotoxicity [64,67]. This discrepancy may have arisen from the fact that autophagy can lead to either cell survival or cell death depending on the context [97]. Finally, cardiovascular senescence may also play a role in DOX cardiotoxicity as it has been shown that cultured neonatal rat cardiomyocytes treated with DOX exhibited characteristic changes similar to cardiomyocytes of aged rats [98]. Although resveratrol has been proposed as an anti-aging molecule [99], little is known about its effect on DOX-induced cardiovascular senescence. We have recently shown that the combined treatment of DOX followed by angiotensin II upregulated several senescence-associated genes including: p21, insulin-like growth factor binding protein 3 (Igfbp3), and growth arrest and DNA damage inducible gamma (Gadd45g) which was corrected by the coadministration of resveratrol with DOX [83].
Numerous studies have shown that DOX reduces cardiac energy reserves, particularly ATP and phosphocreatine, in different animal models of cardiotoxicity as well as in patients receiving DOX treatment [100,101,102]. This energetic deficit caused by DOX has been firstly attributed to compromised mitochondrial function [84]. More recently, DOX-induced cardiotoxicity has been shown to affect the phosphotransfer network of creatine kinase and the AMPK signaling pathway [103]. Intriguingly, resveratrol has been shown to ameliorate DOX-induced mitochondrial dysfunction [60,79] and to activate the AMPK signaling pathway [65], potentially leading to improved myocardial energetics and protection from DOX-induced cardiotoxicity.
2.2. Resveratrol Augments DOX-Induced Anticancer Effects
Protection against cancer treatment-induced toxicity is challenging because any protective treatment or strategy should not reduce the therapeutic benefit of the cancer treatment. In addition to its cardio-protective properties, resveratrol has also been shown to possess significant chemo-preventive and anticancer effects. Therefore, resveratrol holds the promise that it can protect the heart and fight the cancer at the same time. Since the anticancer properties of resveratrol have been discussed in a number of excellent reviews [27,104,105,106], we will focus here on the studies that demonstrated an additive and/or synergistic effect when resveratrol is combined with an anthracycline both in vitro (Table 3) and in vivo (Table 4).

Table 3.
In vitro studies demonstrating anticancer effect of the combination of resveratrol and anthracycline treatment.

Table 4.
In vivo studies demonstrating anticancer effect of resveratrol co-administration with anthracyclines.
A plethora of in vitro studies have shown that resveratrol enhanced the cytotoxic effect of DOX primarily in human breast cancer cells [107,109,110,111,112,114,116,119,121], but also in glioblastoma [107], prostate cancer [107], ovarian cancer [59,125], melanoma [108], hepatocellular carcinoma [109], cervical cancer [109], pancreatic cancer [122], leukemia [107,118], colon carcinoma [113,117], multiple myeloma, and lymphoma [118]. Since DOX is the most commonly used chemotherapeutic agent in canine hemangiosarcoma, we have recently shown that resveratrol can also augment the cytotoxic effect of DOX in two hemangiosarcoma cell lines [123]. Since resveratrol itself has been shown to induce apoptosis in cancer cells, it is not surprising that it had an additive proapoptotic effect when combined with DOX [108,115,118,123]. More intriguingly, however, is the finding that resveratrol increased cellular accumulation of DOX by inhibiting P-glycoprotein (P-gp), multidrug resistance-1 (MDR1), and multidrug resistance-associated protein-1 (MRP1) [109,117,122]. This mechanism has been most noticeable in DOX-resistant subclones such as MCF-7/ADR [112,114,125].
Since a major limitation for the clinical utility of resveratrol is its poor bioavailability, it is important to show that resveratrol can also exert anticancer effects when administered orally in vivo. Indeed, a number of studies have demonstrated that oral resveratrol administration suppressed tumor growth when given alone [128,129,130] and augmented the chemotherapeutic effect of DOX when given in combination to tumor-bearing mice in vivo [112,120,121]. A major limitation to the vast majority of these studies is the use of immunocompromised nude mice implanted with human cancer cells (Table 4). This approach precludes studying the immunomodulatory properties of resveratrol which are increasingly recognized to be important players in its chemo-preventive and anticancer effects [131,132,133].
3. Other Cardiotoxic Cancer Treatments
3.1. Cisplatin
Cisplatin is a first-generation platinum-based chemotherapeutic agent. Unlike anthracyclines, cisplatin-induced cardiotoxicity is uncommon, and its exact prevalence is unknown [134]. Preclinical studies demonstrated that cisplatin-induced cardiotoxicity may be mediated by mitochondrial abnormalities, increased endoplasmic reticulum stress, oxidative stress, and apoptosis [135,136]. In contrast to the large number of studies that reported cardio-protective effects of resveratrol against anthracycline-induced cardiotoxicity, a single study was found on PubMed that reported the effect of resveratrol on cisplatin-induced cardiotoxicity. In this study, oral administration of resveratrol (5, 15, or 45 mg/kg/day for 10 days) protected against cisplatin-induced cardiotoxicity in adult male Wistar rats by alleviating cisplatin-induced oxidative stress [136]. However, resveratrol has been shown to protect against cisplatin-induced nephrotoxicity [137,138,139], gonadotoxicity [140,141,142], and ototoxicity [143,144,145]. Pretreatment with resveratrol did not significantly alter the plasma level of cisplatin; however, it lowered its urinary concentration and its accumulation in the kidneys, which may explain its protective effects [146].
Importantly, resveratrol has also been shown to potentiate the cytotoxic effect of cisplatin in vitro via different mechanisms including: glutamine metabolism inhibition in human hepatoma cells [147], increased mitochondrial oxidative damage in malignant mesothelioma cells [148], enhanced autophagic cell death in A549 cells [149], inducing the mitochondrial apoptotic pathway in human non-small cell lung cancer H838 and H520 cell lines [150], increasing the cellular uptake of cisplatin in parent and cisplatin-resistant HCT-116 colorectal cancer cells [151], induction of dual specificity phosphatase 1 in androgen-independent prostate cancer cells [152], preventing DNA repair of double-strand breaks [153] and phosphorylation of p53 at the serine 20 position in human breast cancer cells [154], and modulation of intrinsic apoptosis in H446 small cell lung cancer [155]. Resveratrol significantly enhanced the antineoplastic effect of cisplatin in a mouse model of ovarian cancer, through inhibition of glucose uptake in ovarian tumor cells with high baseline glycolytic rates [156].
3.2. Cyclophosphamide
Cyclophosphamide is an alkylating agent that is clinically used in the management of autoimmune conditions and a number of malignant diseases like lymphoma, leukemia, and breast cancer [157]. Although conventional doses of cyclophosphamide are rarely associated with cardiotoxicity, symptomatic cardiotoxicity occurs in 5–28% of patients treated with high doses [158,159]. Cyclophosphamide-induced cardiotoxicity presents as myocarditis, pericarditis, cardiomyopathy, reversible myocardial failure, and/or isolated arrhythmias [159,160]. Cyclophosphamide-induced oxidative and nitrosative stress are believed to mediate its cardiotoxicity [161]; however, the exact mechanism is not fully elucidated [157]. Resveratrol (10 mg/kg/day for 8 days) has been reported to protect against acute cyclophosphamide-induced cardiotoxicity, primarily via mitigation of cyclophosphamide-induced oxidative stress [162]. Importantly, resveratrol (50 µM) enhanced the antiproliferative and apoptotic effect of cyclophosphamide in MCF-7 breast cancer cells [163,164].
3.3. Arsenic Trioxide
Arsenic trioxide is a chemotherapeutic agent that is mainly used in the treatment of relapsed or refractory acute promyelocytic leukemia [165]. Its clinical utility has been limited by multiorgan toxicity, particularly cardiotoxicity [166]. Arsenic trioxide-induced cardiotoxicity causes QT prolongation which may lead to ventricular tachycardia, torsade de pointes, and sudden death [167]. The molecular mechanism underlying this adverse effect is believed to be arsenic trioxide-induced increase in calcium currents to cardiomyocytes [168]. In addition, arsenic trioxide has been shown to induce oxidative stress, inflammation, and apoptosis (reviewed in [166]). In a BALB/c mouse model of arsenic trioxide-induced cardiomyopathy in vivo, resveratrol was administered intravenously 3 mg/kg every other day for 3 doses, 1 h before arsenic trioxide administration. In this model, resveratrol ameliorated arsenic trioxide-induced QT interval prolongation, oxidative damage, and cardiomyocyte injury (apoptosis, myofibrillar loss, and vacuolization) [169]. Similarly, in Wistar rats, resveratrol was administered intravenously 8 mg/kg every other day for 4 doses, 1 h before arsenic trioxide administration. Pretreatment with resveratrol ameliorated arsenic trioxide-induced oxidative stress, intracellular calcium accumulation, pathological alterations, and cardiac dysfunction [170]. These protective effects were associated with an induction of antioxidant enzymes and a decrease in myocardial arsenic concentration [170]. In vitro studies have also shown that resveratrol confers protection against arsenic trioxide damage. In H9c2 cardiomyoblasts, resveratrol (1–10 µM) protected against arsenic trioxide-induced oxidative stress, apoptosis, and accumulation of intracellular calcium [169]. In guinea pig ventricular cardiomyocytes, resveratrol ameliorated arsenic trioxide-induced damage of the human ether-a-go-go-related gene (hERG) current, relieved endoplasmic reticulum stress, and shortened the action potential duration [171]. Resveratrol also had a protective effect against arsenic trioxide-induced oxidative damage in the feline brain, lung, and kidney [172,173,174], and nephrotoxicity and hepatotoxicity in rats [175,176].
Resveratrol enhanced the cytotoxic effect of arsenic trioxide in several cancer cell lines, including: human lung adenocarcinoma A549 cells, SK-N-SH neuroblastoma cells, lung adenocarcinoma A549 cells, MCF-7 breast cancer cells, and promyelocytic leukemia NB4 cells through increased ROS and augmented apoptotic response [177,178,179,180,181]. In BALB/c nude mice, treatment with resveratrol (16.5 mg/kg/day), arsenic trioxide (5 mg/kg/day), or the combination for 2 weeks caused significant reduction in tumor growth; however, there was no noticeable difference between the combination therapy and either drug given alone [181]. Intriguingly, Fan et al. concurrently studied the chemosensitizing and cardio-protective effects of resveratrol when combined with arsenic trioxide in vitro. Resveratrol (5 µM) increased ROS and potentiated arsenic trioxide cytotoxicity in human promyelocytic leukemia NB4 cells, whereas it abrogated arsenic trioxide-induced ROS and protected against its toxic effect in neonatal rat ventricular cardiomyocytes [178].
3.4. Tyrosine Kinase Inhibitors
Tyrosine kinase inhibitors (e.g., sunitinib, dasatinib, imatinib, and nilotinib) are a relatively newer group of chemotherapeutic agents that predominantly target tyrosine kinase enzymes. They are used in treatment of several cancers including leukemia, renal cell carcinoma, hepatocellular carcinoma, and melanoma [157]. The occurrence of cardiovascular adverse effects varies among these agents, with sunitinib showing the worst cardiovascular safety profile [182]. Sunitinib is associated with significantly increased risk of heart failure in up to 8%, and hypertension in up to 50% of patients [183]. The main mechanism of sunitinib-induced cardiotoxicity is the inhibition of off-target kinases, such as mitogen-activated protein kinase kinase 7 [184], mitogen-activated protein kinases (p38, JNK, and ERK1/2) [185], and AMP-activated protein kinase (AMPK) [186]. Activation of the AhR signaling pathway has also been reported to mediate sunitinib-induced cardiac hypertrophy in vivo and in vitro [187]. Interestingly, resveratrol (20 µM) prevented sunitinib-induced H9c2 cardiomyoblast hypertrophy and abrogated sunitinib-mediated induction of CYP1A1, an AhR-regulated gene [187]. Conversely, resveratrol did not prevent dasatinib-mediated induction of hypertrophic markers or CYP1A1 genes in H9c2 cardiomyoblasts [188].
There is still a paucity of research determining the impact of resveratrol on the cytotoxic effect of tyrosine kinase inhibitors. Although resveratrol has been shown to prevent sunitinib-mediated induction of CYP1A1 in MCF-7 breast cancer cells, the effect of resveratrol on sunitinib-induced cytotoxicity in these cancer cells was not reported [189]. In chronic myeloid leukemia cells, resveratrol increased dasatinib-, nilotinib-, and imatinib-induced apoptosis [190,191,192]. Interestingly, resveratrol alone showed significant cytotoxic effect in imatinib-resistant chronic myeloid leukemia cells [192,193].
3.5. Radiation
Radiation may induce a number of cardiovascular adverse effects, including: pericarditis, pericardial fibrosis, myocardial fibrosis, coronary artery disease, and microvascular damage (reviewed in [194]). The mechanisms of radiation-induced cardiovascular toxicity include: increased oxidative stress [195,196], inflammation [197,198], apoptotic cell death [199], and cellular senescence [200]. Although resveratrol has the potential to prevent all these detrimental effects, it is rarely studied as a protective agent against radiation-induced cardiovascular toxicity. In a single study, black grape juice with high resveratrol content has been shown to abrogate radiation-induced oxidative stress in the heart tissue of rats [201]. Although this study showed that black grape juice normalized the increase of lactate dehydrogenase, which can be a marker of cardiac damage, it did not report the protective effect on cardiac function or structure [201]. Beyond cardiovascular protection, resveratrol has been shown to protect against radiation-induced erectile dysfunction in male rats [202], immunosuppression in mice [203], intestinal injury in mice [204], hepatotoxicity in rats [205], ovarian toxicity in female rats [206,207], salivary gland damage in mice [208] and rats [209], and hematopoietic stem cell injury in mice [210].
The potential use of resveratrol to protect against radiation-induced cardiovascular toxicity is intriguing, because resveratrol has been largely shown to sensitize cancer cells to radiation (Table 5). The radiomodulatory effect of resveratrol was determined in CHO-k1 and A549 cell lines, wherein the low concentration of resveratrol, 15 µM, protected from radiation-induced genotoxic damage, while the high concentration, 60 µM, augmented radiation-induced genotoxic damage. It is important to mention that while the low concentration protected against genotoxic damage, it did not reduce the cytotoxic effect of radiation as assessed by the MTT cell viability assay [211]. Resveratrol (10–100 µM) and irradiation with 4 Gy alone and in combination significantly decreased cell viability in rodent GH3 and TtT/GF pituitary adenoma cells in vitro [212]. Resveratrol (10 μM) sensitized the human breast cancer cell line MCF7 to the cytotoxic and anti-proliferative effect of 3 Gy ionizing radiation [213]. Supra-additive cytotoxic effect was observed when cervical squamous cell cancer cell line (HeLa) was treated with resveratrol at its CC50 followed by radiotherapy at 2Gy. The same effect was also observed with other mTOR inhibitors including temsirolimus, everolimus, curcumin, and epigallocatechin gallate [214]. Conversely, resveratrol (50 μM) protected lung cancer cell lines A549 and H460 against radiation-induced apoptosis through Sirt1 activation [215].

Table 5.
Studies demonstrating the radiomodulatory effects of resveratrol.
4. Resveratrol Analogs
There are other naturally occurring stilbene-like compounds related to resveratrol that have shown promising anticancer properties (reviewed in [237]). In addition, a number of resveratrol analogs have been synthesized to enhance the bioavailability and pharmacologic properties of resveratrol (reviewed in [238,239]). These analogs have also demonstrated promising chemo-preventive and anticancer properties in preclinical studies (reviewed in [237]).
4.1. Pterostilbene
Pterostilbene (3,5-di-methoxy-4′-hydroxystilbene) is a dimethylated analog of resveratrol that has been extensively studied as a potential chemo-preventive and therapeutic agent against different types of cancer [106,240,241,242]. It has been shown to enhance the chemotherapeutic effect of some potentially cardiotoxic chemotherapies. For instance, pterostilbene demonstrated synergistic anti-proliferative activity with cisplatin in several ovarian cancer cell lines [243]. However, the cardiovascular effects of pterostilbene are not as extensively studied as resveratrol. A few recent studies have suggested that pterostilbene may confer cardio-protection against myocardial ischemia-reperfusion injury through AMPK and SIRT1 activation [244,245], reduction in oxidative stress [246], and anti-inflammatory and anti-apoptotic effects [246,247]. It has also been shown to prevent cardiac hypertrophy and restore right ventricular function in a rat model of cor pulmonale [248]. Nevertheless, there are no studies that report the potential protective effect of pterostilbene against cancer treatment-induced cardiotoxicity.
4.2. Tetramethoxystilbenes
DMU-212 (3,4,5,4′-tetramethoxy-trans-stilbene) is a resveratrol analog that has been shown to be more potent than resveratrol in inhibiting the growth of several cancer cell lines such as breast, ovarian, and melanoma cells [249,250,251,252]. PicMet (3,5,3′,4′-tetramethoxystilbene) is another tetramethoxystilbene that has been shown to enhance the cytotoxic effect of DOX and increased its intra-cellular accumulation in DOX-resistant human adenocarcinoma cell line (LoVo/Dx) [253] and the L5178 mouse lymphoma cell line expressing the human MDR1 gene [254]. TMS (2,4,3′,5′-tetramethoxystilbene) is a selective inhibitor of cytochrome P450 CYP1B1, an enzyme that is overexpressed in some tumors and has protumorigenic activity [255]. TMS has been shown to inhibit cell viability of human breast cancer, leukemia, and prostatic cancer cell lines, largely by inhibiting CYP1B1 [256,257]. Intriguingly, TMS has recently been shown to protect against DOX-induced cardiotoxicity by inhibiting cytochrome P450 CYP1B1 and midchain hydroxyeicosatetraenoic acid (HETE) formation [258]. It has also been shown to protect against isoproterenol- and angiotensin II-induced cardiac hypertrophy [259,260], angiotensin II-induced aortic aneurysm [261], and atherosclerosis and hypertension [262,263] through inhibition of CYP1B1 enzyme.
4.3. Trimethoxystilbene
Trimethoxystilbene (trans-3,5,4′-trimethoxystilbene) is another resveratrol analog that also has promising anticancer properties, reviewed in [264]. However, its potential cardio-protective properties have rarely been studied. In a single study, it has been shown to prevent pulmonary vascular remodeling and right ventricle hypertrophy in a hypoxia-induced rat model of pulmonary arterial hypertension, which contributed to inhibition of oxidative stress and inflammation [265].
4.4. Piceatannol
Piceatannol (trans-3,4,3′,5′-tetrahydroxystilbene) is a naturally occurring stilbene commonly found in grape skins and wine which has potential chemo-preventive and anticancer properties (reviewed in [266]). With regard to its potential cardiovascular benefits, piceatannol has been shown to prevent isoproterenol-induced cardiac hypertrophy [267]. Although piceatannol was shown to possess anti-arrhythmic properties [268], recent reports have shown that it does not have such properties [269] and it may even be pro-arrhythmic [270]. There are no reports of cardio-protective effects of piceatannol against cancer treatment-induced cardiotoxicity. Nevertheless, there are a number of studies showing that it can potentiate the chemotherapeutic effect of cardiotoxic chemotherapies. For instance, it potentiated the apoptotic effect of DOX in DOX-resistant human adenocarcinoma cell line (LoVo/Dx), primarily by inhibiting MDR protein and increasing the intracellular accumulation of DOX [253]. It also enhanced cisplatin sensitivity in ovarian cancer cells by acting on p53, x-linked inhibitor of apoptosis (XIAP), and mitochondrial fission [271].
4.5. Epsilon-Viniferin
Epsilon-viniferin (ε-viniferin) is a resveratrol dimer that has been shown to be more effective than resveratrol in reducing systolic blood pressure and mitigating cardiac hypertrophy in spontaneously hypertensive rats [272]. It enhanced cisplatin-induced apoptosis in C6 cells [273].
5. Challenges to the Clinical Use of Resveratrol in Cardio-Oncology
Despite all the aforementioned studies that support the use of resveratrol in cardio-oncology, there are several challenges to its clinical application in cancer patients receiving cardiotoxic cancer treatments. First, all the previous studies showing cardio-protective effects of resveratrol are preclinical studies conducted either in vitro or in rodent models of human diseases. Unfortunately, a number of agents which had shown significant cardio-protective properties in animals failed in subsequent clinical trials (e.g., vitamin E and N-acetyl cysteine [274,275]). These failures hampered the clinical interest in translating new cardio-protective agents into clinical trials. In clinical trials of resveratrol in cancer patients, modest beneficial effects were demonstrated at the molecular level in colon cancer [276] and breast cancer patients [277]. However, there were very disappointing results in patients with multiple myeloma [278]. Indeed, there was an unexpected renal toxicity in multiple myeloma patients who received SRT501 (micronized resveratrol, 5 g/day). This safety concern was enough to terminate the clinical trial [278]. Moreover, cancer patients are already receiving multiple chemotherapeutic agents ± radiation therapy. Therefore, the oncologist is usually very reluctant to add another drug that may interfere with the therapeutic benefit and/or increase the toxicity of these therapies. For instance, the fear of reducing the therapeutic benefit has hindered the use of dexrazoxane, the only FDA approved drug to prevent anthracycline cardiotoxicity. In one study, dexrazoxane has been shown to be associated with higher rates of secondary malignancy in childhood cancer survivors [279]. Although the results of this study were not corroborated by other studies [280,281], it was enough to hinder more widespread use of dexrazoxane. Furthermore, the predictive and diagnostic value of different approaches such as strain echocardiography, cardiac magnetic resonance imaging, and biomarkers are still not well established in the field of cardio-oncology [282]. Therefore, it is difficult to identify those patients who will benefit the most from a potential cardio-protective therapy.
In addition to these general concerns, resveratrol has its own particular challenges. First, its exact mechanism of action is not known. It has multiple targets that make it extremely difficult to predict how it will interact with the human body, different disease states, and different medications. Another major limitation of resveratrol is its poor bioavailability due to rapid metabolism to its sulfated and glucuronide metabolites [18]. Administration of up to 5 g of resveratrol yielded a plasma concentration of only 500 ng/mL (2 μM) in humans [22]. Despite this poor bioavailability, oral administration of resveratrol to experimental animals was almost as effective as its parenteral administration, creating the “resveratrol paradox” which describes resveratrol as a molecule with very low plasma level, but significant biological effects [283]. A number of mechanisms have been postulated to explain this phenomenon including higher tissue concentration of resveratrol in certain organs [284], conversion of these metabolites back to resveratrol [285], and biological activity exerted by these metabolites [286]. More recently, it has been shown that resveratrol may exert some of its beneficial effects through alteration of the gut microbiome [287,288,289], a mechanism that does not need systemic absorption. It is also important to mention that dietary intake of resveratrol is not likely to result in the aforementioned pharmacological effects, since the average daily intake of resveratrol is estimated to be less than 1 mg [290].
6. Conclusions
There is an obvious mismatch between the plethora of preclinical studies and the limited number of clinical studies of resveratrol that support its use in the field of cardio-oncology. Despite the very promising preclinical findings of resveratrol as a cardio-protective agent, there are still several questions that need to be answered before advancing resveratrol into clinical trials. First and foremost, all the previously reviewed studies showed the cardio-protective effect of resveratrol in tumor-free experimental animals. Although resveratrol has been shown to augment the chemotherapeutic effect of these cardiotoxic cancer treatments, no study has yet demonstrated the cardio-protective and the anticancer effects of resveratrol simultaneously in tumor-bearing models. Taking into account the possible hormetic dose-response properties of resveratrol [291], it is extremely important to show that the same dose of resveratrol is able to protect the heart and fight cancer. Second, it is also important to determine the effect of resveratrol on the pharmacokinetics and tissue distribution of chemotherapeutic agents, taking into account the potential of resveratrol to alter a number of drug metabolizing enzymes [292]. Indeed, canine cancer patients can offer a perfect translational platform to answer these questions. Resveratrol is a common nutritional supplement in dogs and it has been shown to have cytotoxic effects in canine cancer cells [123,293]. Therefore, initiation of clinical trials of resveratrol in canine cancer patients seems a logical first step in translating the preclinical findings to human cancer patients. Finally, the emergence of novel resveratrol analogs with improved bioavailability and enhanced pharmacological properties could open new venues for preclinical testing of these analogs as new armaments in the battle of cardio-oncology.
Author Contributions
Conceptualization, B.N.Z.; literature review, I.Y.A. and B.N.Z.; writing—original draft preparation, I.Y.A., M.K.O.G. and B.N.Z.; writing—review and editing, I.Y.A., M.K.O.G. and B.N.Z.; supervision and funding acquisition, B.N.Z.
Funding
B.N.Z. is a Masonic Cancer Center Women’s Health Scholar, sponsored by the Masonic Cancer Center, a comprehensive cancer center designated by the National Cancer Institute, and administered by the University of Minnesota Women’s Health Research Program. B.N.Z. is also supported by a research grant from the Rally Foundation for Childhood Cancer (Award ID 582420) and an institutional research grant from the American Cancer Society (Award #129819-IRG-16-189-58-IRG91).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Tajiri, K.; Aonuma, K.; Sekine, I. Cardio-oncology: A multidisciplinary approach for detection, prevention and management of cardiac dysfunction in cancer patients. Jpn. J. Clin. Oncol. 2017, 47, 678–682. [Google Scholar] [CrossRef]
- Coviello, J.S. Cardio-oncology: A Subspecialty in its Infancy. J. Adv. Pract. Oncol. 2018, 9, 154–155. [Google Scholar] [PubMed]
- Lefrak, E.A.; Pitha, J.; Rosenheim, S.; Gottlieb, J.A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973, 32, 302–314. [Google Scholar] [CrossRef]
- Gilladoga, A.C.; Manuel, C.; Tan, C.T.; Wollner, N.; Sternberg, S.S.; Murphy, M.L. The cardiotoxicity of adriamycin and daunomycin in children. Cancer 1976, 37, 1070–1078. [Google Scholar] [CrossRef]
- Long, H.D.; Lin, Y.E.; Zhang, J.J.; Zhong, W.Z.; Zheng, R.N. Risk of Congestive Heart Failure in Early Breast Cancer Patients Undergoing Adjuvant Treatment With Trastuzumab: A Meta-Analysis. Oncologist 2016, 21, 547–554. [Google Scholar] [CrossRef] [PubMed]
- De Azambuja, E.; Procter, M.J.; van Veldhuisen, D.J.; Agbor-Tarh, D.; Metzger-Filho, O.; Steinseifer, J.; Untch, M.; Smith, I.E.; Gianni, L.; Baselga, J.; et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01). J. Clin. Oncol. 2014, 32, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Waxman, A.J.; Clasen, S.; Hwang, W.T.; Garfall, A.; Vogl, D.T.; Carver, J.; O’Quinn, R.; Cohen, A.D.; Stadtmauer, E.A.; Ky, B.; et al. Carfilzomib-associated cardiovascular adverse events: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, e174519. [Google Scholar] [CrossRef] [PubMed]
- Catino, A.B.; Hubbard, R.A.; Chirinos, J.A.; Townsend, R.; Keefe, S.; Haas, N.B.; Puzanov, I.; Fang, J.C.; Agarwal, N.; Hyman, D.; et al. Longitudinal assessment of vascular function with sunitinib in patients with metastatic renal cell carcinoma. Circ. Heart Fail. 2018, 11, e004408. [Google Scholar] [CrossRef] [PubMed]
- Ederhy, S.; Cautela, J.; Ancedy, Y.; Escudier, M.; Thuny, F.; Cohen, A. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC Cardiovasc. Imaging 2018, 11, 1187–1190. [Google Scholar] [CrossRef]
- Niska, J.R.; Thorpe, C.S.; Allen, S.M.; Daniels, T.B.; Rule, W.G.; Schild, S.E.; Vargas, C.E.; Mookadam, F. Radiation and the heart: Systematic review of dosimetry and cardiac endpoints. Expert Rev. Cardiovasc. Ther. 2018, 16, 931–950. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, J.; Xu, L.; Ma, J.; Li, J.; Ma, R.; Sun, K.; Wang, Z.; Zhang, H. Ionic liquid-based salt-induced liquid-liquid extraction of polyphenols and anthraquinones in Polygonum cuspidatum. J. Pharm. Biomed. Anal. 2019, 163, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hain, R.; Reif, H.J.; Krause, E.; Langebartels, R.; Kindl, H.; Vornam, B.; Wiese, W.; Schmelzer, E.; Schreier, P.H.; Stocker, R.H.; et al. Disease resistance results from foreign phytoalexin expression in a novel plant. Nature 1993, 361, 153–156. [Google Scholar] [CrossRef]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French paradox revisited. Front. Pharmacol. 2012, 3, 141. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Block, G.; Jensen, C.D.; Norkus, E.P.; Dalvi, T.B.; Wong, L.G.; McManus, J.F.; Hudes, M.L. Usage patterns, health, and nutritional status of long-term multiple dietary supplement users: A cross-sectional study. Nutr. J. 2007, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- La Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—Safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Boocock, D.J.; Patel, K.R.; Faust, G.E.; Normolle, D.P.; Marczylo, T.H.; Crowell, J.A.; Brenner, D.E.; Booth, T.D.; Gescher, A.; Steward, W.P. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J. Chromatogr. B 2007, 848, 182–187. [Google Scholar] [CrossRef]
- Park, E.J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta 2015, 1852, 1071–1113. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef] [PubMed]
- Wicinski, M.; Socha, M.; Walczak, M.; Wodkiewicz, E.; Malinowski, B.; Rewerski, S.; Gorski, K.; Pawlak-Osinska, K. Beneficial Effects of Resveratrol Administration-Focus on Potential Biochemical Mechanisms in Cardiovascular Conditions. Nutrients 2018, 10, 1813. [Google Scholar] [CrossRef]
- Dyck, G.J.B.; Raj, P.; Zieroth, S.; Dyck, J.R.B.; Ezekowitz, J.A. The Effects of Resveratrol in Patients with Cardiovascular Disease and Heart Failure: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 904. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Ndiaye, M.A.; Ahmad, N. Resveratrol and cancer: Challenges for clinical translation. Biochim. Biophys. Acta 2015, 1852, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- De Ligt, M.; Timmers, S.; Schrauwen, P. Resveratrol and obesity: Can resveratrol relieve metabolic disturbances? Biochim. Biophys. Acta 2015, 1852, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.C. Resveratrol supplementation affects bone acquisition and osteoporosis: Pre-clinical evidence toward translational diet therapy. Biochim. Biophys. Acta 2015, 1852, 1186–1194. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Wang, J.; Ho, L.; Zhao, W.; Dubner, L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim. Biophys. Acta 2015, 1852, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Hubbard, B.P. Lifespan and healthspan extension by resveratrol. Biochim. Biophys. Acta 2015, 1852, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Canto, C. The molecular targets of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.; Dolinsky, V.W.; Soltys, C.L.; Viollet, B.; Baksh, S.; Light, P.E.; Dyck, J.R. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J. Biol. Chem. 2008, 283, 24194–24201. [Google Scholar] [CrossRef]
- Ma, S.; Feng, J.; Zhang, R.; Chen, J.; Han, D.; Li, X.; Yang, B.; Li, X.; Fan, M.; Li, C.; et al. SIRT1 Activation by Resveratrol Alleviates Cardiac Dysfunction via Mitochondrial Regulation in Diabetic Cardiomyopathy Mice. Oxid. Med. Cell. Longev. 2017, 2017, 4602715. [Google Scholar] [CrossRef]
- Fourny, N.; Lan, C.; Seree, E.; Bernard, M.; Desrois, M. Protective Effect of Resveratrol against Ischemia-Reperfusion Injury via Enhanced High Energy Compounds and eNOS-SIRT1 Expression in Type 2 Diabetic Female Rat Heart. Nutrients 2019, 11, 105. [Google Scholar] [CrossRef]
- Kitada, M.; Kume, S.; Imaizumi, N.; Koya, D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes 2011, 60, 634–643. [Google Scholar] [CrossRef]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Zhu, M.; Zhang, Q.; Wang, X.; Wang, Y.; Zhang, J.; Li, J.; Yang, L.; Liu, J.; et al. Resveratrol attenuates myocardial ischemia/reperfusion injury through up-regulation of vascular endothelial growth factor B. Free Radic. Biol. Med. 2016, 101, 1–9. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Chakrabarti, S.; Pereira, T.J.; Oka, T.; Levasseur, J.; Beker, D.; Zordoky, B.N.; Morton, J.S.; Nagendran, J.; Lopaschuk, G.D.; et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta 2013, 1832, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Chen, Y.W.; Chi, P.L.; Lin, C.C.; Hsiao, L.D. Resveratrol inhibits BK-induced COX-2 transcription by suppressing acetylation of AP-1 and NF-kappaB in human rheumatoid arthritis synovial fibroblasts. Biochem. Pharmacol. 2017, 132, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, L.; Cui, J.; Huoc, Z.; Xue, J.; Cui, H.; Mao, Q.; Yang, R. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie 2013, 68, 689–694. [Google Scholar] [PubMed]
- Cullen, J.P.; Morrow, D.; Jin, Y.; Curley, B.; Robinson, A.; Sitzmann, J.V.; Cahill, P.A.; Redmond, E.M. Resveratrol, a polyphenolic phytostilbene, inhibits endothelial monocyte chemotactic protein-1 synthesis and secretion. J. Vasc. Res. 2007, 44, 75–84. [Google Scholar] [CrossRef]
- Zordoky, B.N.; El-Kadi, A.O. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and beta-naphthoflavone induce cellular hypertrophy in H9c2 cells by an aryl hydrocarbon receptor-dependant mechanism. Toxicol. In Vitro 2010, 24, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Frojdo, S.; Cozzone, D.; Vidal, H.; Pirola, L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem. J. 2007, 406, 511–518. [Google Scholar] [CrossRef]
- Liu, M.; Wilk, S.A.; Wang, A.; Zhou, L.; Wang, R.H.; Ogawa, W.; Deng, C.; Dong, L.Q.; Liu, F. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J. Biol. Chem. 2010, 285, 36387–36394. [Google Scholar] [CrossRef]
- Haider, U.G.; Sorescu, D.; Griendling, K.K.; Vollmar, A.M.; Dirsch, V.M. Resveratrol suppresses angiotensin II-induced Akt/protein kinase B and p70 S6 kinase phosphorylation and subsequent hypertrophy in rat aortic smooth muscle cells. Mol. Pharmacol. 2002, 62, 772–777. [Google Scholar] [CrossRef]
- Christiansen, S.; Autschbach, R. Doxorubicin in experimental and clinical heart failure. Eur. J. Cardiothorac. Surg. 2006, 30, 611–616. [Google Scholar] [CrossRef]
- Outomuro, D.; Grana, D.R.; Azzato, F.; Milei, J. Adriamycin-induced myocardial toxicity: New solutions for an old problem? Int. J. Cardiol. 2007, 117, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Takemura, G.; Fujiwara, H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 2007, 49, 330–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Shi, J.; Li, Y.J.; Wei, L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch. Immunol. Ther. Exp. 2009, 57, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L., Jr.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ueda, Y.; Juan, Y.; Katsuda, S.; Takahashi, H.; Koh, E. Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: In vivo study. Circulation 2000, 102, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Kakinuma, Y.; Yuhki, K.; Murakoshi, N.; Iemitsu, M.; Miyauchi, T.; Yamaguchi, I. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J. Pharmacol. Sci. 2006, 101, 151–158. [Google Scholar] [CrossRef]
- Ferreira, A.; Cunha-Oliveira, T.; Simoes, R.F.; Carvalho, F.S.; Burgeiro, A.; Nordgren, K.; Wallace, K.B.; Oliveira, P.J. Altered mitochondrial epigenetics associated with subchronic doxorubicin cardiotoxicity. Toxicology 2017, 390, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Li, Y. Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes: Protection against oxidative and electrophilic injury. Eur. J. Pharmacol. 2004, 489, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Rezk, Y.A.; Balulad, S.S.; Keller, R.S.; Bennett, J.A. Use of resveratrol to improve the effectiveness of cisplatin and doxorubicin: Study in human gynecologic cancer cell lines and in rodent heart. Am. J. Obstet. Gynecol. 2006, 194, e23–e26. [Google Scholar] [CrossRef]
- Danz, E.D.; Skramsted, J.; Henry, N.; Bennett, J.A.; Keller, R.S. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic. Biol. Med. 2009, 46, 1589–1597. [Google Scholar] [CrossRef]
- Xu, X.; Chen, K.; Kobayashi, S.; Timm, D.; Liang, Q. Resveratrol attenuates doxorubicin-induced cardiomyocyte death via inhibition of p70 S6 kinase 1-mediated autophagy. J. Pharmacol. Exp. Ther. 2012, 341, 183–195. [Google Scholar] [CrossRef]
- De Angelis, A.; Piegari, E.; Cappetta, D.; Russo, R.; Esposito, G.; Ciuffreda, L.P.; Ferraiolo, F.A.; Frati, C.; Fagnoni, F.; Berrino, L.; et al. SIRT1 activation rescues doxorubicin-induced loss of functional competence of human cardiac progenitor cells. Int. J. Cardiol. 2015, 189, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Wang, Z.; Xu, Y.; Zhou, P.; Cao, J.; Li, Y.; Chen, Y.; Sun, J.; Fu, L. Resveratrol prevents doxorubicin-induced cardiotoxicity in H9c2 cells through the inhibition of endoplasmic reticulum stress and the activation of the Sirt1 pathway. Int. J. Mol. Med. 2015, 36, 873–880. [Google Scholar] [CrossRef]
- Gu, J.; Hu, W.; Song, Z.P.; Chen, Y.G.; Zhang, D.D.; Wang, C.Q. Resveratrol-induced autophagy promotes survival and attenuates doxorubicin-induced cardiotoxicity. Int. Immunopharmacol. 2016, 32, 1–7. [Google Scholar] [CrossRef]
- Liu, M.H.; Lin, X.L.; Guo, D.M.; Zhang, Y.; Yuan, C.; Tan, T.P.; Chen, Y.D.; Wu, S.J.; Ye, Z.F.; He, J. Resveratrol protects cardiomyocytes from doxorubicin-induced apoptosis through the AMPK/P53 pathway. Mol. Med. Rep. 2016, 13, 1281–1286. [Google Scholar] [CrossRef]
- Liu, M.H.; Shan, J.; Li, J.; Zhang, Y.; Lin, X.L. Resveratrol inhibits doxorubicin-induced cardiotoxicity via sirtuin 1 activation in H9c2 cardiomyocytes. Exp. Ther. Med. 2016, 12, 1113–1118. [Google Scholar] [CrossRef]
- Gu, J.; Fan, Y.Q.; Zhang, H.L.; Pan, J.A.; Yu, J.Y.; Zhang, J.F.; Wang, C.Q. Resveratrol suppresses doxorubicin-induced cardiotoxicity by disrupting E2F1 mediated autophagy inhibition and apoptosis promotion. Biochem. Pharmacol. 2018, 150, 202–213. [Google Scholar] [CrossRef]
- Wang, G.Y.; Wang, Y.M.; Zhang, L.N.; Li, Q.; Yue, H.; Song, C.M.; Feng, J.K.; Wang, N. Effect of resveratrol on heart function of rats with adriamycin-induced heart failure. Zhongguo Zhong Yao Za Zhi 2007, 32, 1563–1565. [Google Scholar] [PubMed]
- Olukman, M.; Can, C.; Erol, A.; Oktem, G.; Oral, O.; Cinar, M.G. Reversal of doxorubicin-induced vascular dysfunction by resveratrol in rat thoracic aorta: Is there a possible role of nitric oxide synthase inhibition? Anadolu Kardiyol. Derg. 2009, 9, 260–266. [Google Scholar] [PubMed]
- Osman, A.M.; Al-Harthi, S.E.; AlArabi, O.M.; Elshal, M.F.; Ramadan, W.S.; Alaama, M.N.; Al-Kreathy, H.M.; Damanhouri, Z.A.; Osman, O.H. Chemosensetizing and cardioprotective effects of resveratrol in doxorubicin-treated animals. Cancer Cell Int. 2013, 13, 52. [Google Scholar] [CrossRef]
- Pinarli, F.A.; Turan, N.N.; Pinarli, F.G.; Okur, A.; Sonmez, D.; Ulus, T.; Oguz, A.; Karadeniz, C.; Delibasi, T. Resveratrol and adipose-derived mesenchymal stem cells are effective in the prevention and treatment of doxorubicin cardiotoxicity in rats. Pediatr. Hematol. Oncol. 2013, 30, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Al-Harthi, S.E.; Alarabi, O.M.; Ramadan, W.S.; Alaama, M.N.; Al-Kreathy, H.M.; Damanhouri, Z.A.; Khan, L.M.; Osman, A.M. Amelioration of doxorubicininduced cardiotoxicity by resveratrol. Mol. Med. Rep. 2014, 10, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Dong, C.; Patel, J.; Duan, C.; Wang, X.; Wu, X.; Cao, Y.; Pu, L.; Lu, D.; Shen, T.; et al. SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cell. Physiol. Biochem. 2015, 35, 1116–1124. [Google Scholar] [CrossRef]
- Sin, T.K.; Tam, B.T.; Yung, B.Y.; Yip, S.P.; Chan, L.W.; Wong, C.S.; Ying, M.; Rudd, J.A.; Siu, P.M. Resveratrol protects against doxorubicin-induced cardiotoxicity in aged hearts through the SIRT1-USP7 axis. J. Physiol. 2015, 593, 1887–1899. [Google Scholar] [CrossRef]
- Tatlidede, E.; Sehirli, O.; Velioglu-Ogunc, A.; Cetinel, S.; Yegen, B.C.; Yarat, A.; Suleymanoglu, S.; Sener, G. Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radic. Res. 2009, 43, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, Y.; Qu, S.; Wei, X.; Zhu, H.; Luo, Q.; Liu, M.; Chen, G.; Xiao, X. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc. Res. 2011, 90, 538–545. [Google Scholar] [CrossRef]
- Gu, J.; Song, Z.P.; Gui, D.M.; Hu, W.; Chen, Y.G.; Zhang, D.D. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in lymphoma nude mice by heme oxygenase-1 induction. Cardiovasc. Toxicol. 2012, 12, 341–349. [Google Scholar] [CrossRef]
- Dudka, J.; Gieroba, R.; Korga, A.; Burdan, F.; Matysiak, W.; Jodlowska-Jedrych, B.; Mandziuk, S.; Korobowicz, E.; Murias, M. Different effects of resveratrol on dose-related Doxorubicin-induced heart and liver toxicity. Evid. Based Complement. Alternat. Med. 2012, 2012, 606183. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Rogan, K.J.; Sung, M.M.; Zordoky, B.N.; Haykowsky, M.J.; Young, M.E.; Jones, L.W.; Dyck, J.R. Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E243–E253. [Google Scholar] [CrossRef]
- Arafa, M.H.; Mohammad, N.S.; Atteia, H.H.; Abd-Elaziz, H.R. Protective effect of resveratrol against doxorubicin-induced cardiac toxicity and fibrosis in male experimental rats. J. Physiol. Biochem. 2014, 70, 701–711. [Google Scholar] [CrossRef]
- Cappetta, D.; Esposito, G.; Piegari, E.; Russo, R.; Ciuffreda, L.P.; Rivellino, A.; Berrino, L.; Rossi, F.; De Angelis, A.; Urbanek, K. SIRT1 activation attenuates diastolic dysfunction by reducing cardiac fibrosis in a model of anthracycline cardiomyopathy. Int. J. Cardiol. 2016, 205, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Shoukry, H.S.; Ammar, H.I.; Rashed, L.A.; Zikri, M.B.; Shamaa, A.A.; Abou Elfadl, S.G.; Rub, E.A.; Saravanan, S.; Dhingra, S. Prophylactic supplementation of resveratrol is more effective than its therapeutic use against doxorubicin induced cardiotoxicity. PLoS ONE 2017, 12, e0181535. [Google Scholar] [CrossRef]
- Matsumura, N.; Zordoky, B.N.; Robertson, I.M.; Hamza, S.M.; Parajuli, N.; Soltys, C.M.; Beker, D.L.; Grant, M.K.; Razzoli, M.; Bartolomucci, A.; et al. Co-administration of resveratrol with doxorubicin in young mice attenuates detrimental late-occurring cardiovascular changes. Cardiovasc. Res. 2018, 114, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B. Doxorubicin-induced cardiac mitochondrionopathy. Pharmacol. Toxicol. 2003, 93, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Blake, S.L.; Ichinose, F.; Raher, M.J.; Buys, E.S.; Jassal, D.S.; Furutani, E.; Perez-Sanz, T.M.; Graveline, A.; Janssens, S.P.; et al. Disruption of nitric oxide synthase 3 protects against the cardiac injury, dysfunction, and mortality induced by doxorubicin. Circulation 2007, 116, 506–514. [Google Scholar] [CrossRef]
- Lebrecht, D.; Walker, U.A. Role of mtDNA lesions in anthracycline cardiotoxicity. Cardiovasc. Toxicol. 2007, 7, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, J.H. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res. 1983, 43, 460–472. [Google Scholar]
- Singal, P.K.; Segstro, R.J.; Singh, R.P.; Kutryk, M.J. Changes in lysosomal morphology and enzyme activities during the development of adriamycin-induced cardiomyopathy. Can. J. Cardiol. 1985, 1, 139–147. [Google Scholar]
- Doroshow, J.H.; Locker, G.Y.; Baldinger, J.; Myers, C.E. The effect of doxorubicin on hepatic and cardiac glutathione. Res. Commun. Chem. Pathol. Pharmacol. 1979, 26, 285–295. [Google Scholar]
- Kim, S.Y.; Kim, S.J.; Kim, B.J.; Rah, S.Y.; Chung, S.M.; Im, M.J.; Kim, U.H. Doxorubicin-induced reactive oxygen species generation and intracellular Ca2+ increase are reciprocally modulated in rat cardiomyocytes. Exp. Mol. Med. 2006, 38, 535–545. [Google Scholar] [CrossRef]
- Childs, A.C.; Phaneuf, S.L.; Dirks, A.J.; Phillips, T.; Leeuwenburgh, C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002, 62, 4592–4598. [Google Scholar] [PubMed]
- Deniaud, A.; Sharaf el dein, O.; Maillier, E.; Poncet, D.; Kroemer, G.; Lemaire, C.; Brenner, C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 2008, 27, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mao, W.; Ding, B.; Liang, C.S. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1956–H1965. [Google Scholar] [CrossRef]
- Riad, A.; Bien, S.; Westermann, D.; Becher, P.M.; Loya, K.; Landmesser, U.; Kroemer, H.K.; Schultheiss, H.P.; Tschope, C. Pretreatment with statin attenuates the cardiotoxicity of Doxorubicin in mice. Cancer Res. 2009, 69, 695–699. [Google Scholar] [CrossRef]
- Gustafsson, A.B.; Gottlieb, R.A. Heart mitochondria: Gates of life and death. Cardiovasc. Res. 2008, 77, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Volden, P.; Timm, D.; Mao, K.; Xu, X.; Liang, Q. Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. J. Biol. Chem. 2010, 285, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Anding, A.L.; Baehrecke, E.H. Autophagy in Cell Life and Cell Death. Curr. Top. Dev. Biol. 2015, 114, 67–91. [Google Scholar] [CrossRef]
- Maejima, Y.; Adachi, S.; Ito, H.; Hirao, K.; Isobe, M. Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell 2008, 7, 125–136. [Google Scholar] [CrossRef]
- Li, Y.R.; Li, S.; Lin, C.C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 2018, 44, 69–82. [Google Scholar] [CrossRef]
- Tokarska-Schlattner, M.; Wallimann, T.; Schlattner, U. Alterations in myocardial energy metabolism induced by the anti-cancer drug doxorubicin. C. R. Biol. 2006, 329, 657–668. [Google Scholar] [CrossRef]
- Tokarska-Schlattner, M.; Zaugg, M.; Zuppinger, C.; Wallimann, T.; Schlattner, U. New insights into doxorubicin-induced cardiotoxicity: The critical role of cellular energetics. J. Mol. Cell. Cardiol. 2006, 41, 389–405. [Google Scholar] [CrossRef]
- Maslov, M.Y.; Chacko, V.P.; Hirsch, G.A.; Akki, A.; Leppo, M.K.; Steenbergen, C.; Weiss, R.G. Reduced in vivo high-energy phosphates precede adriamycin-induced cardiac dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H332–H337. [Google Scholar] [CrossRef]
- Tokarska-Schlattner, M.; Zaugg, M.; da Silva, R.; Lucchinetti, E.; Schaub, M.C.; Wallimann, T.; Schlattner, U. Acute toxicity of doxorubicin on isolated perfused heart: Response of kinases regulating energy supply. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H37–H47. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Vlachogiannis, I.A.; Tsiani, E. Effects of Resveratrol against Lung Cancer: In Vitro and In Vivo Studies. Nutrients 2017, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Hwang, K.A.; Choi, K.C. Antitumor Effect of Various Phytochemicals on Diverse Types of Thyroid Cancers. Nutrients 2019, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rimando, A.M.; Levenson, A.S. Resveratrol and pterostilbene as a microRNA-mediated chemopreventive and therapeutic strategy in prostate cancer. Ann. N. Y. Acad. Sci. 2017, 1403, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K.M. Sensitization for anticancer drug-induced apoptosis by the chemopreventive agent resveratrol. Oncogene 2004, 23, 6702–6711. [Google Scholar] [CrossRef]
- Gatouillat, G.; Balasse, E.; Joseph-Pietras, D.; Morjani, H.; Madoulet, C. Resveratrol induces cell-cycle disruption and apoptosis in chemoresistant B16 melanoma. J. Cell. Biochem. 2010, 110, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Al-Abd, A.M.; Mahmoud, A.M.; El-Sherbiny, G.A.; El-Moselhy, M.A.; Nofal, S.M.; El-Latif, H.A.; El-Eraky, W.I.; El-Shemy, H.A. Resveratrol enhances the cytotoxic profile of docetaxel and doxorubicin in solid tumour cell lines in vitro. Cell Prolif. 2011, 44, 591–601. [Google Scholar] [CrossRef]
- Osman, A.M.; Bayoumi, H.M.; Al-Harthi, S.E.; Damanhouri, Z.A.; Elshal, M.F. Modulation of doxorubicin cytotoxicity by resveratrol in a human breast cancer cell line. Cancer Cell Int. 2012, 12, 47. [Google Scholar] [CrossRef]
- Diaz-Chavez, J.; Fonseca-Sanchez, M.A.; Arechaga-Ocampo, E.; Flores-Perez, A.; Palacios-Rodriguez, Y.; Dominguez-Gomez, G.; Marchat, L.A.; Fuentes-Mera, L.; Mendoza-Hernandez, G.; Gariglio, P.; et al. Proteomic profiling reveals that resveratrol inhibits HSP27 expression and sensitizes breast cancer cells to doxorubicin therapy. PLoS ONE 2013, 8, e64378. [Google Scholar] [CrossRef]
- Kim, T.H.; Shin, Y.J.; Won, A.J.; Lee, B.M.; Choi, W.S.; Jung, J.H.; Chung, H.Y.; Kim, H.S. Resveratrol enhances chemosensitivity of doxorubicin in multidrug-resistant human breast cancer cells via increased cellular influx of doxorubicin. Biochim. Biophys. Acta 2014, 1840, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, A.; Marko, D. Resveratrol modulates the topoisomerase inhibitory potential of doxorubicin in human colon carcinoma cells. Molecules 2014, 19, 20054–20072. [Google Scholar] [CrossRef]
- Huang, F.; Wu, X.N.; Chen, J.; Wang, W.X.; Lu, Z.F. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp. Ther. Med. 2014, 7, 1611–1616. [Google Scholar] [CrossRef]
- Tomoaia, G.; Horovitz, O.; Mocanu, A.; Nita, A.; Avram, A.; Racz, C.P.; Soritau, O.; Cenariu, M.; Tomoaia-Cotisel, M. Effects of doxorubicin mediated by gold nanoparticles and resveratrol in two human cervical tumor cell lines. Colloids Surf. B Biointerfaces 2015, 135, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.T.; Jhan, H.J.; Hsieh, C.M.; Wang, C.J.; Ho, H.O. Efficacy of antioxidants as a Complementary and Alternative Medicine (CAM) in combination with the chemotherapeutic agent doxorubicin. Integr. Cancer Ther. 2015, 14, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, S.A.; Al-Abd, A.M.; Ali, A.A.; Abdel-Naim, A.B. Didox and resveratrol sensitize colorectal cancer cells to doxorubicin via activating apoptosis and ameliorating P-glycoprotein activity. Sci. Rep. 2016, 6, 36855. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Karami, S.P.; Delaramifar, A.; Sheidary, A.; Tabrizian, K.; Rezaee, R.; Shahsavand, S.; Arsene, A.L.; Tsatsakis, A.M.; Taghdisi, S.M. Anticancer Effects of Co-Administration of Daunorubicin and Resveratrol in Molt-4, U266 B1 and Raji Cell Lines. Farmacia 2016, 64, 36–42. [Google Scholar]
- Rai, G.; Mishra, S.; Suman, S.; Shukla, Y. Resveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: A mechanistic insight. Phytomedicine 2016, 23, 233–242. [Google Scholar] [CrossRef]
- Xu, J.; Liu, D.; Niu, H.; Zhu, G.; Xu, Y.; Ye, D.; Li, J.; Zhang, Q. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J. Exp. Clin. Cancer Res. 2017, 36, 19. [Google Scholar] [CrossRef]
- Chen, J.M.; Bai, J.Y.; Yang, K.X. Effect of resveratrol on doxorubicin resistance in breast neoplasm cells by modulating PI3K/Akt signaling pathway. IUBMB Life 2018, 70, 491–500. [Google Scholar] [CrossRef]
- Barros, A.S.; Costa, E.C.; Nunes, A.S.; de Melo-Diogo, D.; Correia, I.J. Comparative study of the therapeutic effect of Doxorubicin and Resveratrol combination on 2D and 3D (spheroids) cell culture models. Int. J. Pharm. 2018, 551, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.; Alderete, K.S.; Grant, M.K.O.; Seelig, D.M.; Sharkey, L.C.; Zordoky, B.N.M. Anticancer effects of resveratrol in canine hemangiosarcoma cell lines. Vet. Comp. Oncol. 2018, 16, 253–261. [Google Scholar] [CrossRef] [PubMed]
- El-Readi, M.Z.; Eid, S.; Abdelghany, A.A.; Al-Amoudi, H.S.; Efferth, T.; Wink, M. Resveratrol mediated cancer cell apoptosis, and modulation of multidrug resistance proteins and metabolic enzymes. Phytomedicine 2018, 55, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Pouyafar, A.; Zadi Heydarabad, M.; Aghdam, S.B.; Khaksar, M.; Azimi, A.; Rahbarghazi, R.; Talebi, M. Resveratrol potentially increased the tumoricidal effect of doxorubicin on SKOV3 cancer stem cells in vitro. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wei, Y.; Liu, Y.; Lu, X.; Ding, F.; Wang, J.; Yang, S. Resveratrol promotes sensitization to Doxorubicin by inhibiting epithelial-mesenchymal transition and modulating SIRT1/beta-catenin signaling pathway in breast cancer. Cancer Med. 2019. [Google Scholar] [CrossRef]
- Hallajian, F.; Ghasmi, M.; Abedi, S.M.; Behzadi, R.; Hayati, E.; Sadeghzadeh, N.; Rezazadeh, F.; Karimi, H. Evaluation of the Effect of Resveratrol and Doxorubicin on (99m)Tc-MIBI Uptake in Breast Cancer Cell Xenografts in Mice. Cancer Biother. Radiopharm. 2018, 33, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Jajoo, S.; Kaur, T.; Mukherjea, D.; Sheehan, K.; Rybak, L.P.; Ramkumar, V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS ONE 2012, 7, e51655. [Google Scholar] [CrossRef]
- Weng, Y.L.; Liao, H.F.; Li, A.F.; Chang, J.C.; Chiou, R.Y. Oral administration of resveratrol in suppression of pulmonary metastasis of BALB/c mice challenged with CT26 colorectal adenocarcinoma cells. Mol. Nutr. Food Res. 2010, 54, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Singh, U.P.; Hegde, V.L.; Guan, H.; Hofseth, L.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol (trans-3,5,4′-trihydroxystilbene) suppresses EL4 tumor growth by induction of apoptosis involving reciprocal regulation of SIRT1 and NF-kappaB. Mol. Nutr. Food Res. 2011, 55, 1207–1218. [Google Scholar] [CrossRef]
- Sun, L.; Chen, B.; Jiang, R.; Li, J.; Wang, B. Resveratrol inhibits lung cancer growth by suppressing M2-like polarization of tumor associated macrophages. Cell. Immunol. 2017, 311, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sumiyoshi, M. Resveratrol Prevents Tumor Growth and Metastasis by Inhibiting Lymphangiogenesis and M2 Macrophage Activation and Differentiation in Tumor-associated Macrophages. Nutr. Cancer 2016, 68, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Trung, L.Q.; An, D.T.T. Is Resveratrol a Cancer Immunomodulatory Molecule? Front. Pharmacol. 2018, 9, 1255. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, B.; Zhao, B.; Mei, D.; Gu, Q.; Tian, Z. Cisplatin-induced cardiotoxicity with midrange ejection fraction: A case report and review of the literature. Medicine 2018, 97, e13807. [Google Scholar] [CrossRef]
- Ma, H.; Jones, K.R.; Guo, R.; Xu, P.; Shen, Y.; Ren, J. Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: Role of endoplasmic reticulum stress. Clin. Exp. Pharmacol. Physiol. 2010, 37, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, D.; Zhang, Q.; Han, Y.; Jin, S.; Qi, F. Resveratrol protects against Cisplatin-induced cardiotoxicity by alleviating oxidative damage. Cancer Biother. Radiopharm. 2009, 24, 675–680. [Google Scholar] [CrossRef]
- Hao, Q.; Xiao, X.; Zhen, J.; Feng, J.; Song, C.; Jiang, B.; Hu, Z. Resveratrol attenuates acute kidney injury by inhibiting death receptormediated apoptotic pathways in a cisplatininduced rat model. Mol. Med. Rep. 2016, 14, 3683–3689. [Google Scholar] [CrossRef] [PubMed]
- Valentovic, M.A.; Ball, J.G.; Brown, J.M.; Terneus, M.V.; McQuade, E.; Van Meter, S.; Hedrick, H.M.; Roy, A.A.; Williams, T. Resveratrol attenuates cisplatin renal cortical cytotoxicity by modifying oxidative stress. Toxicol. In Vitro 2014, 28, 248–257. [Google Scholar] [CrossRef]
- Do Amaral, C.L.; Francescato, H.D.; Coimbra, T.M.; Costa, R.S.; Darin, J.D.; Antunes, L.M.; Bianchi Mde, L. Resveratrol attenuates cisplatin-induced nephrotoxicity in rats. Arch. Toxicol. 2008, 82, 363–370. [Google Scholar] [CrossRef]
- Singh, I.; Goyal, Y.; Ranawat, P. Potential chemoprotective role of resveratrol against cisplatin induced testicular damage in mice. Chem. Biol. Interact. 2017, 273, 200–211. [Google Scholar] [CrossRef]
- Reddy, K.P.; Madhu, P.; Reddy, P.S. Protective effects of resveratrol against cisplatin-induced testicular and epididymal toxicity in rats. Food Chem. Toxicol. 2016, 91, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Atli, M.; Engin-Ustun, Y.; Tokmak, A.; Caydere, M.; Hucumenoglu, S.; Topcuoglu, C. Dose dependent effect of resveratrol in preventing cisplatin-induced ovarian damage in rats: An experimental study. Reprod. Biol. 2017, 17, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Yumusakhuylu, A.C.; Yazici, M.; Sari, M.; Binnetoglu, A.; Kosemihal, E.; Akdas, F.; Sirvanci, S.; Yuksel, M.; Uneri, C.; Tutkun, A. Protective role of resveratrol against cisplatin induced ototoxicity in guinea pigs. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Erdem, T.; Bayindir, T.; Filiz, A.; Iraz, M.; Selimoglu, E. The effect of resveratrol on the prevention of cisplatin ototoxicity. Eur. Arch. Otorhinolaryngol. 2012, 269, 2185–2188. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.S.; An, Y.S.; Chang, J.; Choi, J.; Im, G.J. Protective effect of resveratrol against cisplatin-induced ototoxicity in HEI-OC1 auditory cells. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.A.; Abo-Youssef, A.M.; Khalaf, M.M.; Abo-Saif, A.A.; Saleh, I.G.; Abdelghany, T.M. Resveratrol influences platinum pharmacokinetics: A novel mechanism in protection against cisplatin-induced nephrotoxicity. Toxicol. Lett. 2018, 290, 73–82. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, Q.; Li, Y.; Gao, Y. Resveratrol enhances cisplatin-induced apoptosis in human hepatoma cells via glutamine metabolism inhibition. BMB Rep. 2018, 51, 474–479. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, G.J.; Yi, S.S.; Heo, S.H.; Park, C.R.; Nam, H.S.; Cho, M.K.; Lee, S.H. Cisplatin and resveratrol induce apoptosis and autophagy following oxidative stress in malignant mesothelioma cells. Food Chem. Toxicol. 2016, 97, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, X.; Xu, R.; Ye, L.; Kong, H.; Zeng, X.; Wang, H.; Xie, W. The synergistic effect of resveratrol in combination with cisplatin on apoptosis via modulating autophagy in A549 cells. Acta Biochim. Biophys. Sin. 2016, 48, 528–535. [Google Scholar] [CrossRef]
- Ma, L.; Li, W.; Wang, R.; Nan, Y.; Wang, Q.; Liu, W.; Jin, F. Resveratrol enhanced anticancer effects of cisplatin on non-small cell lung cancer cell lines by inducing mitochondrial dysfunction and cell apoptosis. Int. J. Oncol. 2015, 47, 1460–1468. [Google Scholar] [CrossRef]
- Osman, A.M.; Al-Malki, H.S.; Al-Harthi, S.E.; El-Hanafy, A.A.; Elashmaoui, H.M.; Elshal, M.F. Modulatory role of resveratrol on cytotoxic activity of cisplatin, sensitization and modification of cisplatin resistance in colorectal cancer cells. Mol. Med. Rep. 2015, 12, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, D.; Soto, A.; Gil-Araujo, B.; Gallego, B.; Chiloeches, A.; Lasa, M. Resveratrol promotes apoptosis through the induction of dual specificity phosphatase 1 and sensitizes prostate cancer cells to cisplatin. Food Chem. Toxicol. 2018, 124, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Leon-Galicia, I.; Diaz-Chavez, J.; Albino-Sanchez, M.E.; Garcia-Villa, E.; Bermudez-Cruz, R.; Garcia-Mena, J.; Herrera, L.A.; Garcia-Carranca, A.; Gariglio, P. Resveratrol decreases Rad51 expression and sensitizes cisplatinresistant MCF7 breast cancer cells. Oncol. Rep. 2018, 39, 3025–3033. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Valencia, J.; Garcia-Villa, E.; Arenas-Hernandez, A.; Garcia-Mena, J.; Diaz-Chavez, J.; Gariglio, P. Induction of p53 Phosphorylation at Serine 20 by Resveratrol Is Required to Activate p53 Target Genes, Restoring Apoptosis in MCF-7 Cells Resistant to Cisplatin. Nutrients 2018, 10, 1148. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Y.; Wang, R.; Pan, L.; Ma, L.; Jin, F. Resveratrol promotes the sensitivity of small-cell lung cancer H446 cells to cisplatin by regulating intrinsic apoptosis. Int. J. Oncol. 2018, 53, 2123–2130. [Google Scholar] [CrossRef]
- Tan, L.; Wang, W.; He, G.; Kuick, R.D.; Gossner, G.; Kueck, A.S.; Wahl, H.; Opipari, A.W.; Liu, J.R. Resveratrol inhibits ovarian tumor growth in an in vivo mouse model. Cancer 2016, 122, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Higgins, A.Y.; O’Halloran, T.D.; Chang, J.D. Chemotherapy-induced cardiomyopathy. Heart Fail. Rev. 2015, 20, 721–730. [Google Scholar] [CrossRef]
- Ayash, L.J.; Wright, J.E.; Tretyakov, O.; Gonin, R.; Elias, A.; Wheeler, C.; Eder, J.P.; Rosowsky, A.; Antman, K.; Frei, E., 3rd. Cyclophosphamide pharmacokinetics: Correlation with cardiac toxicity and tumor response. J. Clin. Oncol. 1992, 10, 995–1000. [Google Scholar] [CrossRef]
- Tiersten, A.; Wo, J.; Jacobson, C.; Weitzman, A.; Horwich, T.; Hesdorffer, C.; Savage, D.; Troxel, A. Cardiac toxicity observed in association with high-dose cyclophosphamide-based chemotherapy for metastatic breast cancer. Breast 2004, 13, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Cazin, B.; Gorin, N.C.; Laporte, J.P.; Gallet, B.; Douay, L.; Lopez, M.; Najman, A.; Duhamel, G. Cardiac complications after bone marrow transplantation. A report on a series of 63 consecutive transplantations. Cancer 1986, 57, 2061–2069. [Google Scholar] [CrossRef]
- Nagi, M.N.; Al-Shabanah, O.A.; Hafez, M.M.; Sayed-Ahmed, M.M. Thymoquinone supplementation attenuates cyclophosphamide-induced cardiotoxicity in rats. J. Biochem. Mol. Toxicol. 2011, 25, 135–142. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.A.; Morsy, M.A.; Okasha, A.M. Inhibition of NF-kappaB/TNF-alpha pathway may be involved in the protective effect of resveratrol against cyclophosphamide-induced multi-organ toxicity. Immunopharmacol. Immunotoxicol. 2017, 39, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Nigam, M.; Ranjan, V.; Zaidi, D.; Garg, V.K.; Sharma, S.; Chaturvedi, R.; Shankar, R.; Kumar, S.; Sharma, R.; et al. Resveratrol as an adjunct therapy in cyclophosphamide-treated MCF-7 cells and breast tumor explants. Cancer Sci. 2011, 102, 1059–1067. [Google Scholar] [CrossRef]
- Singh, N.; Nigam, M.; Ranjan, V.; Sharma, R.; Balapure, A.K.; Rath, S.K. Caspase mediated enhanced apoptotic action of cyclophosphamide- and resveratrol-treated MCF-7 cells. J. Pharmacol. Sci. 2009, 109, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Chen, Z. Acute promyelocytic leukemia: From highly fatal to highly curable. Blood 2008, 111, 2505–2515. [Google Scholar] [CrossRef] [PubMed]
- Vineetha, V.P.; Raghu, K.G. An Overview on Arsenic Trioxide-Induced Cardiotoxicity. Cardiovasc. Toxicol. 2019. [Google Scholar] [CrossRef]
- Unnikrishnan, D.; Dutcher, J.P.; Varshneya, N.; Lucariello, R.; Api, M.; Garl, S.; Wiernik, P.H.; Chiaramida, S. Torsades de pointes in 3 patients with leukemia treated with arsenic trioxide. Blood 2001, 97, 1514–1516. [Google Scholar] [CrossRef]
- Ficker, E.; Kuryshev, Y.A.; Dennis, A.T.; Obejero-Paz, C.; Wang, L.; Hawryluk, P.; Wible, B.A.; Brown, A.M. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol. Pharmacol. 2004, 66, 33–44. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Li, G.Y.; Liu, Y.; Chai, L.M.; Chen, J.X.; Zhang, Y.; Du, Z.M.; Lu, Y.J.; Yang, B.F. Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Br. J. Pharmacol. 2008, 154, 105–113. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, C.; Gao, R.; Ge, M.; Zhu, Y.; Zhang, Z. The Protective Role of Resveratrol against Arsenic Trioxide-Induced Cardiotoxicity. Evid. Based Complement. Altern. Med. 2013, 2013, 407839. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, K.P.; Huang, T.; Yan, C.C.; Liu, L.R.; Zhu, Q.L.; Guo, F.F.; Liu, C.; Li, B.X. The rescuable function and mechanism of resveratrol on As(2)O(3)-induced hERG K(+) channel deficiency. Naunyn. Schmiedebergs Arch. Pharmacol. 2014, 387, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xue, J.; Jiang, H.; Wang, M.; Gao, L.; Ma, D.; Zhang, Z. Neuroprotective effect of resveratrol on arsenic trioxide-induced oxidative stress in feline brain. Hum. Exp. Toxicol. 2014, 33, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xue, J.; Yao, C.; Gao, L.; Ma, D.; Liu, Y.; Zhang, Z. Resveratrol ameliorates the oxidative damage induced by arsenic trioxide in the feline lung. J. Vet. Med. Sci. 2013, 75, 1139–1146. [Google Scholar] [CrossRef]
- Yu, M.; Xue, J.; Li, Y.; Zhang, W.; Ma, D.; Liu, L.; Zhang, Z. Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch. Toxicol. 2013, 87, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xue, J.; Ge, M.; Yu, M.; Liu, L.; Zhang, Z. Resveratrol attenuates hepatotoxicity of rats exposed to arsenic trioxide. Food Chem. Toxicol. 2013, 51, 87–92. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Ge, M.; Jing, J.; Chen, Y.; Jiang, H.; Yu, H.; Li, N.; Zhang, Z. Protective effect of resveratrol on arsenic trioxide-induced nephrotoxicity in rats. Nutr. Res. Pract. 2014, 8, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Chen, C.; Jiang, X.; Zhang, Z. ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction underlie apoptosis induced by resveratrol and arsenic trioxide in A549 cells. Chem. Biol. Interact. 2016, 245, 100–109. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, M.; Meng, J.; Yu, L.; Tu, Y.; Wan, L.; Fang, K.; Zhu, W. Arsenic trioxide and resveratrol show synergistic anti-leukemia activity and neutralized cardiotoxicity. PLoS ONE 2014, 9, e105890. [Google Scholar] [CrossRef]
- Yen, C.M.; Tsai, C.W.; Chang, W.S.; Yang, Y.C.; Hung, Y.W.; Lee, H.T.; Shen, C.C.; Sheu, M.L.; Wang, J.Y.; Gong, C.L.; et al. Novel Combination of Arsenic Trioxide (As2O3) Plus Resveratrol in Inducing Programmed Cell Death of Human Neuroblastoma SK-N-SH Cells. Cancer Genom. Proteom. 2018, 15, 453–460. [Google Scholar] [CrossRef]
- Gu, S.; Chen, C.; Jiang, X.; Zhang, Z. Resveratrol synergistically triggers apoptotic cell death with arsenic trioxide via oxidative stress in human lung adenocarcinoma A549 cells. Biol. Trace Elem. Res. 2015, 163, 112–123. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Yang, S.; Chen, Y.R.; Li, P.C.; Dou, M.M.; Zhang, J. Resveratrol and arsenic trioxide act synergistically to kill tumor cells in vitro and in vivo. PLoS ONE 2014, 9, e98925. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Vasina, V.; Ursino, M.G.; Boriani, G.; Martoni, A.; De Ponti, F. Anticancer drugs and cardiotoxicity: Insights and perspectives in the era of targeted therapy. Pharmacol. Ther. 2010, 125, 196–218. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.F.; Rupnick, M.A.; Kerkela, R.; Dallabrida, S.M.; Zurakowski, D.; Nguyen, L.; Woulfe, K.; Pravda, E.; Cassiola, F.; Desai, J.; et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007, 370, 2011–2019. [Google Scholar] [CrossRef]
- Cooper, S.L.; Sandhu, H.; Hussain, A.; Mee, C.; Maddock, H. Involvement of mitogen activated kinase kinase 7 intracellular signalling pathway in Sunitinib-induced cardiotoxicity. Toxicology 2018, 394, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Korashy, H.M.; Al-Suwayeh, H.A.; Maayah, Z.H.; Ansari, M.A.; Ahmad, S.F.; Bakheet, S.A. Mitogen-activated protein kinases pathways mediate the sunitinib-induced hypertrophy in rat cardiomyocyte H9c2 cells. Cardiovasc. Toxicol. 2015, 15, 41–51. [Google Scholar] [CrossRef]
- Kerkela, R.; Woulfe, K.C.; Durand, J.B.; Vagnozzi, R.; Kramer, D.; Chu, T.F.; Beahm, C.; Chen, M.H.; Force, T. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin. Transl. Sci. 2009, 2, 15–25. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Ansari, M.A.; El Gendy, M.A.; Al-Arifi, M.N.; Korashy, H.M. Development of cardiac hypertrophy by sunitinib in vivo and in vitro rat cardiomyocytes is influenced by the aryl hydrocarbon receptor signaling pathway. Arch. Toxicol. 2014, 88, 725–738. [Google Scholar] [CrossRef]
- Alsaad, A.M.S. Dasatinib induces gene expression of CYP1A1, CYP1B1, and cardiac hypertrophy markers (BNP, beta-MHC) in rat cardiomyocyte H9c2 cells. Toxicol. Mech. Methods 2018, 28, 678–684. [Google Scholar] [CrossRef]
- Maayah, Z.H.; El Gendy, M.A.; El-Kadi, A.O.; Korashy, H.M. Sunitinib, a tyrosine kinase inhibitor, induces cytochrome P450 1A1 gene in human breast cancer MCF7 cells through ligand-independent aryl hydrocarbon receptor activation. Arch. Toxicol. 2013, 87, 847–856. [Google Scholar] [CrossRef]
- Damiano, S.; Montagnaro, S.; Puzio, M.V.; Severino, L.; Pagnini, U.; Barbarino, M.; Cesari, D.; Giordano, A.; Florio, S.; Ciarcia, R. Effects of antioxidants on apoptosis induced by dasatinib and nilotinib in K562 cells. J. Cell. Biochem. 2018, 119, 4845–4854. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, Y.H. Inhibition of human chronic myelogenous leukemia K562 cell growth following combination treatment with resveratrol and imatinib mesylate. Genet. Mol. Res. 2015, 14, 6413–6418. [Google Scholar] [CrossRef] [PubMed]
- Puissant, A.; Grosso, S.; Jacquel, A.; Belhacene, N.; Colosetti, P.; Cassuto, J.P.; Auberger, P. Imatinib mesylate-resistant human chronic myelogenous leukemia cell lines exhibit high sensitivity to the phytoalexin resveratrol. FASEB J. 2008, 22, 1894–1904. [Google Scholar] [CrossRef]
- Can, G.; Cakir, Z.; Kartal, M.; Gunduz, U.; Baran, Y. Apoptotic effects of resveratrol, a grape polyphenol, on imatinib-sensitive and resistant K562 chronic myeloid leukemia cells. Anticancer Res. 2012, 32, 2673–2678. [Google Scholar] [PubMed]
- Tapio, S. Pathology and biology of radiation-induced cardiac disease. J. Radiat. Res. 2016, 57, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.H.; Tawfik, S.S. Early treatment of radiation-induced heart damage in rats by caffeic acid phenethyl ester. Eur. J. Pharmacol. 2012, 692, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, K.; Ko, K.C.; Choi, M.H.; Kang, J.A.; Chung, Y.J.; Park, S.H. Protective effect of hesperidin, a citrus flavanoglycone, against gamma-radiation-induced tissue damage in Sprague-Dawley rats. J. Med. Food 2012, 15, 419–427. [Google Scholar] [CrossRef]
- Michalowski, A.S. On radiation damage to normal tissues and its treatment. II. Anti-inflammatory drugs. Acta Oncol. 1994, 33, 139–157. [Google Scholar] [CrossRef]
- Meeren, A.V.; Bertho, J.M.; Vandamme, M.; Gaugler, M.H. Ionizing radiation enhances IL-6 and IL-8 production by human endothelial cells. Mediat. Inflamm. 1997, 6, 185–193. [Google Scholar] [CrossRef]
- Panganiban, R.A.; Mungunsukh, O.; Day, R.M. X-irradiation induces ER stress, apoptosis, and senescence in pulmonary artery endothelial cells. Int. J. Radiat. Biol. 2013, 89, 656–667. [Google Scholar] [CrossRef]
- Suzuki, K.; Mori, I.; Nakayama, Y.; Miyakoda, M.; Kodama, S.; Watanabe, M. Radiation-induced senescence-like growth arrest requires TP53 function but not telomere shortening. Radiat. Res. 2001, 155, 248–253. [Google Scholar] [CrossRef]
- De Freitas, R.B.; Boligon, A.A.; Rovani, B.T.; Piana, M.; de Brum, T.F.; da Silva Jesus, R.; Rother, F.C.; Alves, N.M.; Teixeira da Rocha, J.B.; Athayde, M.L.; et al. Effect of black grape juice against heart damage from acute gamma TBI in rats. Molecules 2013, 18, 12154–12167. [Google Scholar] [CrossRef] [PubMed]
- Sener, T.E.; Tavukcu, H.H.; Atasoy, B.M.; Cevik, O.; Kaya, O.T.; Cetinel, S.; Dagli Degerli, A.; Tinay, I.; Simsek, F.; Akbal, C.; et al. Resveratrol treatment may preserve the erectile function after radiotherapy by restoring antioxidant defence mechanisms, SIRT1 and NOS protein expressions. Int. J. Impot. Res. 2018, 30, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, H.; Ying, J.; Du, L.; Zhang, C.; Yang, Y.; Wang, H.; Wang, H. Resveratrol ameliorates ionizing irradiation-induced long-term immunosuppression in mice. Int. J. Radiat. Biol. 2018, 94, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, H.; Zhou, X.; Wang, H.; Yang, Y.; Zhang, J.; Wang, H. The protective effects of Resveratrol against radiation-induced intestinal injury. BMC Complement. Altern. Med. 2017, 17, 410. [Google Scholar] [CrossRef]
- Velioglu-Ogunc, A.; Sehirli, O.; Toklu, H.Z.; Ozyurt, H.; Mayadagli, A.; Eksioglu-Demiralp, E.; Erzik, C.; Cetinel, S.; Yegen, B.C.; Sener, G. Resveratrol protects against irradiation-induced hepatic and ileal damage via its anti-oxidative activity. Free Radic Res. 2009, 43, 1060–1071. [Google Scholar] [CrossRef]
- Said, R.S.; El-Demerdash, E.; Nada, A.S.; Kamal, M.M. Resveratrol inhibits inflammatory signaling implicated in ionizing radiation-induced premature ovarian failure through antagonistic crosstalk between silencing information regulator 1 (SIRT1) and poly(ADP-ribose) polymerase 1 (PARP-1). Biochem. Pharmacol. 2016, 103, 140–150. [Google Scholar] [CrossRef]
- Simsek, Y.; Gurocak, S.; Turkoz, Y.; Akpolat, N.; Celik, O.; Ozer, A.; Yilmaz, E.; Turhan, U.; Ozyalin, F. Ameliorative effects of resveratrol on acute ovarian toxicity induced by total body irradiation in young adult rats. J. Pediatr. Adolesc. Gynecol. 2012, 25, 262–266. [Google Scholar] [CrossRef]
- Xu, L.; Yang, X.; Cai, J.; Ma, J.; Cheng, H.; Zhao, K.; Yang, L.; Cao, Y.; Qin, Q.; Zhang, C.; et al. Resveratrol attenuates radiation-induced salivary gland dysfunction in mice. Laryngoscope 2013, 123, E23–E29. [Google Scholar] [CrossRef]
- Simsek, G.; Gurocak, S.; Karadag, N.; Karabulut, A.B.; Demirtas, E.; Karatas, E.; Pepele, E. Protective effects of resveratrol on salivary gland damage induced by total body irradiation in rats. Laryngoscope 2012, 122, 2743–2748. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Z.; Wang, Y.; Zhang, J.; Wu, H.; Wang, Y.; Li, C.; Li, D.; Lu, L.; Wang, X.; et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic. Biol. Med. 2013, 54, 40–50. [Google Scholar] [CrossRef]
- Banegas, Y.C.; Ocolotobiche, E.E.; Padula, G.; Cordoba, E.E.; Fernandez, E.; Guerci, A.M. Evaluation of resveratrol radiomodifying potential for radiotherapy treatment. Mutat. Res. 2018, 836, 79–83. [Google Scholar] [CrossRef]
- Voellger, B.; Waldt, N.; Rupa, R.; Kirches, E.; Melhem, O.; Ochel, H.J.; Mawrin, C.; Firsching, R. Combined effects of resveratrol and radiation in GH3 and TtT/GF pituitary adenoma cells. J. Neurooncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Araldi, I.C.; Bordin, F.P.R.; Cadona, F.C.; Barbisan, F.; Azzolin, V.F.; Teixeira, C.F.; Baumhardt, T.; da Cruz, I.B.M.; Duarte, M.; Bauermann, L.F. The in vitro radiosensitizer potential of resveratrol on MCF-7 breast cancer cells. Chem. Biol. Interact. 2018, 282, 85–92. [Google Scholar] [CrossRef]
- Assad, D.X.; Borges, G.A.; Avelino, S.R.; Guerra, E.N.S. Additive cytotoxic effects of radiation and mTOR inhibitors in a cervical cancer cell line. Pathol. Res. Pract. 2018, 214, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Sun, X.; Liu, Y.; Du, L.; Wang, Y.; He, N.; Wang, J.; Xu, C.; Liu, Q. Regulation of Apoptosis and Radiation Sensitization in Lung Cancer Cells via the Sirt1/NF-kappaB/Smac Pathway. Cell. Physiol. Biochem. 2018, 48, 304–316. [Google Scholar] [CrossRef]
- Zoberi, I.; Bradbury, C.M.; Curry, H.A.; Bisht, K.S.; Goswami, P.C.; Roti Roti, J.L.; Gius, D. Radiosensitizing and anti-proliferative effects of resveratrol in two human cervical tumor cell lines. Cancer Lett. 2002, 175, 165–173. [Google Scholar] [CrossRef]
- Baatout, S.; Derradji, H.; Jacquet, P.; Ooms, D.; Michaux, A.; Mergeay, M. Enhanced radiation-induced apoptosis of cancer cell lines after treatment with resveratrol. Int. J. Mol. Med. 2004, 13, 895–902. [Google Scholar] [CrossRef]
- Baatout, S.; Derradji, H.; Jacquet, P.; Mergeay, M. Increased radiation sensitivity of an eosinophilic cell line following treatment with epigallocatechin-gallate, resveratrol and curcuma. Int. J. Mol. Med. 2005, 15, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.F.; Kuo, C.D.; Yang, Y.C.; Lin, C.P.; Tai, H.C.; Chen, Y.Y.; Chen, Y.J. Resveratrol enhances radiosensitivity of human non-small cell lung cancer NCI-H838 cells accompanied by inhibition of nuclear factor-kappa B activation. J. Radiat. Res. 2005, 46, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Scarlatti, F.; Sala, G.; Ricci, C.; Maioli, C.; Milani, F.; Minella, M.; Botturi, M.; Ghidoni, R. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett. 2007, 253, 124–130. [Google Scholar] [CrossRef]
- Lu, K.H.; Chen, Y.W.; Tsai, P.H.; Tsai, M.L.; Lee, Y.Y.; Chiang, C.Y.; Kao, C.L.; Chiou, S.H.; Ku, H.H.; Lin, C.H.; et al. Evaluation of radiotherapy effect in resveratrol-treated medulloblastoma cancer stem-like cells. Childs Nerv. Syst. 2009, 25, 543–550. [Google Scholar] [CrossRef]
- Kao, C.L.; Huang, P.I.; Tsai, P.H.; Tsai, M.L.; Lo, J.F.; Lee, Y.Y.; Chen, Y.J.; Chen, Y.W.; Chiou, S.H. Resveratrol-induced apoptosis and increased radiosensitivity in CD133-positive cells derived from atypical teratoid/rhabdoid tumor. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 219–228. [Google Scholar] [CrossRef]
- Rashid, A.; Liu, C.; Sanli, T.; Tsiani, E.; Singh, G.; Bristow, R.G.; Dayes, I.; Lukka, H.; Wright, J.; Tsakiridis, T. Resveratrol enhances prostate cancer cell response to ionizing radiation. Modulation of the AMPK, Akt and mTOR pathways. Radiat. Oncol. 2011, 6, 144. [Google Scholar] [CrossRef]
- Fang, Y.; DeMarco, V.G.; Nicholl, M.B. Resveratrol enhances radiation sensitivity in prostate cancer by inhibiting cell proliferation and promoting cell senescence and apoptosis. Cancer Sci. 2012, 103, 1090–1098. [Google Scholar] [CrossRef]
- Yang, Y.P.; Chang, Y.L.; Huang, P.I.; Chiou, G.Y.; Tseng, L.M.; Chiou, S.H.; Chen, M.H.; Chen, M.T.; Shih, Y.H.; Chang, C.H.; et al. Resveratrol suppresses tumorigenicity and enhances radiosensitivity in primary glioblastoma tumor initiating cells by inhibiting the STAT3 axis. J. Cell. Physiol. 2012, 227, 976–993. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.K.; Lee, J.H.; Park, J.W. Resveratrol and piperine enhance radiosensitivity of tumor cells. BMB Rep. 2012, 45, 242–246. [Google Scholar] [CrossRef]
- Fang, Y.; Herrick, E.J.; Nicholl, M.B. A possible role for perforin and granzyme B in resveratrol-enhanced radiosensitivity of prostate cancer. J. Androl. 2012, 33, 752–760. [Google Scholar] [CrossRef]
- Fang, Y.; Bradley, M.J.; Cook, K.M.; Herrick, E.J.; Nicholl, M.B. A potential role for resveratrol as a radiation sensitizer for melanoma treatment. J. Surg. Res. 2013, 183, 645–653. [Google Scholar] [CrossRef]
- Luo, H.; Wang, L.; Schulte, B.A.; Yang, A.; Tang, S.; Wang, G.Y. Resveratrol enhances ionizing radiation-induced premature senescence in lung cancer cells. Int. J. Oncol. 2013, 43, 1999–2006. [Google Scholar] [CrossRef]
- Magalhaes, V.D.; Rogero, S.O.; Cruz, A.S.; Vieira, D.P.; Okazaki, K.; Rogero, J.R. In vitro tests of resveratrol radiomodifying effect on rhabdomyosarcoma cells by comet assay. Toxicol. In Vitro 2014, 28, 1436–1442. [Google Scholar] [CrossRef]
- Heiduschka, G.; Lill, C.; Seemann, R.; Brunner, M.; Schmid, R.; Houben, R.; Bigenzahn, J.; Thurnher, D. The effect of resveratrol in combination with irradiation and chemotherapy: Study using Merkel cell carcinoma cell lines. Strahlenther. Onkol. 2014, 190, 75–80. [Google Scholar] [CrossRef]
- Atienzar, A.N.; Camacho-Alonso, F.; Lopez-Jornet, P. Effects of resveratrol and irradiation upon oral squamous cell carcinoma cells. Acta Odontol. Scand. 2014, 72, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Long, L.; Wang, W.; Liang, Z. Resveratrol, a potential radiation sensitizer for glioma stem cells both in vitro and in vivo. J. Pharmacol. Sci. 2015, 129, 216–225. [Google Scholar] [CrossRef]
- Baek, S.H.; Ko, J.H.; Lee, H.; Jung, J.; Kong, M.; Lee, J.W.; Lee, J.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; et al. Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine 2016, 23, 566–577. [Google Scholar] [CrossRef]
- Chen, Y.A.; Lien, H.M.; Kao, M.C.; Lo, U.G.; Lin, L.C.; Lin, C.J.; Chang, S.J.; Chen, C.C.; Hsieh, J.T.; Lin, H.; et al. Sensitization of Radioresistant Prostate Cancer Cells by Resveratrol Isolated from Arachis hypogaea Stems. PLoS ONE 2017, 12, e0169204. [Google Scholar] [CrossRef]
- Tan, Y.; Wei, X.; Zhang, W.; Wang, X.; Wang, K.; Du, B.; Xiao, J. Resveratrol enhances the radiosensitivity of nasopharyngeal carcinoma cells by downregulating E2F1. Oncol. Rep. 2017, 37, 1833–1841. [Google Scholar] [CrossRef]
- Fulda, S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today 2010, 15, 757–765. [Google Scholar] [CrossRef]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic Versatility of Resveratrol Derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef] [PubMed]
- Ogas, T.; Kondratyuk, T.P.; Pezzuto, J.M. Resveratrol analogs: Promising chemopreventive agents. Ann. N. Y. Acad. Sci. 2013, 1290, 21–29. [Google Scholar] [CrossRef]
- Butt, N.A.; Kumar, A.; Dhar, S.; Rimando, A.M.; Akhtar, I.; Hancock, J.C.; Lage, J.M.; Pound, C.R.; Lewin, J.R.; Gomez, C.R.; et al. Targeting MTA1/HIF-1alpha signaling by pterostilbene in combination with histone deacetylase inhibitor attenuates prostate cancer progression. Cancer Med. 2017, 6, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Kumar, A.; Zhang, L.; Rimando, A.M.; Lage, J.M.; Lewin, J.R.; Atfi, A.; Zhang, X.; Levenson, A.S. Dietary pterostilbene is a novel MTA1-targeted chemopreventive and therapeutic agent in prostate cancer. Oncotarget 2016, 7, 18469–18484. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Kumar, A.; Rimando, A.M.; Zhang, X.; Levenson, A.S. Resveratrol and pterostilbene epigenetically restore PTEN expression by targeting oncomiRs of the miR-17 family in prostate cancer. Oncotarget 2015, 6, 27214–27226. [Google Scholar] [CrossRef]
- Wen, W.; Lowe, G.; Roberts, C.M.; Finlay, J.; Han, E.S.; Glackin, C.A.; Dellinger, T.H. Pterostilbene Suppresses Ovarian Cancer Growth via Induction of Apoptosis and Blockade of Cell Cycle Progression Involving Inhibition of the STAT3 Pathway. Int. J. Mol. Sci. 2018, 19, 1983. [Google Scholar] [CrossRef]
- Kosuru, R.; Cai, Y.; Kandula, V.; Yan, D.; Wang, C.; Zheng, H.; Li, Y.; Irwin, M.G.; Singh, S.; Xia, Z. AMPK Contributes to Cardioprotective Effects of Pterostilbene Against Myocardial Ischemia-Reperfusion Injury in Diabetic Rats by Suppressing Cardiac Oxidative Stress and Apoptosis. Cell. Physiol. Biochem. 2018, 46, 1381–1397. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L.; Li, F.; Hu, C.P.; Zhang, Z. Restoration of sirt1 function by pterostilbene attenuates hypoxia-reoxygenation injury in cardiomyocytes. Eur. J. Pharmacol. 2016, 776, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, S.; Zhang, X.; Li, Y.; Zhao, Q.; Liu, T. Pterostilbene protects against myocardial ischemia/reperfusion injury via suppressing oxidative/nitrative stress and inflammatory response. Int. Immunopharmacol. 2017, 43, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Lu, S.; Zhong, J.; Huang, K.; Zhang, S. Protective Effects of Pterostilbene Against Myocardial Ischemia/Reperfusion Injury in Rats. Inflammation 2017, 40, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Lacerda, D.; Turck, P.; Gazzi de Lima-Seolin, B.; Colombo, R.; Duarte Ortiz, V.; Poletto Bonetto, J.H.; Campos-Carraro, C.; Bianchi, S.E.; Bello-Klein, A.; Linck Bassani, V.; et al. Pterostilbene reduces oxidative stress, prevents hypertrophy and preserves systolic function of right ventricle in cor pulmonale model. Br. J. Pharmacol. 2017, 174, 3302–3314. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Kempisty, H.; Klupczynska, A.; Trzybulska, D.; Kulcenty, K.; Sulej-Suchomska, A.M.; Kucinska, M.; Mikstacka, R.; Wierzchowski, M.; Murias, M.; Baer-Dubowska, W.; et al. Role of CYP1A1 in the biological activity of methylated resveratrol analogue, 3,4,5,4′-tetramethoxystilbene (DMU-212) in ovarian cancer A-2780 and non-cancerous HOSE cells. Toxicol. Lett. 2017, 267, 59–66. [Google Scholar] [CrossRef]
- Androutsopoulos, V.P.; Fragiadaki, I.; Spandidos, D.A.; Tosca, A. The resveratrol analogue, 3,4,5,4’trans-tetramethoxystilbene, inhibits the growth of A375 melanoma cells through multiple anticancer modes of action. Int. J. Oncol. 2016, 49, 1305–1314. [Google Scholar] [CrossRef]
- Piotrowska, H.; Myszkowski, K.; Ziolkowska, A.; Kulcenty, K.; Wierzchowski, M.; Kaczmarek, M.; Murias, M.; Kwiatkowska-Borowczyk, E.; Jodynis-Liebert, J. Resveratrol analogue 3,4,4′,5-tetramethoxystilbene inhibits growth, arrests cell cycle and induces apoptosis in ovarian SKOV-3 and A-2780 cancer cells. Toxicol. Appl. Pharmacol. 2012, 263, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Molavi, O.; Haddadi, A.; Lai, R.; Gossage, R.A.; Lavasanifar, A. Resveratrol analog trans 3,4,5,4′-tetramethoxystilbene (DMU-212) mediates anti-tumor effects via mechanism different from that of resveratrol. Cancer Chemother. Pharmacol. 2008, 63, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, O.; Wisniewski, J.; Bielawska-Pohl, A.; Paprocka, M.; Duarte, N.; Ferreira, M.J.; Dus, D.; Michalak, K. Stilbenes as multidrug resistance modulators and apoptosis inducers in human adenocarcinoma cells. Anticancer Res. 2010, 30, 4587–4593. [Google Scholar]
- Ferreira, M.J.; Duarte, N.; Gyemant, N.; Radics, R.; Cherepnev, G.; Varga, A.; Molnar, J. Interaction between doxorubicin and the resistance modifier stilbene on multidrug resistant mouse lymphoma and human breast cancer cells. Anticancer Res. 2006, 26, 3541–3546. [Google Scholar] [PubMed]
- D’Uva, G.; Baci, D.; Albini, A.; Noonan, D.M. Cancer chemoprevention revisited: Cytochrome P450 family 1B1 as a target in the tumor and the microenvironment. Cancer Treat. Rev. 2018, 63, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Cho, N.H.; Ye, D.J.; Baek, H.S.; Ryu, Y.S.; Chun, Y.J. Cytochrome P450 1B1 promotes cancer cell survival via specificity protein 1 (Sp1)-mediated suppression of death receptor 4. J. Toxicol. Environ. Health A 2018, 81, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Jung, H.K.; Kim, M.Y. Induction of p27(kip1) by 2,4,3′,5′-tetramethoxystilbene is regulated by protein phosphatase 2A-dependent Akt dephosphorylation in PC-3 prostate cancer cells. Arch. Pharm. Res. 2008, 31, 1187–1194. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Althurwi, H.N.; Abdelhamid, G.; Lesyk, G.; Jurasz, P.; El-Kadi, A.O. CYP1B1 inhibition attenuates doxorubicin-induced cardiotoxicity through a mid-chain HETEs-dependent mechanism. Pharmacol. Res. 2016, 105, 28–43. [Google Scholar] [CrossRef]
- Elkhatali, S.; Maayah, Z.H.; El-Sherbeni, A.A.; Elshenawy, O.H.; Abdelhamid, G.; Shoieb, S.M.; El-Kadi, A.O.S. Inhibition of Mid-chain HETEs Protects Against Angiotensin II-induced Cardiac Hypertrophy. J. Cardiovasc. Pharmacol. 2017, 70, 16–24. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Althurwi, H.N.; El-Sherbeni, A.A.; Abdelhamid, G.; Siraki, A.G.; El-Kadi, A.O. The role of cytochrome P450 1B1 and its associated mid-chain hydroxyeicosatetraenoic acid metabolites in the development of cardiac hypertrophy induced by isoproterenol. Mol. Cell. Biochem. 2017, 429, 151–165. [Google Scholar] [CrossRef]
- Thirunavukkarasu, S.; Khan, N.S.; Song, C.Y.; Ghafoor, H.U.; Brand, D.D.; Gonzalez, F.J.; Malik, K.U. Cytochrome P450 1B1 Contributes to the Development of Angiotensin II-Induced Aortic Aneurysm in Male Apoe(-/-) Mice. Am. J. Pathol. 2016, 186, 2204–2219. [Google Scholar] [CrossRef]
- Song, C.Y.; Ghafoor, K.; Ghafoor, H.U.; Khan, N.S.; Thirunavukkarasu, S.; Jennings, B.L.; Estes, A.M.; Zaidi, S.; Bridges, D.; Tso, P.; et al. Cytochrome P450 1B1 Contributes to the Development of Atherosclerosis and Hypertension in Apolipoprotein E-Deficient Mice. Hypertension 2016, 67, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Jennings, B.L.; Montanez, D.E.; May, M.E., Jr.; Estes, A.M.; Fang, X.R.; Yaghini, F.A.; Kanu, A.; Malik, K.U. Cytochrome P450 1B1 contributes to increased blood pressure and cardiovascular and renal dysfunction in spontaneously hypertensive rats. Cardiovasc. Drugs Ther. 2014, 28, 145–161. [Google Scholar] [CrossRef]
- Aldawsari, F.S.; Velazquez-Martinez, C.A. 3,4′,5-trans-Trimethoxystilbene; a natural analogue of resveratrol with enhanced anticancer potency. Investig. New Drugs 2015, 33, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Luo, X.J.; Yang, Z.B.; Zhang, J.J.; Li, T.B.; Zhang, X.J.; Ma, Q.L.; Zhang, G.G.; Hu, C.P.; Peng, J. Inhibition of NOX/VPO1 pathway and inflammatory reaction by trimethoxystilbene in prevention of cardiovascular remodeling in hypoxia-induced pulmonary hypertensive rats. J. Cardiovasc. Pharmacol. 2014, 63, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Seyed, M.A.; Jantan, I.; Bukhari, S.N.; Vijayaraghavan, K. A Comprehensive Review on the Chemotherapeutic Potential of Piceatannol for Cancer Treatment, with Mechanistic Insights. J. Agric. Food Chem. 2016, 64, 725–737. [Google Scholar] [CrossRef]
- Kee, H.J.; Park, S.; Kang, W.; Lim, K.S.; Kim, J.H.; Ahn, Y.; Jeong, M.H. Piceatannol attenuates cardiac hypertrophy in an animal model through regulation of the expression and binding of the transcription factor GATA binding factor 6. FEBS Lett. 2014, 588, 1529–1536. [Google Scholar] [CrossRef]
- Chen, W.P.; Hung, L.M.; Hsueh, C.H.; Lai, L.P.; Su, M.J. Piceatannol, a derivative of resveratrol, moderately slows I(Na) inactivation and exerts antiarrhythmic action in ischaemia-reperfused rat hearts. Br. J. Pharmacol. 2009, 157, 381–391. [Google Scholar] [CrossRef]
- Ellermann, C.; Wolfes, J.; Kochhauser, S.; Dechering, D.G.; Reinke, F.; Wasmer, K.; Eckardt, L.; Frommeyer, G. Divergent antiarrhythmic effects of resveratrol and piceatannol in a whole-heart model of long QT syndrome. Int. J. Cardiol. 2017, 243, 233–238. [Google Scholar] [CrossRef]
- Chou, C.C.; Chang, P.C.; Wen, M.S.; Lee, H.L.; Wo, H.T.; Yeh, S.J.; Wu, D. Piceatannol facilitates conduction block and ventricular fibrillation induction in ischemia-reperfused rabbit hearts with pacing-induced heart failure. Int. J. Cardiol. 2014, 171, 250–258. [Google Scholar] [CrossRef]
- Farrand, L.; Byun, S.; Kim, J.Y.; Im-Aram, A.; Lee, J.; Lim, S.; Lee, K.W.; Suh, J.Y.; Lee, H.J.; Tsang, B.K. Piceatannol enhances cisplatin sensitivity in ovarian cancer via modulation of p53, X-linked inhibitor of apoptosis protein (XIAP), and mitochondrial fission. J. Biol. Chem. 2013, 288, 23740–23750. [Google Scholar] [CrossRef]
- Zghonda, N.; Yoshida, S.; Ezaki, S.; Otake, Y.; Murakami, C.; Mliki, A.; Ghorbel, A.; Miyazaki, H. epsilon-Viniferin is more effective than its monomer resveratrol in improving the functions of vascular endothelial cells and the heart. Biosci. Biotechnol. Biochem. 2012, 76, 954–960. [Google Scholar] [CrossRef]
- Ozdemir, F.; Apaydin, E.; Onder, N.I.; Sen, M.; Ayrim, A.; Ogunc, Y.; Incesu, Z. Apoptotic effects of epsilon-viniferin in combination with cis-platin in C6 cells. Cytotechnology 2018, 70, 1061–1073. [Google Scholar] [CrossRef]
- Whittaker, J.A.; Al-Ismail, S.A. Effect of digoxin and vitamin E in preventing cardiac damage caused by doxorubicin in acute myeloid leukaemia. Br. Med. J. (Clin. Res. Ed.) 1984, 288, 283–284. [Google Scholar] [CrossRef]
- Dresdale, A.R.; Barr, L.H.; Bonow, R.O.; Mathisen, D.J.; Myers, C.E.; Schwartz, D.E.; d’Angelo, T.; Rosenberg, S.A. Prospective randomized study of the role of N-acetyl cysteine in reversing doxorubicin-induced cardiomyopathy. Am. J. Clin. Oncol. 1982, 5, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Tebbi, C.K.; London, W.B.; Friedman, D.; Villaluna, D.; De Alarcon, P.A.; Constine, L.S.; Mendenhall, N.P.; Sposto, R.; Chauvenet, A.; Schwartz, C.L. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J. Clin. Oncol. 2007, 25, 493–500. [Google Scholar] [CrossRef]
- Barry, E.V.; Vrooman, L.M.; Dahlberg, S.E.; Neuberg, D.S.; Asselin, B.L.; Athale, U.H.; Clavell, L.A.; Larsen, E.C.; Moghrabi, A.; Samson, Y.; et al. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J. Clin. Oncol. 2008, 26, 1106–1111. [Google Scholar] [CrossRef]
- Chow, E.J.; Asselin, B.L.; Schwartz, C.L.; Doody, D.R.; Leisenring, W.M.; Aggarwal, S.; Baker, K.S.; Bhatia, S.; Constine, L.S.; Freyer, D.R.; et al. Late Mortality After Dexrazoxane Treatment: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2015, 33, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Biasillo, G.; Cipolla, C.M.; Cardinale, D. Cardio-oncology: Gaps in Knowledge, Goals, Advances, and Educational Efforts. Curr. Oncol. Rep. 2017, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Azorin-Ortuno, M.; Yanez-Gascon, M.J.; Vallejo, F.; Pallares, F.J.; Larrosa, M.; Lucas, R.; Morales, J.C.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Metabolites and tissue distribution of resveratrol in the pig. Mol. Nutr. Food Res. 2011, 55, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, Y.; Liu, W. Tissue distribution and excretion of resveratrol in rat after oral administration of Polygonum cuspidatum extract (PCE). Phytomedicine 2008, 15, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Andreadi, C.; Britton, R.G.; Horner-Glister, E.; Karmokar, A.; Sale, S.; Brown, V.A.; Brenner, D.E.; Singh, R.; Steward, W.P.; et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci. Transl. Med. 2013, 5, 205ra133. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Calani, L.; Bocchi, L.; Delucchi, F.; Savi, M.; Ray, S.; Brighenti, F.; Stilli, D.; Del Rio, D. Bioaccumulation of resveratrol metabolites in myocardial tissue is dose-time dependent and related to cardiac hemodynamics in diabetic rats. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 408–415. [Google Scholar] [CrossRef]
- Sung, M.M.; Kim, T.T.; Denou, E.; Soltys, C.M.; Hamza, S.M.; Byrne, N.J.; Masson, G.; Park, H.; Wishart, D.S.; Madsen, K.L.; et al. Improved Glucose Homeostasis in Obese Mice Treated With Resveratrol Is Associated With Alterations in the Gut Microbiome. Diabetes 2017, 66, 418–425. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. MBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef]
- Kim, T.T.; Parajuli, N.; Sung, M.M.; Bairwa, S.C.; Levasseur, J.; Soltys, C.M.; Wishart, D.S.; Madsen, K.; Schertzer, J.D.; Dyck, J.R.B. Fecal transplant from resveratrol-fed donors improves glycaemia and cardiovascular features of the metabolic syndrome in mice. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E511–E519. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventos, R.M.; Berenguer, T.; Jakszyn, P.; Martinez, C.; Sanchez, M.J.; Navarro, C.; Chirlaque, M.D.; Tormo, M.J.; et al. Concentrations of resveratrol and derivatives in foods and estimation of dietary intake in a Spanish population: European Prospective Investigation into Cancer and Nutrition (EPIC)-Spain cohort. Br. J. Nutr. 2008, 100, 188–196. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Garland, L.L.; Hsu, C.H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Empl, M.T.; Macke, S.; Winterhalter, P.; Puff, C.; Lapp, S.; Stoica, G.; Baumgartner, W.; Steinberg, P. The growth of the canine glioblastoma cell line D-GBM and the canine histiocytic sarcoma cell line DH82 is inhibited by the resveratrol oligomers hopeaphenol and r2-viniferin. Vet. Comp. Oncol. 2014, 12, 149–159. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).