Suboptimal Nutrition and Low Physical Activity Are Observed Together with Reduced Plasma Brain-Derived Neurotrophic Factor (BDNF) Concentration in Children with Severe Cerebral Palsy (CP)
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethics Approval and Consent to Participate
2.3. Measurements of Height and Body Weight
2.4. Diet and Activity Registration
2.5. Maximal Oxygen Uptake
2.6. Blood Sampling
2.7. Analysis
2.8. Calculations
2.9. Statistics
3. Results
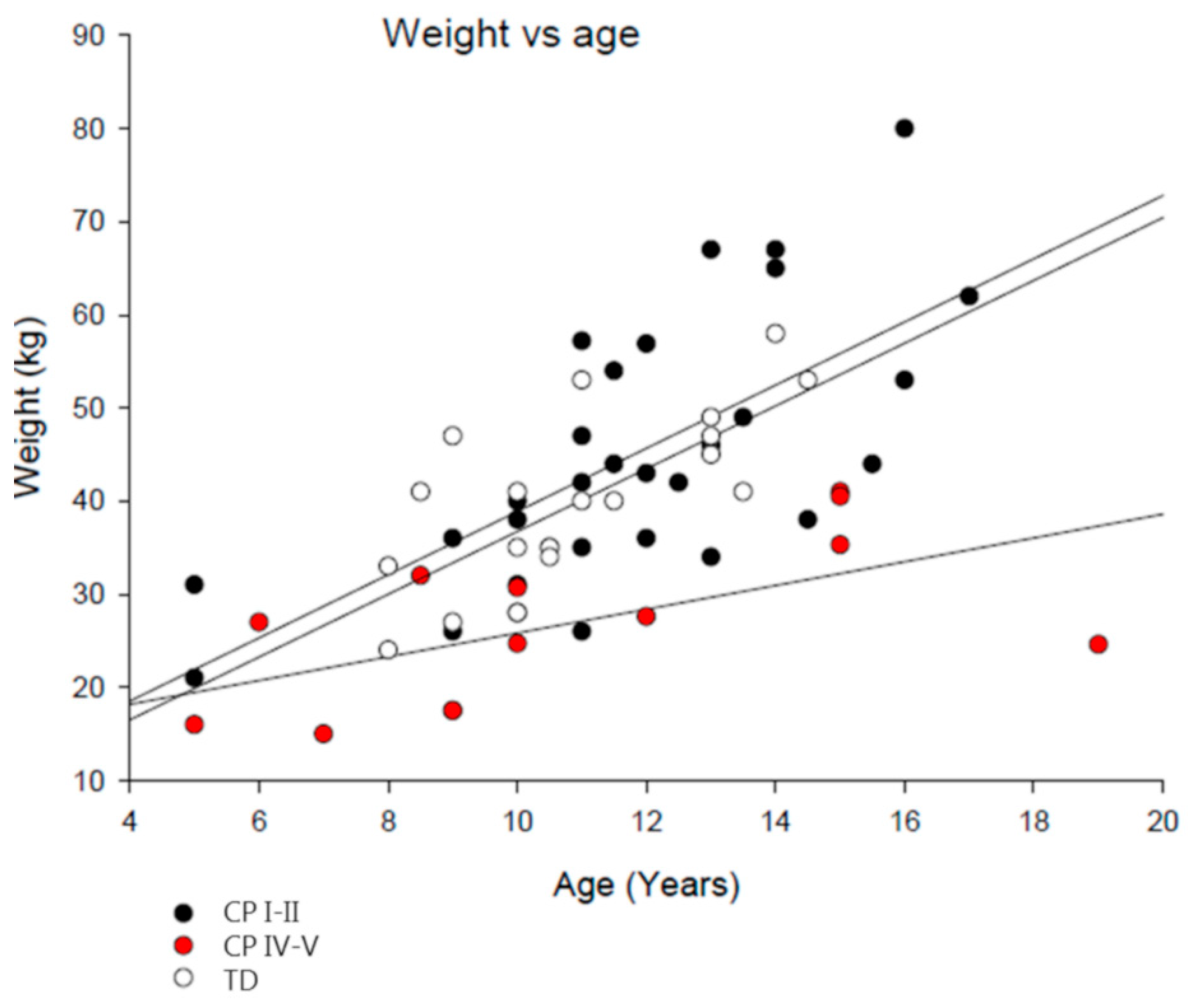
3.1. Subject Characteristics
3.2. Resting Energy Expenditure (REE) and Daily Energy Expenditure (EE)
3.3. Evaluation of Physical Fitness Capacity
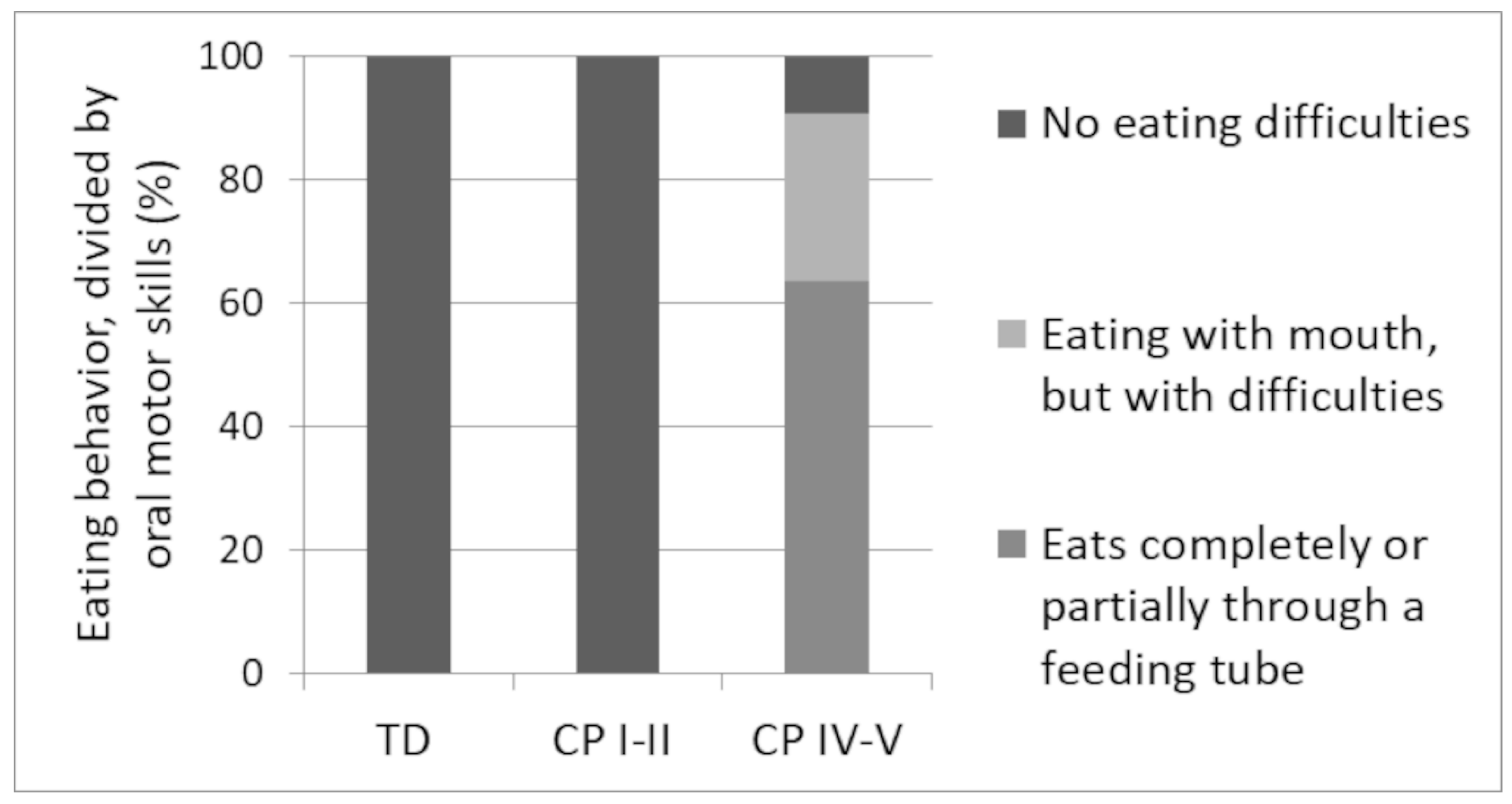
3.4. Eating Patterns
3.5. Daily Intake of Energy and Macronutrients
3.6. Daily Intake of Vitamins and Minerals
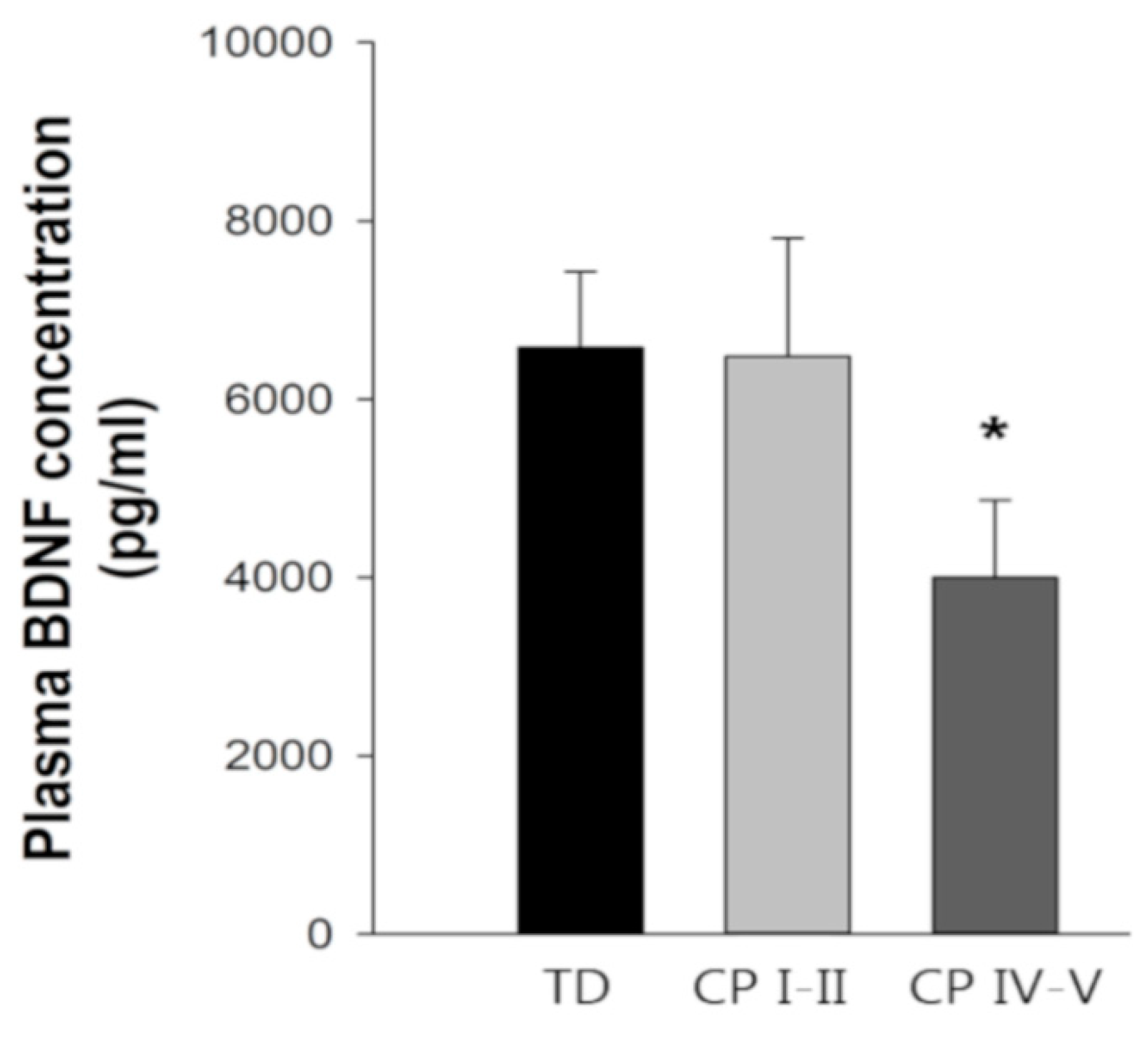
3.7. Fasting Blood Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stein, D.; Weinberger-Litman, S.L.; Latzer, Y. Psychosocial perspectives and the issue of prevention in childhood obesity. Front Public Health 2014, 2, 104. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, A.L.; Ahn, S. The effects of physical activity and physical fitness on children’s achievement and cognitive outcomes: A meta-analysis. Res. Q. Exerc. Sport 2011, 82, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar] [PubMed]
- Martin, J.H.; Chakrabarty, S.; Friel, K.M. Harnessing activity-dependent plasticity to repair the damaged corticospinal tract in an animal model of cerebral palsy. Dev. Med. Child Neurol. 2011, 53 (Suppl. 4), 9–13. [Google Scholar] [CrossRef]
- Martin, J.H.; Friel, K.M.; Salimi, I.; Chakrabarty, S. Activity- and use-dependent plasticity of the developing corticospinal system. Neurosci. Biobehav. Rev. 2007, 31, 1125–1135. [Google Scholar] [CrossRef]
- Stolp, H.; Neuhaus, A.; Sundramoorthi, R.; Molnar, Z. The Long and the Short of it: Gene and Environment Interactions During Early Cortical Development and Consequences for Long-Term Neurological Disease. Front Psychiatry 2012, 3, 50. [Google Scholar] [CrossRef]
- Eyre, J.A. Corticospinal tract development and its plasticity after perinatal injury. Neurosci. Biobehav. Rev. 2007, 31, 1136–1149. [Google Scholar] [CrossRef]
- Van den Berg-Emons, H.J.; Saris, W.H.; de Barbanson, D.C.; Westerterp, K.R.; Huson, A.; van Baak, M.A. Daily physical activity of schoolchildren with spastic diplegia and of healthy control subjects. J. Pediatr. 1995, 127, 578–584. [Google Scholar] [CrossRef]
- Verschuren, O.; Takken, T. Aerobic capacity in children and adolescents with cerebral palsy. Res. Dev. Disabil. 2010, 31, 1352–1357. [Google Scholar] [CrossRef]
- Garcia, C.C.; Alcocer-Gamboa, A.; Ruiz, M.P.; Caballero, I.M.; Faigenbaum, A.D.; Esteve-Lanao, J.; Saiz, B.M.; Lorenzo, T.M.; Lara, S.L. Metabolic, cardiorespiratory, and neuromuscular fitness performance in children with cerebral palsy: A comparison with healthy youth. J. Exerc. Rehabil. 2016, 12, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Hoofwijk, M.; Unnithan, V.B.; Bar-Or, O. Maximal treadmill performance in children with cerebral palsy. Pediatr. Exerc. Sci. 1995, 7, 305–315. [Google Scholar] [CrossRef]
- Sullivan, P.B.; Juszczak, E.; Lambert, B.R.; Rose, M.; Ford-Adams, M.E.; Johnson, A. Impact of feeding problems on nutritional intake and growth: Oxford Feeding Study II. Dev. Med. Child Neurol. 2002, 44, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Muhit, M.; Karim, T.; Smithers-Sheedy, H.; Novak, I.; Jones, C.; Badawi, N.; Khandaker, G. What makes children with cerebral palsy vulnerable to malnutrition? Findings from the Bangladesh cerebral palsy register (BCPR). Disabil. Rehabil. 2018, 1–8. [Google Scholar] [CrossRef]
- Herrera-Anaya, E.; Angarita-Fonseca, A.; Herrera-Galindo, V.M.; Martinez-Marin, R.D.; Rodriguez-Bayona, C.N. Association between gross motor function and nutritional status in children with cerebral palsy: A cross-sectional study from Colombia. Dev. Med. Child Neurol. 2016, 58, 936–941. [Google Scholar] [CrossRef]
- Polack, S.; Adams, M.; O’banion, D.; Baltussen, M.; Asante, S.; Kerac, M.; Gladstone, M.; Zuurmond, M. Children with cerebral palsy in Ghana: Malnutrition, feeding challenges, and caregiver quality of life. Dev. Med. Child Neurol. 2018, 60, 914–921. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Cansev, M.; Ulus, I.H. Synapse formation is enhanced by oral administration of uridine and DHA, the circulating precursors of brain phosphatides. J. Nutr. Health Aging 2009, 13, 189–197. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Cansev, M.; Sakamoto, T.; Ulus, I.H. Use of phosphatide precursors to promote synaptogenesis. Annu. Rev. Nutr. 2009, 29, 59–87. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma 2004, 21, 1457–1467. [Google Scholar] [CrossRef]
- Jiang, L.H.; Shi, Y.; Wang, L.S.; Yang, Z.R. The influence of orally administered docosahexaenoic acid on cognitive ability in aged mice. J. Nutr. Biochem. 2009, 20, 735–741. [Google Scholar] [CrossRef]
- Sakamoto, T.; Cansev, M.; Wurtman, R.J. Oral supplementation with docosahexaenoic acid and uridine-5′-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res. 2007, 1182, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Pagliusi, S.R.; Hohn, A.; Leibrock, J.; Barde, Y.A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990, 9, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Acheson, A.; Conover, J.C.; Fandl, J.P.; DeChiara, T.M.; Russell, M.; Thadani, A.; Squinto, S.P.; Yancopoulos, G.D.; Lindsay, R.M. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 1995, 374, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Suzuki, S.; Kumamaru, E.; Adachi, N.; Richards, M.; Kunugi, H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010, 25, 237–258. [Google Scholar] [PubMed]
- Zhang, Q.; Liu, G.; Wu, Y.; Sha, H.; Zhang, P.; Jia, J. BDNF promotes EGF-induced proliferation and migration of human fetal neural stem/progenitor cells via the PI3K/Akt pathway. Molecules 2011, 16, 10146–10156. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Lhotsky, A.; Wang, Y.; Dal, F.G.; An, Y.; Metter, E.J.; Ferrucci, L.; O’Brien, R.; Zonderman, A.B. Association of adiposity status and changes in early to mid-adulthood with incidence of Alzheimer’s disease. Am. J. Epidemiol. 2008, 168, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Tortorella, A.; Martiadis, V.; Serritella, C.; Fuschino, A.; Maj, M. Opposite changes in the serum brain-derived neurotrophic factor in anorexia nervosa and obesity. Psychosom. Med. 2004, 66, 744–748. [Google Scholar] [CrossRef]
- Sandberg, J.C.; Bjorck, I.M.E.; Nilsson, A.C. Increased Plasma Brain-Derived Neurotrophic Factor 10.5 h after Intake of Whole Grain Rye-Based Products in Healthy Subjects. Nutrients 2018, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Raine, L.B.; Drollette, E.S.; Scudder, M.R.; Kramer, A.F.; Hillman, C.H. Dietary fiber is positively associated with cognitive control among prepubertal children. J. Nutr. 2015, 145, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Castelli, D.M.; Buck, S.M. Aerobic fitness and neurocognitive function in healthy preadolescent children. Med. Sci. Sports Exerc. 2005, 37, 1967–1974. [Google Scholar] [CrossRef]
- Hillman, C.H.; Buck, S.M.; Themanson, J.R.; Pontifex, M.B.; Castelli, D.M. Aerobic fitness and cognitive development: Event-related brain potential and task performance indices of executive control in preadolescent children. Dev. Psychol. 2009, 45, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Coe, D.P.; Peterson, T.; Blair, C.; Schutten, M.C.; Peddie, H. Physical fitness, academic achievement, and socioeconomic status in school-aged youth. J. Sch. Health 2013, 83, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Greene, J.L.; Gibson, C.A.; Smith, B.K.; Washburn, R.A.; Sullivan, D.K.; DuBose, K.; Mayo, M.S.; Schmelzle, K.H.; Ryan, J.J.; et al. Physical Activity Across the Curriculum (PAAC): A randomized controlled trial to promote physical activity and diminish overweight and obesity in elementary school children. Prev. Med. 2009, 49, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Araya, A.V.; Orellana, X.; Godoy, D.; Soto, L.; Fiedler, J. Effect of exercise on circulating levels of brain-derived neurotrophic factor (BDNF) in overweight and obese subjects. Horm. Metab. Res. 2013, 45, 541–544. [Google Scholar] [CrossRef]
- Kuo, F.C.; Lee, C.H.; Hsieh, C.H.; Kuo, P.; Chen, Y.C.; Hung, Y.J. Lifestyle modification and behavior therapy effectively reduce body weight and increase serum level of brain-derived neurotrophic factor in obese non-diabetic patients with schizophrenia. Psychiatry Res. 2013, 209, 150–154. [Google Scholar] [CrossRef]
- Swift, D.L.; Johannsen, N.M.; Myers, V.H.; Earnest, C.P.; Smits, J.A.; Blair, S.N.; Church, T.S. The effect of exercise training modality on serum brain derived neurotrophic factor levels in individuals with type 2 diabetes. PLoS ONE 2012, 7, e42785. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 2008, 155, 751–759. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Exercise facilitates the action of dietary DHA on functional recovery after brain trauma. Neuroscience 2013, 248, 655–663. [Google Scholar] [CrossRef]
- Palisano, R.J.; Copeland, W.P.; Galuppi, B.E. Performance of physical activities by adolescents with cerebral palsy. Phys. Ther. 2007, 87, 77–87. [Google Scholar] [CrossRef]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012, Integrating Nutrition and Physical Activity; Nordic council of Ministers: Copenhagen, Denmark, 2012. [Google Scholar]
- Hansen, S.; Ritterband-Rosenbaum, A.; Voigt, C.; Hellgren, L.; Sørensen, A.; Jacobsen, C.; Greve, L.; Jørgensen, K.; Bilde, P.; Kiens, B.; et al. Supplementation of docosahexaenoc acid (DHA), vitamin D3 and uridine in combination with six weeks of cognitive and motor training in prepubecent children: A pilot study. BMC Nutr. 2017. [Google Scholar] [CrossRef]
- Henry, C.J. Basal metabolic rate studies in humans: Measurement and development of new equations. Public Health Nutr. 2005, 8, 1133–1152. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Karagiozoglou-Lampoudi, T.; Daskalou, E.; Vargiami, E.; Zafeiriou, D. Identification of feeding risk factors for impaired nutrition status in paediatric patients with cerebral palsy. Acta Paediatr. 2012, 101, 649–654. [Google Scholar] [CrossRef]
- Dahlseng, M.O.; Finbraten, A.K.; Juliusson, P.B.; Skranes, J.; Andersen, G.; Vik, T. Feeding problems, growth and nutritional status in children with cerebral palsy. Acta Paediatr. 2012, 101, 92–98. [Google Scholar] [CrossRef]
- Thommessen, M.; Kase, B.F.; Riis, G.; Heiberg, A. The impact of feeding problems on growth and energy intake in children with cerebral palsy. Eur. J. Clin. Nutr. 1991, 45, 479–487. [Google Scholar]
- Stallings, V.A.; Cronk, C.E.; Zemel, B.S.; Charney, E.B. Body composition in children with spastic quadriplegic cerebral palsy. J. Pediatr. 1995, 126, 833–839. [Google Scholar] [CrossRef]
- Krick, J.; van Duyn, M.A. The relationship between oral-motor involvement and growth: A pilot study in a pediatric population with cerebral palsy. J. Am. Diet. Assoc. 1984, 84, 555–559. [Google Scholar]
- Reilly, S.; Skuse, D. Characteristics and management of feeding problems of young children with cerebral palsy. Dev. Med. Child Neurol. 1992, 34, 379–388. [Google Scholar] [CrossRef]
- Cheng, H.L.; Amatoury, M.; Steinbeck, K. Energy expenditure and intake during puberty in healthy nonobese adolescents: A systematic review. Am. J. Clin. Nutr. 2016, 104, 1061–1074. [Google Scholar] [CrossRef]
- Saxena, S.; Kumaran, S.; Rao, B.K. Energy expenditure during standing in children with cerebral palsy: A brief report1. J. Pediatr. Rehabil. Med. 2016, 9, 241–245. [Google Scholar] [CrossRef]
- Verschuren, O.; Peterson, M.D.; Leferink, S.; Darrah, J. Muscle activation and energy-requirements for varying postures in children and adolescents with cerebral palsy. J. Pediatr. 2014, 165, 1011–1016. [Google Scholar] [CrossRef]
- Unnithan, V.B.; Dowling, J.J.; Frost, G.; Bar-Or, O. Role of cocontraction in the O2 cost of walking in children with cerebral palsy. Med. Sci. Sports Exerc. 1996, 28, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Johnston, T.E.; Moore, S.E.; Quinn, L.T.; Smith, B.T. Energy cost of walking in children with cerebral palsy: Relation to the Gross Motor Function Classification System. Dev. Med. Child Neurol. 2004, 46, 34–38. [Google Scholar] [CrossRef]
- Glomset, J.A. Role of docosahexaenoic acid in neuronal plasma membranes. Sci. STKE 2006, 2006, e6. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.J. A nutrient combination that can affect synapse formation. Nutrients 2014, 6, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Rathod, R.S.; Khaire, A.A.; Kale, A.A.; Joshi, S.R. Beneficial effects of omega-3 fatty acids and vitamin B12 supplementation on brain docosahexaenoic acid, brain derived neurotrophic factor, and cognitive performance in the second-generation Wistar rats. Biofactors 2015, 41, 261–272. [Google Scholar] [CrossRef]
- Holguin, S.; Huang, Y.; Liu, J.; Wurtman, R. Chronic administration of DHA and UMP improves the impaired memory of environmentally impoverished rats. Behav. Brain Res. 2008, 191, 11–16. [Google Scholar] [CrossRef]
- Holguin, S.; Martinez, J.; Chow, C.; Wurtman, R. Dietary uridine enhances the improvement in learning and memory produced by administering DHA to gerbils. FASEB J. 2008, 22, 3938–3946. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; Alexander, D.D.; van Elswyk, M.E. Docosahexaenoic acid and adult memory: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0120391. [Google Scholar] [CrossRef]
- Stonehouse, W.; Conlon, C.A.; Podd, J.; Hill, S.R.; Minihane, A.M.; Haskell, C.; Kennedy, D. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.E.; Tye, C.; Kuntsi, J.; Vassos, E.; Asherson, P. Omega-3 polyunsaturated fatty acid supplementation and cognition: A systematic review and meta-analysis. J. Psychopharmacol. 2015, 29, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W. Does consumption of LC omega-3 PUFA enhance cognitive performance in healthy school-aged children and throughout adulthood? Evidence from clinical trials. Nutrients 2014, 6, 2730–2758. [Google Scholar] [CrossRef]
- Ruottinen, S.; Lagstrom, H.K.; Niinikoski, H.; Ronnemaa, T.; Saarinen, M.; Pahkala, K.A.; Hakanen, M.; Viikari, J.S.; Simell, O. Dietary fiber does not displace energy but is associated with decreased serum cholesterol concentrations in healthy children. Am. J. Clin. Nutr. 2010, 91, 651–661. [Google Scholar] [CrossRef]
- Nelson, K.B.; Grether, J.K.; Croen, L.A.; Dambrosia, J.M.; Dickens, B.F.; Jelliffe, L.L.; Hansen, R.L.; Phillips, T.M. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann. Neurol. 2001, 49, 597–606. [Google Scholar] [CrossRef]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Matthews, V.B.; Astrom, M.B.; Chan, M.H.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Akerstrom, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef]
- Chaldakov, G.N.; Tonchev, A.B.; Aloe, L. NGF and BDNF: From nerves to adipose tissue, from neurokines to metabokines. Riv. Psichiatr. 2009, 44, 79–87. [Google Scholar]
- Sung, K.H.; Chung, C.Y.; Lee, K.M.; Cho, B.C.; Moon, S.J.; Kim, J.; Park, M.S. Differences in Body Composition According to Gross Motor Function in Children With Cerebral Palsy. Arch. Phys. Med. Rehabil. 2017, 98, 2295–2300. [Google Scholar] [CrossRef]
- Sullivan, P.B.; Juszczak, E.; Bachlet, A.M.; Lambert, B.; Vernon-Roberts, A.; Grant, H.W.; Eltumi, M.; McLean, L.; Alder, N.; Thomas, A.G. Gastrostomy tube feeding in children with cerebral palsy: A prospective, longitudinal study. Dev. Med. Child Neurol. 2005, 47, 77–85. [Google Scholar] [CrossRef]
- Caselli, T.B.; Lomazi, E.A.; Montenegro, M.A.S.; Bellomo-Brandao, M.A. Comparative study on gastrostomy and orally nutrition of children and adolescents with tetraparesis cerebral palsy. Arquivos Gastroenterol. 2017, 54, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Day, S.M.; Strauss, D.J.; Vachon, P.J.; Rosenbloom, L.; Shavelle, R.M.; Wu, Y.W. Growth patterns in a population of children and adolescents with cerebral palsy. Dev. Med. Child Neurol. 2007, 49, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.B.; Vitolo, M.R.; Aguirre, A.N.; Fagundes-Neto, U. Measurement of low dietary fiber intake as a risk factor for chronic constipation in children. J. Pediatr. Gastroenterol. Nutr. 1999, 29, 132–135. [Google Scholar] [CrossRef]
- Vande, V.S.; Van, R.K.; Van, W.M.; De, B.R.; Van, B.S. Constipation and fecal incontinence in children with cerebral palsy. Overview of literature and flowchart for a stepwise approach. Acta Gastroenterol. Belg. 2018, 81, 415–418. [Google Scholar]
- Andrew, M.J.; Parr, J.R.; Sullivan, P.B. Feeding difficulties in children with cerebral palsy. Arch. Dis. Child Educ. Pract. Ed. 2012, 97, 222–229. [Google Scholar] [CrossRef]
- Fardy, P.S.; White, R.E.; Haltiwanger-Schmitz, K.; Magel, J.R.; McDermott, K.J.; Clark, L.T.; Hurster, M.M. Coronary disease risk factor reduction and behavior modification in minority adolescents: The PATH program. J. Adolesc. Health 1996, 18, 247–253. [Google Scholar] [CrossRef]
- Eliakim, A.; Barstow, T.J.; Brasel, J.A.; Ajie, H.; Lee, W.N.; Renslo, R.; Berman, N.; Cooper, D.M. Effect of exercise training on energy expenditure, muscle volume, and maximal oxygen uptake in female adolescents. J. Pediatr. 1996, 129, 537–543. [Google Scholar] [CrossRef]
- Trevino, R.P.; Yin, Z.; Hernandez, A.; Hale, D.E.; Garcia, O.A.; Mobley, C. Impact of the Bienestar school-based diabetes mellitus prevention program on fasting capillary glucose levels: A randomized controlled trial. Arch. Pediatr. Adolesc. Med. 2004, 158, 911–917. [Google Scholar] [CrossRef]
- Sharma, V.K.; Subramanian, S.K.; Radhakrishnan, K.; Rajendran, R.; Ravindran, B.S.; Arunachalam, V. Comparison of structured and unstructured physical activity training on predicted VO2max and heart rate variability in adolescents—A randomized control trial. J. Basic Clin. Physiol. Pharm. 2017, 28, 225–238. [Google Scholar] [CrossRef]
- Satish, V.; Rao, R.M.; Manjunath, N.K.; Amritanshu, R.; Vivek, U.; Shreeganesh, H.R.; Deepashree, S. Yoga versus physical exercise for cardio-respiratory fitness in adolescent school children: A randomized controlled trial. Int. J. Adolesc. Med. Health 2018. [Google Scholar] [CrossRef]
- Walther, C.; Gaede, L.; Adams, V.; Gelbrich, G.; Leichtle, A.; Erbs, S.; Sonnabend, M.; Fikenzer, K.; Korner, A.; Kiess, W.; et al. Effect of increased exercise in school children on physical fitness and endothelial progenitor cells: A prospective randomized trial. Circulation 2009, 120, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- McManus, A.M.; Masters, R.S.; Laukkanen, R.M.; Yu, C.C.; Sit, C.H.; Ling, F.C. Using heart-rate feedback to increase physical activity in children. Prev. Med. 2008, 47, 402–408. [Google Scholar] [CrossRef] [PubMed]
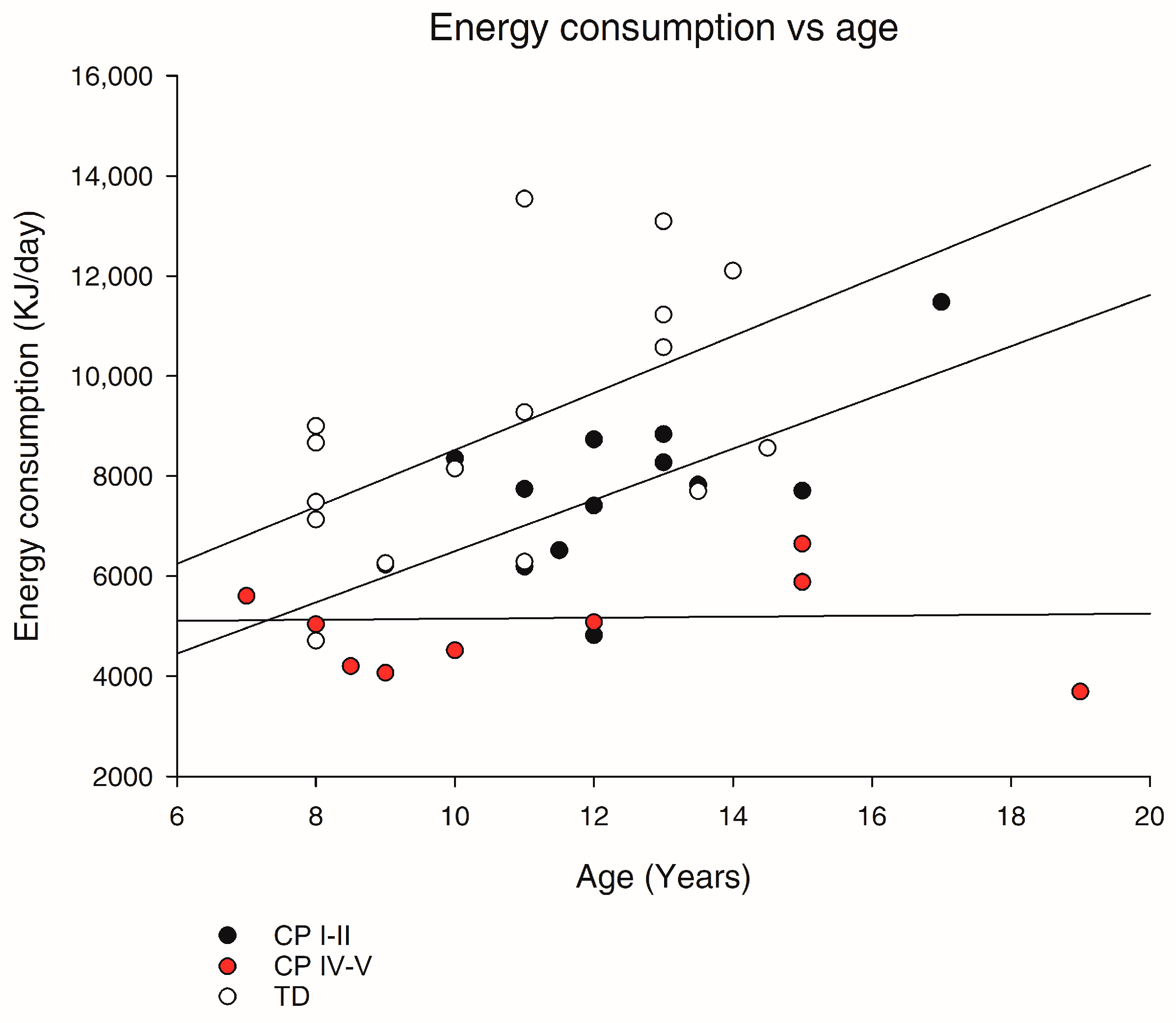
| TD | CP I–II | CP IV–V | |
|---|---|---|---|
| Number of children | 22 | 31 | 14 |
| Boys/girls | 13/9 | 19/12 | 7/7 |
| Age (years) | 10.9 ± 0.6 | 10.6 ± 0.6 | 10.9 ± 1.0 |
| Height (cm) | 147 ± 3 | 152 ± 3 | 131 ± 5 *,### |
| Age–Height (z-score) | 0.1 ± 0.2 | 0.0 ± 0.2 | −2.4 ± 0.7 ***,## |
| Body weight (kg) | 40 ± 2 | 45 ± 3 | 27 ± 2 **,### |
| Age–Body weight (z-score) | 0.2 ± 0.2 | 0.2 ± 0.3 | −4.0 ± 1.2 **,### |
| BMI (kg/m2) | 18 ± 1 | 19 ± 0.6 | 16 ± 1 (*),## |
| Age–BMI (z-score) | 0.1 ± 0.3 | 0.3 ± 0.2 | −1.2 ± 0.5 *,## |
| TD | CP I–II | |
|---|---|---|
| Sport-related activities (hours per week) | 2.5 ± 0.8 | 2.3 ± 0.5 |
| Heart rate max | 197 ± 3 | 197 ± 3 |
| RER | 1.2 ± 0.02 | 1.1 ± 0.02 |
| VO2 max (L/min) | 2.0 ± 0.2 | 2.2 ± 0.1 |
| VO2 max (mL/min/kg) | 52.5 ± 2.5 | 51.4 ± 1.5 |
| TD | CP I–II | CP IV–V | RI a | |
|---|---|---|---|---|
| Energy Intake (KJ) | 8983 ± 633 | 8144 ± 540 | 5165 ± 291 ***,## | Girls: 8600 Boys: 9300 |
| Macronutrients | ||||
| Protein (E%) | 16.7 ± 0.6 | 15.4 ± 0.6 | 15.3 ± 1.4 | 10–20 |
| Protein (g/kg BW) | 2.22 ± 0.14 | 1.81 ± 0.14 | 1.77 ± 0.53 | 0.75 |
| Carbohydrates (E%) | 52.5 ± 1.4 | 50.3 ± 0.9 | 46.9 ± 1.9 * | 45–60 |
| Dietary fibers (E%) | 2.6 ± 0.2 | 2.4 ± 0.1 | 1.5 ± 0.5 * | |
| Dietary fibers (g) | 28.5 ± 1.8 | 23.9 ± 2.0 | 9.6 ± 2.9 ***,# | >10 |
| Dietary fibers (g/MJ) | 3.3 ± 0.2 | 2.9 ± 0.1 | 2.0 ± 0.4 * | 2–3 |
| Fat (E%) | 28.3 ± 1.4 | 32.0 ± 1.0 | 36.2 ± 10.9 * | 25–40 |
| Saturated fatty acid (E%) | 10.1 ± 0.7 | 10.5 ± 2.5 | 11.1 ± 3.4 | <10 |
| Monounsaturated fatty acid (E%) | 8.0 ± 0.5 | 8.9 ± 0.5 | 12.0 ± 3.8 | 10→20 |
| Polyunsaturated fatty acid (E%) | 3.7 ± 0.2 | 4.6 ± 0.4 | 5.1 ± 1.6 | 5→10 |
| n-3 fatty acids (g) | 1.5 ± 0.2 | 1.6 ± 0.2 | 0.6 ± 0.1 *** | |
| Cholesterol (mg) | 198 ± 22 | 213 ± 22 | 103 ± 26 *,# | <300 |
| Added sugar (E%) | 8.9 ± 1.3 | 8.8 ± 1.8 | 4.9 ± 3.9 ***,## | <10 |
| TD (n = 16) | CP I–II (n = 13) | CP IV–V (n = 11) | RI a | ||||
|---|---|---|---|---|---|---|---|
| Dietary Intake | Incl. Dietary Supplements | Dietary Intake | Incl. Dietary Supplements | Dietary Intake | Incl. Dietary Supplements | ||
| Vitamins | |||||||
| Vitamin A (RE (µg)) | 945 ± 124 | 1045 ± 130 | 737 ± 146 | 890 ± 167 | 865 ± 214 | 1083 ± 205 | 600 |
| Vitamin D (µg) | 3.3 ± 0.9 | 6.4 ± 1.3 | 3.0 ± 1.0 | 11.3 ± 4.4 | 8.7 ± 1.7 **,## | 20.0 ± 5.6 | 10 |
| Vitamin E (aTE (mg)) | 6.5 ± 0.6 | 7.6 ± 1.1 | 8.0 ± 1.1 | 10.7 ± 2.0 | 8.8 ± 1.7 | 11.4 ± 1.6 | 7 |
| Vitamin B1 (thiamin) (mg) | 1.4 ± 0.1 | 1.6 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.2 | 1.2 ± 0.2 | 1.5 ± 0.2 | 1.2 |
| Vitamin B2 (riboflavin) (mg) | 1.5 ± 0.1 | 1.7 ± 0.1 | 1.4 ± 0.2 | 1.8 ± 0.3 | 1.2 ± 0.2 | 1.6 ± 0.2 | 1.4 |
| Vitamin B3 (niacin) (mg) | 15.0 ± 1.8 | 17.9 ± 1.8 | 13.0 ± 2.3 | 17.5 ± 2.4 | 12.1 ± 1.8 | 16.6 ± 2.0 | Girls: 14, Boys: 16 |
| Vitamin B6 (mg) | 1.6 ± 0.2 | 1.9 ± 0.2 | 1.4 ± 0.1 | 1.8 ± 0.2 | 1.4 ± 0.2 | 1.8 ± 0.2 | 1.3 |
| Vitamin B12 (µg) | 4.4 ± 0.4 | 9.2 ± 1.5 | 4.5 ± 0.6 | 5.2 ± 0.7 | 2.5 ± 0.4 **,## | 3.1 ± 0.8 | 2 |
| Vitamin C (mg) | 120 ± 17 | 137 ± 15 | 115 ± 8 | 143 ± 18 | 94 ± 12 | 116 ± 13 | 50 |
| Ascorbic acid (mg) | 70 ± 3 | 70 ± 9 | 27 ± 8 *,## | ||||
| β-carotene (µg) | 7241 ± 1391 | 3707 ± 790 | 1879 ± 698 ** | ||||
| Minerals | |||||||
| Calcium (mg) | 960 ± 88 | 979 ± 82 | 794 ± 117 | 842 ± 117 | 751 ± 102 | 987 ± 113 | 900 |
| Phosphorus (mg) | 1359 ± 86 | 1359 ± 85 | 1163 ± 116 | 1174 ± 114 | 675 ± 60 ***,## | 675 ± 59 | 700 |
| Potassium (mg) | 2827 ± 224 | 2503 ± 212 | 1294 ± 163 ***,# | Girls: 2900, Boys: 3300 | |||
| Magnesium (mg) | 328 ± 24 | 335 ± 24 | 272 ± 25 | 295 ± 33 | 187 ± 17 | 190 ± 16 | 280 |
| Iron (mg) | 9.5 ± 0.7 | 11.2 ± 0.9 | 7.9 ± 0.5 | 11.9 ± 1.9 | 10.1 ± 1.7 | 14.1 ± 1.7 | 11 |
| Zinc (mg) | 10.7 ± 0.9 | 11.3 ± 0.8 | 8.6 ± 0.8 | 10.7 ± 1.2 | 8.6 ± 1.4 | 11.3 ± 1.3 | 11 |
| Copper (mg) | 2.7 ± 0.4 | 2.8 ± 0.4 | 2.1 ± 0.3 | 2.3 ± 0.4 | 2.8 ± 0.5 | 3.0 ± 0.5 | 0.7 |
| Iodine (µg) | 137 ± 13 | 155 ± 12 | 138 ± 22 | 178 ± 29 | 108 ± 14 | 148 ± 21 | 150 |
| Selenium (µg) | 46 ± 5 | 51 ± 5 | 38 ± 4 | 52 ± 7 | 40 ± 7 | 54 ± 7 | 40 |
| TD | CP I–II | CP IV–V | |
|---|---|---|---|
| Plasma glucose (mmol/L) | 4.9 ± 0.1 | 5.0 ± 0.2 | 5.0 ± 0.1 |
| Plasma insulin (µlU/mL) | 5.8 ± 0.8 | 7.5 ± 1.0 | 9.0 ± 3.0 |
| HOMA-IR | 1.3 ± 0.2 | 1.9 ± 0.1 | 2.01 ± 0.7 |
| Plasma hematocrit (%) | 43.8 ± 1.0 | 43.3 ± 1.2 | 42.2 ± 1.4 |
| Plasma hemoglobin (mmol/L) | 8.9 ± 0.2 | 8.8 ± 0.3 | 8.5 ± 0.3 |
| Serum sodium (mmol/L) | 133 ± 1.0 | 130 ± 1 | 125 ± 2 * |
| Serum potassium (mmol/L) | 3.88 ± 0.12 | 4.05 ± 0.08 | 4.34 ± 0.26 |
| Plasma total Cholesterol (mmol/L) | 3.90 ± 0.18 | 3.64 ± 0.18 | 3.57 ± 0.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, S.L.; Lorentzen, J.; Pedersen, L.T.; Hendrich, F.L.; Jorsal, M.; Pingel, J.; Nielsen, J.B.; Kiens, B. Suboptimal Nutrition and Low Physical Activity Are Observed Together with Reduced Plasma Brain-Derived Neurotrophic Factor (BDNF) Concentration in Children with Severe Cerebral Palsy (CP). Nutrients 2019, 11, 620. https://doi.org/10.3390/nu11030620
Hansen SL, Lorentzen J, Pedersen LT, Hendrich FL, Jorsal M, Pingel J, Nielsen JB, Kiens B. Suboptimal Nutrition and Low Physical Activity Are Observed Together with Reduced Plasma Brain-Derived Neurotrophic Factor (BDNF) Concentration in Children with Severe Cerebral Palsy (CP). Nutrients. 2019; 11(3):620. https://doi.org/10.3390/nu11030620
Chicago/Turabian StyleHansen, Solvejg L., Jakob Lorentzen, Lin T. Pedersen, Frederikke L. Hendrich, Martin Jorsal, Jessica Pingel, Jens B. Nielsen, and Bente Kiens. 2019. "Suboptimal Nutrition and Low Physical Activity Are Observed Together with Reduced Plasma Brain-Derived Neurotrophic Factor (BDNF) Concentration in Children with Severe Cerebral Palsy (CP)" Nutrients 11, no. 3: 620. https://doi.org/10.3390/nu11030620
APA StyleHansen, S. L., Lorentzen, J., Pedersen, L. T., Hendrich, F. L., Jorsal, M., Pingel, J., Nielsen, J. B., & Kiens, B. (2019). Suboptimal Nutrition and Low Physical Activity Are Observed Together with Reduced Plasma Brain-Derived Neurotrophic Factor (BDNF) Concentration in Children with Severe Cerebral Palsy (CP). Nutrients, 11(3), 620. https://doi.org/10.3390/nu11030620




