A Scientific Perspective of Personalised Gene-Based Dietary Recommendations for Weight Management
Abstract
1. Body Weight Regulation
2. Dietary Intervention and Weight Loss
3. Individual Metabolic Response to Dietary Intervention
4. Purpose of This Work
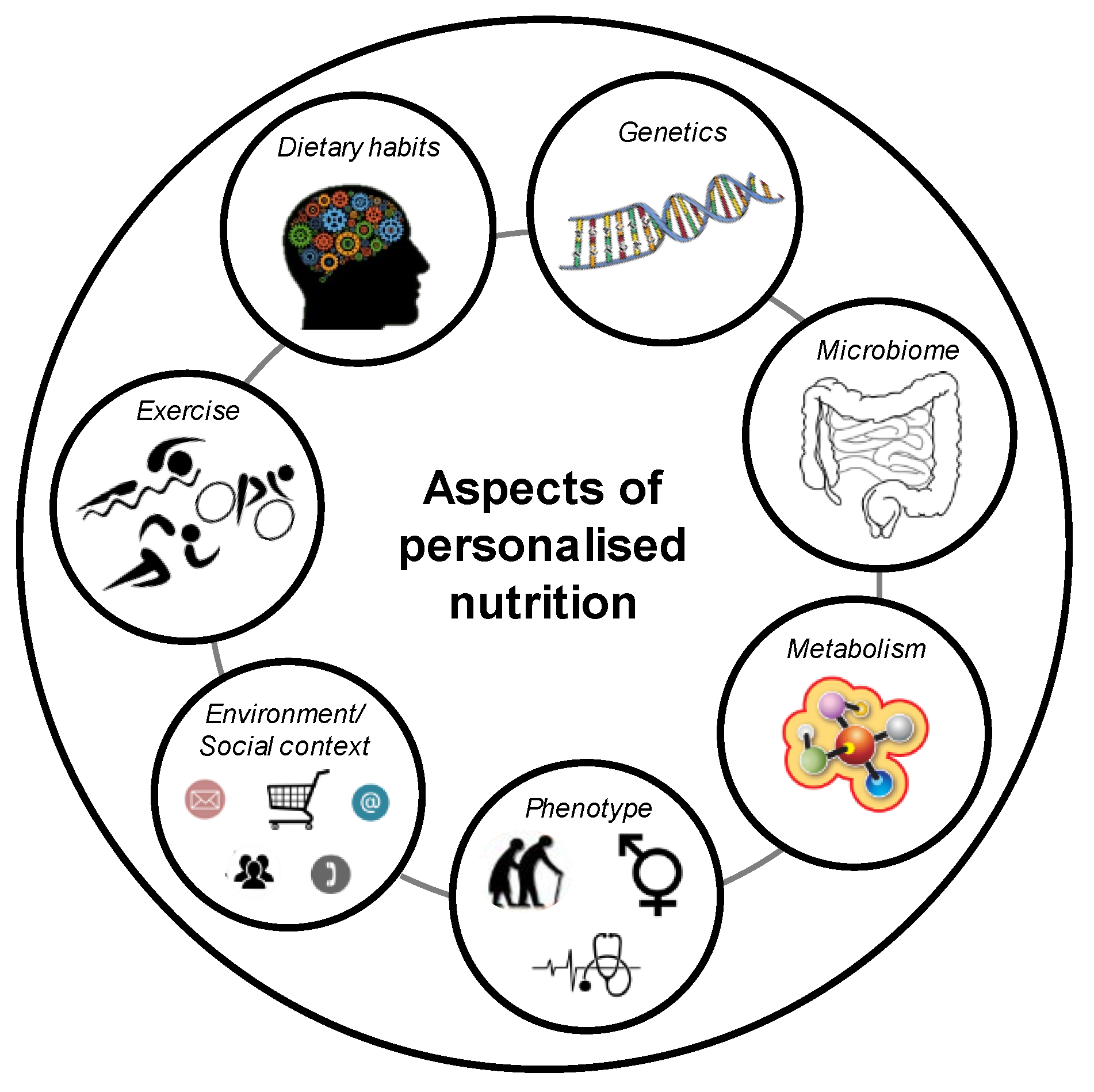
5. Definition of a Gene-Based Personalised Diet
6. Genetics and Obesity
7. Genetics and Weight Loss
8. Genetics and Dietary Intake
9. Direct-to-Consumer Tests
10. Current Opinions for Gene-Based Diets
11. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lam, Y.Y.; Ravussin, E. Analysis of energy metabolism in humans: A review of methodologies. Mol. Metab. 2016, 5, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Crowley, V.E. Overview of human obesity and central mechanisms regulating energy homeostasis. Ann Clin. Biochem. 2008, 45, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Montague, C.T.; Farooqi, I.S.; Whitehead, J.P.; Soos, M.A.; Rau, H.; Wareham, N.J.; Sewter, C.P.; Digby, J.E.; Mohammed, S.N.; Hurst, J.A.; et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997, 387, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, P.; Nogueiras, R.; Broglio, F.; D’Alessio, D.; Tschop, M.H. Ghrelin, obesity and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; D’Alessio, D.A. Central control of body weight and appetite. J. Clin. Endocrinol. Metab. 2008, 93, S37–S50. [Google Scholar] [CrossRef] [PubMed]
- Raybould, H.E. Mechanisms of CCK signaling from gut to brain. Curr. Opin. Pharmacol. 2007, 7, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.C.; Kanters, S.; Bandayrel, K.; Wu, P.; Naji, F.; Siemieniuk, R.A.; Ball, G.D.; Busse, J.W.; Thorlund, K.; Guyatt, G.; et al. Comparison of weight loss among named diet programs in overweight and obese adults: A meta-analysis. JAMA 2014, 312, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-Fat Diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association with Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Kastorini, C.M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and weight loss: Meta-analysis of randomized controlled trials. Metab. Syndr. Relat. Disord. 2011, 9, 1–12. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wong, J.M.; Kendall, C.W.; Esfahani, A.; Ng, V.W.; Leong, T.C.; Faulkner, D.A.; Vidgen, E.; Greaves, K.A.; Paul, G.; et al. The effect of a plant-based low-carbohydrate (“Eco-Atkins”) diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch. Intern. Med. 2009, 169, 1046–1054. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wong, J.M.; Kendall, C.W.; Esfahani, A.; Ng, V.W.; Leong, T.C.; Faulkner, D.A.; Vidgen, E.; Paul, G.; Mukherjea, R.; et al. Effect of a 6-month vegan low-carbohydrate (‘Eco-Atkins’) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: A randomised controlled trial. BMJ Open 2014, 4, e003505. [Google Scholar] [CrossRef]
- Harris, W.S.; Mozaffarian, D.; Rimm, E.; Kris-Etherton, P.; Rudel, L.L.; Appel, L.J.; Engler, M.B.; Sacks, F. Omega-6 fatty acids and risk for cardiovascular disease: A science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009, 119, 902–907. [Google Scholar] [CrossRef]
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Reduces Lipids in Healthy Caucasian Subjects, Independent of Recommended Macronutrient Replacement and Time Point of Consumption: A Prospective, Randomized, Controlled Trial. Nutrients 2017, 9, 097. [Google Scholar] [CrossRef]
- Holzmann, S.L.; Pröll, K.; Hauner, H.; Holzapfel, C. Nutrition apps: Quality and limitations. An explorative investigation on the basis of selected apps. Ernaehrungs Umsch. 2017, 64, 80–89. [Google Scholar] [CrossRef]
- Krug, S.; Kastenmuller, G.; Stuckler, F.; Rist, M.J.; Skurk, T.; Sailer, M.; Raffler, J.; Romisch-Margl, W.; Adamski, J.; Prehn, C.; et al. The dynamic range of the human metabolome revealed by challenges. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 2607–2619. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Korem, T.; Zeevi, D.; Zmora, N.; Weissbrod, O.; Bar, N.; Lotan-Pompan, M.; Avnit-Sagi, T.; Kosower, N.; Malka, G.; Rein, M.; et al. Bread Affects Clinical Parameters and Induces Gut Microbiome-Associated Personal Glycemic Responses. Cell Metab. 2017, 25, 1243.e1245–1253.e1245. [Google Scholar] [CrossRef]
- Wopereis, S.; Stroeve, J.H.M.; Stafleu, A.; Bakker, G.C.M.; Burggraaf, J.; van Erk, M.J.; Pellis, L.; Boessen, R.; Kardinaal, A.A.F.; van Ommen, B. Multi-parameter comparison of a standardized mixed meal tolerance test in healthy and type 2 diabetic subjects: The PhenFlex challenge. Genes Nutr. 2017, 12, 21. [Google Scholar] [CrossRef]
- De Toro-Martin, J.; Arsenault, B.J.; Despres, J.P.; Vohl, M.C. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Nizel, A.E. Personalized nutrition counseling. ASDC J. Dent. Child. 1972, 39, 353–360. [Google Scholar] [PubMed]
- Brug, J.; Campbell, M.; van Assema, P. The application and impact of computer-generated personalized nutrition education: A review of the literature. Patient Educ. Couns. 1999, 36, 145–156. [Google Scholar] [CrossRef]
- Stewart-Knox, B.; Kuznesof, S.; Robinson, J.; Rankin, A.; Orr, K.; Duffy, M.; Poinhos, R.; de Almeida, M.D.; Macready, A.; Gallagher, C.; et al. Factors influencing European consumer uptake of personalised nutrition. Results of a qualitative analysis. Appetite 2013, 66, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Hu, F.B. Precision nutrition for prevention and management of type 2 diabetes. Lancet. Diabetes Endocrinol. 2018, 6, 416–426. [Google Scholar] [CrossRef]
- Van Ommen, B.; van den Broek, T.; de Hoogh, I.; van Erk, M.; van Someren, E.; Rouhani-Rankouhi, T.; Anthony, J.C.; Hogenelst, K.; Pasman, W.; Boorsma, A.; et al. Systems biology of personalized nutrition. Nutr. Rev. 2017, 75, 579–599. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.; Klein, U. Personalisierte Ernährung. J. Für Ernährungsmedizin 2016, 13, 6–9. [Google Scholar]
- Nielsen, D.E.; El-Sohemy, A. Disclosure of genetic information and change in dietary intake: A randomized controlled trial. PLoS ONE 2014, 9, e112665. [Google Scholar] [CrossRef]
- Poinhos, R.; van der Lans, I.A.; Rankin, A.; Fischer, A.R.; Bunting, B.; Kuznesof, S.; Stewart-Knox, B.; Frewer, L.J. Psychological determinants of consumer acceptance of personalised nutrition in 9 European countries. PLoS ONE 2014, 9, e110614. [Google Scholar] [CrossRef]
- Janz, N.K.; Becker, M.H. The Health Belief Model: A decade later. Health Educ. Q. 1984, 11, 1–47. [Google Scholar] [CrossRef]
- Anderson, A.S. How to implement dietary changes to prevent the development of metabolic syndrome. Br. J. Nutr. 2000, 83 (Suppl. 1), S165–S168. [Google Scholar] [CrossRef]
- Bouchard, C.; Tremblay, A. Genetic influences on the response of body fat and fat distribution to positive and negative energy balances in human identical twins. J. Nutr. 1997, 127, 943s–947s. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Sorensen, T.I.; Hanis, C.; Teasdale, T.W.; Chakraborty, R.; Schull, W.J.; Schulsinger, F. An adoption study of human obesity. N. Engl. J. Med. 1986, 314, 193–198. [Google Scholar] [CrossRef]
- Levy, E.; Menard, D.; Delvin, E.; Stan, S.; Mitchell, G.; Lambert, M.; Ziv, E.; Feoli-Fonseca, J.C.; Seidman, E. The polymorphism at codon 54 of the FABP2 gene increases fat absorption in human intestinal explants. J. Biol. Chem. 2001, 276, 39679–39684. [Google Scholar] [CrossRef]
- Hegele, R.A.; Harris, S.B.; Hanley, A.J.; Sadikian, S.; Connelly, P.W.; Zinman, B. Genetic variation of intestinal fatty acid-binding protein associated with variation in body mass in aboriginal Canadians. J. Clin. Endocrinol. Metab. 1996, 81, 4334–4337. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Graves, R.A.; Budavari, A.I.; Spiegelman, B.M. mPPAR gamma 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994, 8, 1224–1234. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Deeb, S.S.; Fajas, L.; Nemoto, M.; Pihlajamaki, J.; Mykkanen, L.; Kuusisto, J.; Laakso, M.; Fujimoto, W.; Auwerx, J. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat. Genet. 1998, 20, 284–287. [Google Scholar] [CrossRef]
- Hagg, S.; Ganna, A.; Van Der Laan, S.W.; Esko, T.; Pers, T.H.; Locke, A.E.; Berndt, S.I.; Justice, A.E.; Kahali, B.; Siemelink, M.A.; et al. Gene-based meta-analysis of genome-wide association studies implicates new loci involved in obesity. Hum. Mol. Genet. 2015, 24, 6849–6860. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Thorleifsson, G.; Walters, G.B.; Gudbjartsson, D.F.; Steinthorsdottir, V.; Sulem, P.; Helgadottir, A.; Styrkarsdottir, U.; Gretarsdottir, S.; Thorlacius, S.; Jonsdottir, I.; et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 2009, 41, 18–24. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Willer, C.J.; Berndt, S.I.; Monda, K.L.; Thorleifsson, G.; Jackson, A.U.; Lango Allen, H.; Lindgren, C.M.; Luan, J.; Magi, R.; et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010, 42, 937–948. [Google Scholar] [CrossRef]
- Loos, R.J. The genetics of adiposity. Curr. Opin. Genet. Dev. 2018, 50, 86–95. [Google Scholar] [CrossRef]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef]
- Dina, C.; Meyre, D.; Gallina, S.; Durand, E.; Korner, A.; Jacobson, P.; Carlsson, L.M.; Kiess, W.; Vatin, V.; Lecoeur, C.; et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 2007, 39, 724–726. [Google Scholar] [CrossRef]
- Scuteri, A.; Sanna, S.; Chen, W.M.; Uda, M.; Albai, G.; Strait, J.; Najjar, S.; Nagaraja, R.; Orru, M.; Usala, G.; et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007, 3, e115. [Google Scholar] [CrossRef] [PubMed]
- Claussnitzer, M.; Dankel, S.N.; Kim, K.H.; Quon, G.; Meuleman, W.; Haugen, C.; Glunk, V.; Sousa, I.S.; Beaudry, J.L.; Puviindran, V.; et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N. Engl. J. Med. 2015, 373, 895–907. [Google Scholar] [CrossRef]
- Larder, R.; Sim, M.F.M.; Gulati, P.; Antrobus, R.; Tung, Y.C.L.; Rimmington, D.; Ayuso, E.; Polex-Wolf, J.; Lam, B.Y.H.; Dias, C.; et al. Obesity-associated gene TMEM18 has a role in the central control of appetite and body weight regulation. Proc. Natl. Acad. Sci. USA 2017, 114, 9421–9426. [Google Scholar] [CrossRef]
- Wiemerslage, L.; Gohel, P.A.; Maestri, G.; Hilmarsson, T.G.; Mickael, M.; Fredriksson, R.; Williams, M.J.; Schioth, H.B. The Drosophila ortholog of TMEM18 regulates insulin and glucagon-like signaling. J. Endocrinol. 2016, 229, 233–243. [Google Scholar] [CrossRef]
- Cone, R.D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005, 8, 571–578. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Keogh, J.M.; Yeo, G.S.; Lank, E.J.; Cheetham, T.; O’Rahilly, S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 2003, 348, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wu, H.; Pan, A.; Patel, B.; Xiang, G.; Qi, L.; Kaplan, R.C.; Hu, F.; Wylie-Rosett, J.; Qi, Q. FTO genotype and weight loss in diet and lifestyle interventions: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 1162–1170. [Google Scholar] [CrossRef]
- Livingstone, K.M.; Celis-Morales, C.; Papandonatos, G.D.; Erar, B.; Florez, J.C.; Jablonski, K.A.; Razquin, C.; Marti, A.; Heianza, Y.; Huang, T.; et al. FTO genotype and weight loss: Systematic review and meta-analysis of 9563 individual participant data from eight randomised controlled trials. BMJ 2016, 354, i4707. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, T.I.; Boutin, P.; Taylor, M.A.; Larsen, L.H.; Verdich, C.; Petersen, L.; Holst, C.; Echwald, S.M.; Dina, C.; Toubro, S.; et al. Genetic polymorphisms and weight loss in obesity: A randomised trial of hypo-energetic high- versus low-fat diets. PLoS Clin. Trials 2006, 1, e12. [Google Scholar] [CrossRef]
- Papandonatos, G.D.; Pan, Q.; Pajewski, N.M.; Delahanty, L.M.; Peter, I.; Erar, B.; Ahmad, S.; Harden, M.; Chen, L.; Fontanillas, P.; et al. Genetic Predisposition to Weight Loss and Regain with Lifestyle Intervention: Analyses From the Diabetes Prevention Program and the Look AHEAD Randomized Controlled Trials. Diabetes 2015, 64, 4312–4321. [Google Scholar] [CrossRef]
- Livingstone, K.M.; Celis-Morales, C.; Lara, J.; Ashor, A.W.; Lovegrove, J.A.; Martinez, J.A.; Saris, W.H.; Gibney, M.; Manios, Y.; Traczyk, I.; et al. Associations between FTO genotype and total energy and macronutrient intake in adults: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Drabsch, T.; Gatzemeier, J.; Pfadenhauer, L.; Hauner, H.; Holzapfel, C. Associations between Single Nucleotide Polymorphisms and Total Energy, Carbohydrate, and Fat Intakes: A Systematic Review. Adv. Nutr. 2018, 9, 425–453. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Penney, K.L.; Giovannucci, E.; Kraft, P.; Wilson, K.M. A genome-wide association study of energy intake and expenditure. PLoS ONE 2018, 13, e0201555. [Google Scholar] [CrossRef]
- Merino, J.; Dashti, H.S.; Li, S.X.; Sarnowski, C.; Justice, A.E.; Graff, M.; Papoutsakis, C.; Smith, C.E.; Dedoussis, G.V.; Lemaitre, R.N.; et al. Genome-wide meta-analysis of macronutrient intake of 91,114 European ancestry participants from the cohorts for heart and aging research in genomic epidemiology consortium. Mol. Psychiatry 2018. [Google Scholar] [CrossRef]
- Larsen, L.H.; Angquist, L.; Vimaleswaran, K.S.; Hager, J.; Viguerie, N.; Loos, R.J.; Handjieva-Darlenska, T.; Jebb, S.A.; Kunesova, M.; Larsen, T.M.; et al. Analyses of single nucleotide polymorphisms in selected nutrient-sensitive genes in weight-regain prevention: The DIOGENES study. Am. J. Clin. Nutr. 2012, 95, 1254–1260. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, Q.; Zhang, C.; Smith, S.R.; Hu, F.B.; Sacks, F.M.; Bray, G.A.; Qi, L. FTO genotype and 2-year change in body composition and fat distribution in response to weight-loss diets: The POUNDS LOST Trial. Diabetes 2012, 61, 3005–3011. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Bray, G.A.; Smith, S.R.; Hu, F.B.; Sacks, F.M.; Qi, L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: The Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2011, 124, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.; Livingstone, K.M.; Marsaux, C.F.; Forster, H.; O’Donovan, C.B.; Woolhead, C.; Macready, A.L.; Fallaize, R.; Navas-Carretero, S.; San-Cristobal, R.; et al. Design and baseline characteristics of the Food4Me study: A web-based randomised controlled trial of personalised nutrition in seven European countries. Genes Nutr. 2015, 10, 450. [Google Scholar] [CrossRef]
- Saukko, P. State of play in direct-to-consumer genetic testing for lifestyle-related diseases: Market, marketing content, user experiences and regulation. Proc. Nutr. Soc. 2013, 72, 53–60. [Google Scholar] [CrossRef]
- Frankwich, K.A.; Egnatios, J.; Kenyon, M.L.; Rutledge, T.R.; Liao, P.S.; Gupta, S.; Herbst, K.L.; Zarrinpar, A. Differences in Weight Loss Between Persons on Standard Balanced vs Nutrigenetic Diets in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2015, 13, 1625–1632.e1621. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.; Scott, A.; Honcz, J.; Spettell, C.; Pradhan, S. Reducing Metabolic Syndrome Risk Using a Personalized Wellness Program. J. Occup. Environ. Med. 2015, 57, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Covolo, L.; Rubinelli, S.; Ceretti, E.; Gelatti, U. Internet-Based Direct-to-Consumer Genetic Testing: A Systematic Review. J. Med. Internet Res. 2015, 17, e279. [Google Scholar] [CrossRef]
- Reis, A. Stellungnahme der Deutschen Gesellschaft fuer Humangenetik (GfH) zu “Direct-to-Consumer” (DTC)-Gentests. Dtsch. Ges. Fuer Humangenet. E.V., 2011. Available online: https://www.gfhev.de/de/leitlinien/LL_und_Stellungnahmen/2011_12_02_GfH-Stellungnahme_DTC-Gentests.pdf (accessed on 25 January 2019).
- Camp, K.M.; Trujillo, E. Position of the Academy of Nutrition and Dietetics: Nutritional genomics. J. Acad. Nutr. Diet. 2014, 114, 299–312. [Google Scholar] [CrossRef]
- Rafiq, M.; Ianuale, C.; Ricciardi, W.; Boccia, S. Direct-to-consumer genetic testing: A systematic review of european guidelines, recommendations, and position statements. Genet. Test. Mol. Biomark. 2015, 19, 535–547. [Google Scholar] [CrossRef]
- Bloss, C.S.; Ornowski, L.; Silver, E.; Cargill, M.; Vanier, V.; Schork, N.J.; Topol, E.J. Consumer perceptions of direct-to-consumer personalized genomic risk assessments. Genet. Med. Off. J. Am. Coll. Med. Genet. 2010, 12, 556–566. [Google Scholar] [CrossRef]
- Bray, M.S.; Loos, R.J.; McCaffery, J.M.; Ling, C.; Franks, P.W.; Weinstock, G.M.; Snyder, M.P.; Vassy, J.L.; Agurs-Collins, T. NIH working group report-using genomic information to guide weight management: From universal to precision treatment. Obesity 2016, 24, 14–22. [Google Scholar] [CrossRef] [PubMed]
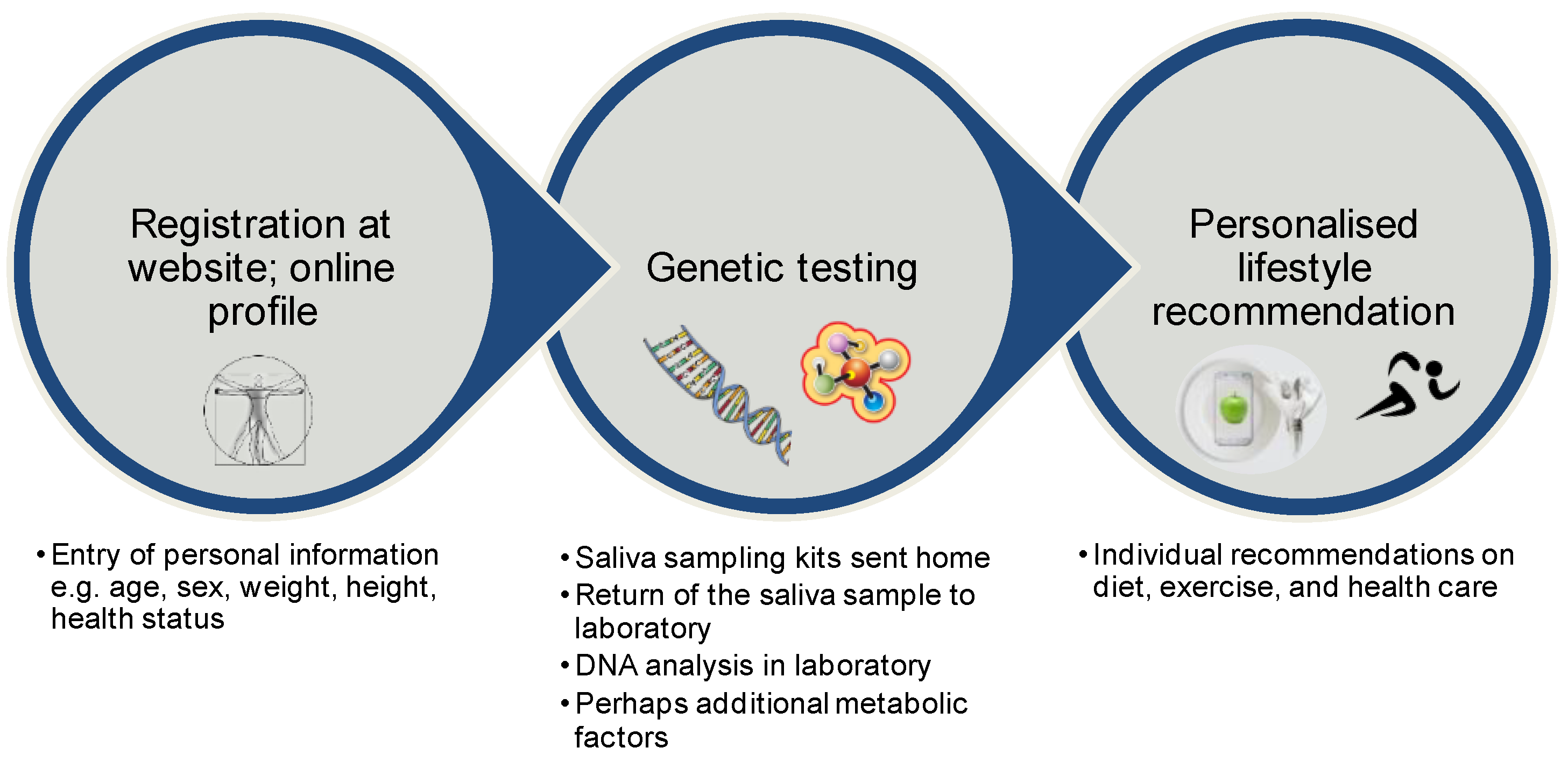
| Study | Investigated SNPs | Intervention | Result | Reference |
|---|---|---|---|---|
| NUGENOB | 42 SNPs at 26 genetic loci | Ten-week dietary intervention based on two hypocaloric diets of 600 kcal/d each and percentage of energy derived from fat of 20–25% (low fat) or 40–45% (high fat) | No SNP–diet interaction on weight change | Sorensen et al. (2006) [54] |
| DiOGenes | 651 SNPs at 69 genetic loci | Five different ad libitum diets consisting of different glycaemic indices (GI) and contents of dietary protein (P): low P/low GI vs. low P/high GI vs. high P/.ow GI vs. high P/high GI vs. control diet | No SNP–diet interaction on weight change | Larsen et al. (2012) [60] |
| Food4Me | 5 SNPs at 5 genetic loci (FTO, FADS1, TCF7L2, ApoE(e4), MTHFR) | Four different diet groups: (1) Non-personalised dietary recommendation (2) Personalised dietary advice based on dietary habit (3) Personalised dietary advice based on dietary habit and phenotypic data (4) Personalised dietary advice based on dietary habit, phenotypic and genotypic data | No significant difference of weight change between risk and non-risk allele carriers; level of personal dietary advice had no effect on weight change | Celis-Morales et al. (2015) [63] |
| DIETFITS | 3 SNPs at 3 genetic loci (PPARG, ADRB2, FABP2) | Low-fat diet or a low-carbohydrate diet | Similar weight change between groups independent of genetic pattern | Gardner et al. (2018) [10] |
| Company | Genetic Approach | Dietary Recommendation Based on | Homepage |
|---|---|---|---|
| Pathway Genomics | SNPs at genetic loci such as ADIPOQ (rs17300539, rs17366568), APOA2 (rs5082), FADS1 (rs174547), FTO (rs9939609, rs1121980), MC4R (rs17782313), PPARG (rs1801282) | Genetic profile matched to a low-fat, low-carbohydrate, Mediterranean or balanced diet, including genetic risks for metabolic health factors (e.g., blood sugar, lipids) | https://www.pathway.com |
| Thinner Gene | SNPs at genetic loci such as FTO, PPARG, PLIN, ADRB2, ADIPOQ, FABP2, PPARG, IRS1, APOA2/5, TCF7L2 | Genetic profile and sensitivity for carbohydrates, fats, and proteins matched with healthy food and fat control | http://www.thinnergene.com |
| Genetic Balance | SNPs at genetic loci associated with fat and carbohydrate metabolism | Genetic make-up matched to good or bad burning of carbohydrates or fats | https://www.genetic-balance.com |
| Bodykey by NUTRILITE | SNPs at genetic loci such as FABP2 (rs1799883), PPARG (rs1801282), ADRB2 (rs1042713), ADRB2 (rs1042714), ADRB3 (rs4994) | Genetic profile matched to diets with different macronutrient compositions | https://www.bodykey.at |
| Nutrigenes | 100 SNPs at genetic loci such as FADS1 | Genetic predisposition to food and nutrient needs, intolerances and sensitivities | http://www.nutrigenes.ch |
| My Kirée | Eight genetic loci associated with body weight | Genetic profile for fat or carbohydrate sensitivity, including supplementation with fat and carbohydrate blockers | https://my-kiree.com |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drabsch, T.; Holzapfel, C. A Scientific Perspective of Personalised Gene-Based Dietary Recommendations for Weight Management. Nutrients 2019, 11, 617. https://doi.org/10.3390/nu11030617
Drabsch T, Holzapfel C. A Scientific Perspective of Personalised Gene-Based Dietary Recommendations for Weight Management. Nutrients. 2019; 11(3):617. https://doi.org/10.3390/nu11030617
Chicago/Turabian StyleDrabsch, Theresa, and Christina Holzapfel. 2019. "A Scientific Perspective of Personalised Gene-Based Dietary Recommendations for Weight Management" Nutrients 11, no. 3: 617. https://doi.org/10.3390/nu11030617
APA StyleDrabsch, T., & Holzapfel, C. (2019). A Scientific Perspective of Personalised Gene-Based Dietary Recommendations for Weight Management. Nutrients, 11(3), 617. https://doi.org/10.3390/nu11030617





