Nutrient Composition Comparison between the Low Saturated Fat Swank Diet for Multiple Sclerosis and Healthy U.S.-Style Eating Pattern
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Overview
2.2. Nutritional Adequacy of Seven-Day Swank Menus
2.3. Nutritional Adequacy of Swank Diet and HEP Using Food Pattern Modeling
2.4. Healthy Eating Index-2015 (HEI-2015) and Alternate Healthy Eating Index-2010 (AHEI-2010)
2.5. Data Analysis
3. Results
3.1. Swank Menu Composition
3.2. Nutritional Adequacy
3.2.1. Swank Menus
3.2.2. Swank Menus plus Dietary Supplements
3.2.3. Food Pattern Modeling
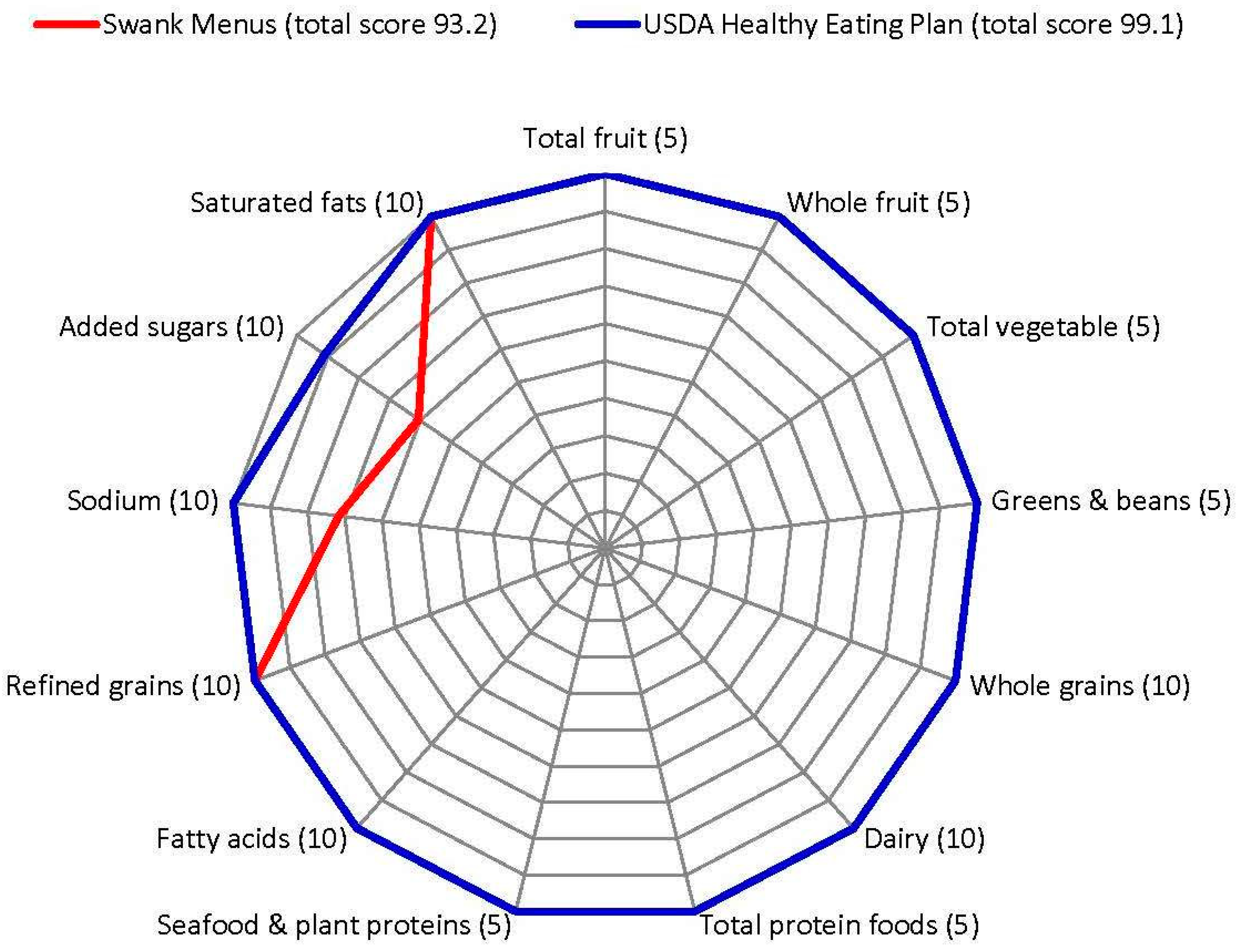
3.3. HEI-2015 and AHEI-2010
4. Discussion
4.1. Menu Composition
4.2. Nutritional Adequacy
4.3. HEI-2015 and AHEI-2010
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Capriotti, T.; Noel, J.; Brissenden, S. Multiple Sclerosis: An Update for Home Healthcare Clinicians. Home Healthc. Now 2018, 36, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, A.; Stahnisch, F.W. A history of multiple sclerosis investigations in Canada between 1850 and 1950. Can. J. Neurol. Sci. 2014, 41, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.J. The history of multiple sclerosis: The changing frame of the disease over the centuries. J. Neurol. Sci. 2009, 277 (Suppl. 1), S3–S8. [Google Scholar] [CrossRef]
- Talley, C.L. The emergence of multiple sclerosis, 1870–1950: A puzzle of historical epidemiology. Perspect. Biol. Med. 2005, 48, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Bourdette, D. Roy Laver Swank, MD, PhD (1909–2008). Neurology 2009, 72, 1120. [Google Scholar] [CrossRef]
- Swank, R.L. Multiple sclerosis; A correlation of its incidence with dietary fat. Am. J. Med. Sci. 1950, 220, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Swank, R.L.; Lerstad, O.; Strom, A.; Backer, J. Multiple sclerosis in rural Norway its geographic and occupational incidence in relation to nutrition. N. Engl. J. Med. 1952, 246, 722–728. [Google Scholar] [CrossRef]
- Swank, R.L.; Goodwin, J. Review of MS patient survival on a Swank low saturated fat diet. Nutrition 2003, 19, 161–162. [Google Scholar] [CrossRef]
- Swank, R.L. Treatment of multiple sclerosis with low-fat diet. AMA Arch. Neurol. Psychiatry 1953, 69, 91–103. [Google Scholar] [CrossRef]
- Swank, R.L. Treatment of multiple sclerosis with low-fat diet; results of five and one-half years’ experience. AMA Arch. Neurol. Psychiatry 1955, 73, 631–644. [Google Scholar] [CrossRef]
- Swank, R.L. Treatment of multiple sclerosis with low-fat diet: Result of seven years’ experience. Ann. Intern. Med. 1956, 45, 812–824. [Google Scholar] [PubMed]
- Swank, R.L. Treatment of multiple sclerosis with a low-fat diet. J. Am. Diet. Assoc. 1960, 36, 322–325. [Google Scholar]
- Swank, R.L.; Bourdillon, R.B. Multiple sclerosis: Assessment of treatment with a modified low-fat diet. J. Nerv. Ment. Dis. 1960, 131, 468–488. [Google Scholar] [CrossRef]
- Swank, R.L. Multiple sclerosis: Twenty years on low fat diet. Arch. Neurol. 1970, 23, 460–474. [Google Scholar] [CrossRef]
- Swank, R.L.; Grimsgaard, A. Multiple sclerosis: The lipid relationship. Am. J. Clin. Nutr. 1988, 48, 1387–1393. [Google Scholar] [CrossRef]
- Swank, R.L.; Dugan, B.B. Effect of low satured fat diet in early and late cases of multiple sclerosis. Lancet 1990, 336, 37–39. [Google Scholar] [CrossRef]
- Swank, R.L. Multiple sclerosis: Fat-oil relationship. Nutrition 1991, 7, 368–376. [Google Scholar]
- Swank, R.L.; Goodwin, J.W. How saturated fats may be a causative factor in multiple sclerosis and other diseases. Nutrition 2003, 19, 478. [Google Scholar] [CrossRef]
- [Editorial]. Lipids and multiple sclerosis. Lancet 1990, 336, 25–26. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Smith, G.D.; Marmot, M.G. Dietary fat in the epidemiology of multiple sclerosis: Has the situation been adequately assessed? Neuroepidemiology 1992, 11, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Wahls, T.L.; Chenard, C.A.; Snetselaar, L.G. Review of Two Popular Eating Plans within the Multiple Sclerosis Community: Low Saturated Fat and Modified Paleolithic. Nutrients 2019, 11, 352. [Google Scholar] [CrossRef]
- Wilmot, V.A.; Swank, R.L. The influence of low-fat diet on blood lipid levels in health and in multiple sclerosis. Am. J. Med. Sci. 1952, 223, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Swank, R.L.; Grimsgaard, A. Changes in blood produced by a fat meal and by intravenous heparin. Am. J. Physiol. 1951, 164, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Swank, R.L.; Franklin, A.E.; Quastel, J.H. Effects of fat meals and heparin on blood plasma composition as shown by paper chromatography. Proc. Soc. Exp. Biol. Med. 1950, 75, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Swank, R.L.; Franklin, A.E.; Quastel, J.H. Paper chromatography of blood plasmas in multiple sclerosis. Proc. Soc. Exp. Biol. Med. 1951, 76, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Swank, R.L.; Wilmot, V. Chylomicra: their composition and their fate after intravenous injection of small amounts of heparin. Am. J. Physiol. 1951, 167, 403–412. [Google Scholar] [CrossRef]
- Ganesh, A.; Stahnisch, F.W. On the historical succession of vessel-based therapies in the treatment of multiple sclerosis. Eur. Neurol. 2013, 70, 48–58. [Google Scholar] [CrossRef]
- Corthals, A.P. Multiple sclerosis is not a disease of the immune system. Q. Rev. Biol. 2011, 86, 287–321. [Google Scholar] [CrossRef]
- Zhornitsky, S.; McKay, K.A.; Metz, L.M.; Teunissen, C.E.; Rangachari, M. Cholesterol and markers of cholesterol turnover in multiple sclerosis: Relationship with disease outcomes. Mult. Scler. Relat. Disord. 2016, 5, 53–65. [Google Scholar] [CrossRef]
- Tettey, P.; Simpson, S., Jr.; Taylor, B.; Blizzard, L.; Ponsonby, A.L.; Dwyer, T.; Kostner, K.; van der Mei, I. An adverse lipid profile is associated with disability and progression in disability, in people with MS. Mult. Scler. 2014, 20, 1737–1744. [Google Scholar] [CrossRef]
- Weinstock-Guttman, B.; Zivadinov, R.; Mahfooz, N.; Carl, E.; Drake, A.; Schneider, J.; Teter, B.; Hussein, S.; Mehta, B.; Weiskopf, M.; et al. Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J. Neuroinflamm. 2011, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Tettey, P.; Simpson, S., Jr.; Taylor, B.; Blizzard, L.; Ponsonby, A.L.; Dwyer, T.; Kostner, K.; van der Mei, I. Adverse lipid profile is not associated with relapse risk in MS: Results from an observational cohort study. J. Neurol. Sci. 2014, 340, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Azary, S.; Schreiner, T.; Graves, J.; Waldman, A.; Belman, A.; Guttman, B.W.; Aaen, G.; Tillema, J.M.; Mar, S.; Hart, J.; et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K. Diet and disease modification in multiple sclerosis: A nutritional epidemiology perspective. J. Neurol. Neurosurg. Psychiatry 2018, 89, 3. [Google Scholar] [CrossRef]
- Swank, R.L.; Dugan, B.B. The Multiple Sclerosis Diet Book. A Low-Fat Diet for the Treatment of MS; Doubleday: New York, NY, USA, 1987. [Google Scholar]
- Swank MS Foundation. The Swank Low-Fat Diet for the Treatment of MS. Available online: http://www.swankmsdiet.org/the-diet/ (accessed on 17 October 2017).
- Nayak, S.; Matheis, R.J.; Schoenberger, N.E.; Shiflett, S.C. Use of unconventional therapies by individuals with multiple sclerosis. Clin. Rehabil. 2003, 17, 181–191. [Google Scholar] [CrossRef]
- Masullo, L.; Papas, M.A.; Cotugna, N.; Baker, S.; Mahoney, L.; Trabulsi, J. Complementary and alternative medicine use and nutrient intake among individuals with multiple sclerosis in the United States. J. Community Health 2015, 40, 153–160. [Google Scholar] [CrossRef]
- Leong, E.M.; Semple, S.J.; Angley, M.; Siebert, W.; Petkov, J.; McKinnon, R.A. Complementary and alternative medicines and dietary interventions in multiple sclerosis: What is being used in South Australia and why? Complement. Ther. Med. 2009, 17, 216–223. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Tyry, T.; Salter, A.; Cofield, S.S.; Cutter, G.; Fox, R.J.; Marrie, R.A. A survey of dietary characteristics in a large population of people with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 22, 12–18. [Google Scholar] [CrossRef]
- Yadav, V.; Marracci, G.; Kim, E.; Spain, R.; Cameron, M.; Overs, S.; Riddehough, A.; Li, D.K.B.; McDougall, J.; Lovera, J.; et al. Low-fat, plant-based diet in multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2016, 9, 80–90. [Google Scholar] [CrossRef]
- Sedaghat, F.; Jessri, M.; Behrooz, M.; Mirghotbi, M.; Rashidkhani, B. Mediterranean diet adherence and risk of multiple sclerosis: A case-control study. Asia Pac. J. Clin. Nutr. 2016, 25, 377–384. [Google Scholar]
- Katz Sand, I. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150–160. [Google Scholar] [CrossRef]
- Storoni, M.; Plant, G.T. The Therapeutic Potential of the Ketogenic Diet in Treating Progressive Multiple Sclerosis. Mult. Scler. Int. 2015, 2015, 681289. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Vizthum, D.; Henry-Barron, B.; Schweitzer, A.; Cassard, S.D.; Kossoff, E.; Hartman, A.L.; Kapogiannis, D.; Sullivan, P.; Baer, D.J.; et al. Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 23, 33–39. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235. [Google Scholar] [CrossRef]
- Saadatnia, M.; Etemadifar, M.; Fatehi, F.; Ashtari, F.; Shaygannejad, V.; Chitsaz, A.; Maghzi, A.H. Short-term effects of prolonged fasting on multiple sclerosis. Eur. Neurol. 2009, 61, 230. [Google Scholar] [CrossRef]
- Bisht, B.; Darling, W.G.; Grossmann, R.E.; Shivapour, E.T.; Lutgendorf, S.K.; Snetselaar, L.G.; Hall, M.J.; Zimmerman, M.B.; Wahls, T.L. A multimodal intervention for patients with secondary progressive multiple sclerosis: Feasibility and effect on fatigue. J. Altern. Complement. Med. 2014, 20, 347–355. [Google Scholar] [CrossRef]
- Bisht, B.; Darling, W.G.; Shivapour, E.T.; Lutgendorf, S.K.; Snetselaar, L.G.; Chenard, C.A.; Wahls, T.L. Multimodal intervention improves fatigue and quality of life in subjects with progressive multiple sclerosis: A pilot study. Degener. Neurol. Neuromuscul. Dis. 2015, 5, 19–35. [Google Scholar]
- Irish, A.K.; Erickson, C.M.; Wahls, T.L.; Snetselaar, L.G.; Darling, W.G. Randomized control trial evaluation of a modified Paleolithic dietary intervention in the treatment of relapsing-remitting multiple sclerosis: A pilot study. Degener. Neurol. Neuromuscul. Dis. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- Lee, J.E.; Bisht, B.; Hall, M.J.; Rubenstein, L.M.; Louison, R.; Klein, D.T.; Wahls, T.L. A Multimodal, Nonpharmacologic Intervention Improves Mood and Cognitive Function in People with Multiple Sclerosis. J. Am. Coll. Nutr. 2017, 36, 150–168. [Google Scholar] [CrossRef]
- Bisht, B.; Darling, W.G.; White, E.C.; White, K.A.; Shivapour, E.T.; Zimmerman, M.B.; Wahls, T.L. Effects of a multimodal intervention on gait and balance of subjects with progressive multiple sclerosis: A prospective longitudinal pilot study. Degener. Neurol. Neuromuscul. Dis. 2017, 7, 79–93. [Google Scholar] [CrossRef]
- Farinotti, M.; Vacchi, L.; Simi, S.; Di Pietrantonj, C.; Brait, L.; Filippini, G. Dietary interventions for multiple sclerosis. Cochrane Database Syst. Rev. 2012, 12, Cd004192. [Google Scholar] [CrossRef]
- Venasse, M.; Edwards, T.; Pilutti, L.A. Exploring Wellness Interventions in Progressive Multiple Sclerosis: An Evidence-Based Review. Curr. Treat. Options Neurol. 2018, 20, 13. [Google Scholar] [CrossRef]
- Bhargava, P. Diet and Multiple Sclerosis. Available online: http://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Documents/Diet-and-Multiple-Sclerosis-Bhargava-06-26-15.pdf (accessed on 25 June 2015).
- Diet & Nutrition. Available online: https://www.nationalmssociety.org/Living-Well-With-MS/Diet-Exercise-Healthy-Behaviors/Diet-Nutrition#section-0 (accessed on 16 October 2017).
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans; Skyhorse Publishing Inc.: Washington, DC, USA, 2015.
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Miller, N.H.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk. Circulation 2014, 129, S76–S99. [Google Scholar] [CrossRef]
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; Gapstur, S.; Patel, A.V.; Andrews, K.; Gansler, T. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin. 2012, 62, 30–67. [Google Scholar] [CrossRef]
- Wahls, T.; Scott, M.O.; Alshare, Z.; Rubenstein, L.; Darling, W.; Carr, L.; Smith, K.; Chenard, C.A.; LaRocca, N.; Snetselaar, L. Dietary approaches to treat MS-related fatigue: Comparing the modified Paleolithic (Wahls Elimination) and low saturated fat (Swank) diets on perceived fatigue in persons with relapsing-remitting multiple sclerosis: Study protocol for a randomized controlled trial. Trials 2018, 19, 309. [Google Scholar]
- Wahls, T.L. Dietary Approaches to Treat Multiple Sclerosis-Related Fatigue Study. Available online: https://clinicaltrials.gov/ct2/show/NCT02914964 (accessed on 15 June 2018).
- Lichtenstein, A.H.; Van Horn, L. Very Low Fat Diets. Circulation 1998, 98, 935–939. [Google Scholar] [CrossRef]
- Mueller-Cunningham, W.M.; Quintana, R.; Kasim-Karakas, S.E. An ad libitum, very low-fat diet results in weight loss and changes in nutrient intakes in postmenopausal women. J. Am. Diet. Assoc. 2003, 103, 1600–1606. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes: EAR, RDA, AI, Acceptable Macronutrient Distribution Ranges, and UL. Available online: http://www.nationalacademies.org/hmd/~/media/Files/Activity%20Files/Nutrition/DRI-Tables/5Summary%20TableTables%2014.pdf?la=en (accessed on 16 June 2018).
- Institute of Medicine. Dietary Reference Intakes: Applications in Dietary Planning; The National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef]
- Center for Nutrition Policy and Promotion. Healthy US-Style Pattern: Recommended Intake Amounts. Available online: https://www.cnpp.usda.gov/sites/default/files/usda_food_patterns/HealthyUS-StylePattern-RecommendedIntakeAmounts.pdf (accessed on 19 September 2017).
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. Nutrients in Healthy US-Style Food Pattern: Nutrients in the Pattern at Each Calorie Level and Comparison of Nutrient Content to the Nutritional Goals for That Pattern. Available online: https://www.cnpp.usda.gov/sites/default/files/usda_food_patterns/NutrientsInHealthyUS-StyleFoodPattern.pdf (accessed on 22 September 2017).
- Reedy, J.; Lerman, J.L.; Krebs-Smith, S.M.; Kirkpatrick, S.I.; Pannucci, T.E.; Wilson, M.M.; Subar, A.F.; Kahle, L.L.; Tooze, J.A. Evaluation of the Healthy Eating Index-2015. J. Acad. Nutr. Diet. 2018, 118, 1622–1633. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, ARS Item Clusters, Percent of Consumption, and Representative Foods for USDA Food Pattern Food Groups and Subgroups. Available online: https://www.cnpp.usda.gov/sites/default/files/usda_food_patterns/ItemClustersPercentOfConsumptionAndRepresentativeFoodsCorrected5-16-17.pdf (accessed on 22 September 2017).
- Nutrition Coordinating Center (NCC). Nutrition Data System for Research (NDSR) Software; University of Minnesota: Minneapolis, MN, USA, 2017. [Google Scholar]
- Bowman, S.A.; Clemens, J.C.; Shimizu, M.; Friday, J.E.; Alanna, J.; Moshfegh, A.J. Food Patterns Equivalents Database 2015–2016: Methodology and User Guide. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/fped/FPED_1516.pdf (accessed on 27 September 2018).
- Murphy, S.P. Using DRIs as the basis for dietary guidelines. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 52–54. [Google Scholar]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. Estimated Calorie Needs per Day—Energy Levels Used for Assignment of Individuals to USDA Food Patterns. Available online: https://www.cnpp.usda.gov/sites/default/files/usda_food_patterns/EstimatedCalorieNeedsPerDay.pdf (accessed on 19 September 2017).
- Krebs-Smith, S.M.; Clark, L.D. Validation of a nutrient adequacy score for use with women and children. J. Am. Diet. Assoc. 1989, 89, 775–783. [Google Scholar]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee Appendix E-3.1: Adequacy of USDA Food Patterns. Available online: https://health.gov/dietaryguidelines/2015-scientific-report/PDFs/Appendix-E-3.1.pdf (accessed on 5 July 2018).
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. Nutrient Profiles for Food Groups and Subgroups in the 2015 USDA Food Patterns. Available online: https://www.cnpp.usda.gov/sites/default/files/usda_food_patterns/NutrientProfiles.pdf (accessed on 22 September 2017).
- Britten, P.; Cleveland, L.E.; Koegel, K.L.; Kuczynski, K.J.; Nickols-Richardson, S.M. Updated US Department of Agriculture Food Patterns meet goals of the 2010 dietary guidelines. J. Acad. Nutr. Diet. 2012, 112, 1648–1655. [Google Scholar] [CrossRef]
- Pannucci, T.; USDA, Alexandria, VA, USA. Personal communication, 2018.
- National Cancer Institute. The Healthy Eating Index—Population Ratio Method. Available online: https://epi.grants.cancer.gov/hei/population-ratio-method.html (accessed on 25 October 2017).
- McCullough, M.L.; Willett, W.C. Evaluating adherence to recommended diets in adults: The Alternate Healthy Eating Index. Public Health Nutr. 2006, 9, 152–157. [Google Scholar] [CrossRef]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; Colditz, G.A.; Willett, W.C. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [CrossRef]
- McCullough, M.L.; Feskanich, D.; Rimm, E.B.; Giovannucci, E.L.; Ascherio, A.; Variyam, J.N.; Spiegelman, D.; Stampfer, M.J.; Willett, W.C. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in men. Am. J. Clin. Nutr. 2000, 72, 1223–1231. [Google Scholar] [CrossRef]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Rosner, B.A.; Hu, F.B.; Hunter, D.J.; Variyam, J.N.; Colditz, G.A.; Willett, W.C. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in women. Am. J. Clin. Nutr. 2000, 72, 1214–1222. [Google Scholar] [CrossRef]
- Kennedy, E.T.; Ohls, J.; Carlson, S.; Fleming, K. The Healthy Eating Index: Design and Applications. J. Am. Diet. Assoc. 1995, 95, 1103. [Google Scholar] [CrossRef]
- Shivappa, N.; Hebert, J.R.; Kivimaki, M.; Akbaraly, T. Alternate Healthy Eating Index 2010, Dietary Inflammatory Index and risk of mortality: Results from the Whitehall II cohort study and meta-analysis of previous Dietary Inflammatory Index and mortality studies. Br. J. Nutr. 2017, 118, 210–221. [Google Scholar] [CrossRef]
- Mears, M.; Tussing-Humphreys, L.; Cerwinske, L.; Tangney, C.; Hughes, S.L.; Fitzgibbons, M.; Gomez-Perez, S. Associations between Alternate Healthy Eating Index-2010, Body Composition, Osteoarthritis Severity, and Interleukin-6 in Older Overweight and Obese African American Females with Self-Reported Osteoarthritis. Nutrients 2018, 11, 26. [Google Scholar] [CrossRef]
- McCullough, M.L.; American Cancer Society, Inc., Atlanta, GA, USA. Personal communication, 2019.
- Hoy, M.K.; Goldman, J.D.; Murayi, T.; Rhodes, D.G.; Moshfegh, A.J. Sodium Intake of the U.S. Population: What We Eat In America, NHANES 2007-2008. Food Surveys Research Group Dietary Data Brief No. 8. October 2011. Available online: http://ars usda gov/Services/docs htm?docid=19476 (accessed on 5 March 2019).
- Schwingshackl, L.; Bogensberger, B.; Hoffmann, G. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: An Updated Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2018, 118, 74–100. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS 9.4; SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- Microsoft Corporation. Microsoft Excel, 14.0.7208.5000 (32-bit); Microsoft Corporation: Albuquerque, NM, USA, 2010. [Google Scholar]
- Brand-Miller, J.C.; Stockmann, K.; Atkinson, F.; Petocz, P.; Denyer, G. Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: Analysis of a database of more than 1000 foods. Am. J. Clin. Nutr. 2009, 89, 97–105. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes: Macronutrients. Available online: http://www.nationalacademies.org/hmd/~/media/Files/Activity%20Files/Nutrition/DRI-Tables/8_Macronutrient%20Summary.pdf?la=en (accessed on 16 June 2018).
- Jacobson, T.A.; Maki, K.C.; Orringer, C.E.; Jones, P.H.; Kris-Etherton, P.; Sikand, G.; La Forge, R.; Daniels, S.R.; Wilson, D.P.; Morris, P.B.; et al. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 2. J. Clin. Lipidol. 2015, 9, S1–S122. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Hadgkiss, E.J.; Jelinek, G.A.; Weiland, T.J.; Pereira, N.G.; Marck, C.H.; van der Meer, D.M. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr. Neurosci. 2015, 18, 125–136. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Tyry, T.; Salter, A.; Cofield, S.S.; Cutter, G.; Fox, R.; Marrie, R.A. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018, 90, e1–e11. [Google Scholar] [CrossRef]
- Marck, C.H.; De Livera, A.M.; Brown, C.R.; Neate, S.L.; Taylor, K.L.; Weiland, T.J.; Hadgkiss, E.J.; Jelinek, G.A. Health outcomes and adherence to a healthy lifestyle after a multimodal intervention in people with multiple sclerosis: Three year follow-up. PLoS ONE 2018, 13, e0197759. [Google Scholar] [CrossRef]
- Thau-Zuchman, O.; Gomes, R.N.; Dyall, S.C.; Davies, M.; Priestley, J.V.; Groenendijk, M.; De Wilde, M.C.; Tremoleda, J.L.; Michael-Titus, A.T. Brain Phospholipid Precursors Administered Post-Injury Reduce Tissue Damage and Improve Neurological Outcome in Experimental Traumatic Brain Injury. J. Neurotrauma 2019, 36, 25–42. [Google Scholar] [CrossRef]
- Skripuletz, T.; Manzel, A.; Gropengiesser, K.; Schafer, N.; Gudi, V.; Singh, V.; Salinas Tejedor, L.; Jorg, S.; Hammer, A.; Voss, E.; et al. Pivotal role of choline metabolites in remyelination. Brain 2015, 138, 398–413. [Google Scholar] [CrossRef]
- Skripuletz, T.; Linker, R.A.; Stangel, M. The choline pathway as a strategy to promote central nervous system (CNS) remyelination. Neural Regener. Res. 2015, 10, 1369–1370. [Google Scholar]
- Hollenbeck, C.B. An introduction to the nutrition and metabolism of choline. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc. Natl. Acad. Sci. USA 2006, 103, 17589–17594. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Optimal micronutrients delay mitochondrial decay and age-associated diseases. Mech. Ageing Dev. 2010, 131, 473–479. [Google Scholar] [CrossRef] [PubMed]
- NIH Office of Dietary Supplements. Choline Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Choline-HealthProfessional/#en11 (accessed on 25 September 2018).
- Wen, W.; Wan, Z.; Ren, K.; Zhou, D.; Gao, Q.; Wu, Y.; Wang, L.; Yuan, Z.; Zhou, J. Potassium supplementation inhibits IL-17A production induced by salt loading in human T lymphocytes via p38/MAPK-SGK1 pathway. Exp. Mol. Pathol. 2016, 100, 370–377. [Google Scholar] [CrossRef]
- Gijsbers, L.; Dower, J.I.; Schalkwijk, C.G.; Kusters, Y.H.; Bakker, S.J.; Hollman, P.C.; Geleijnse, J.M. Effects of sodium and potassium supplementation on endothelial function: A fully controlled dietary intervention study. Br. J. Nutr. 2015, 114, 1419–1426. [Google Scholar] [CrossRef]
- Khalili, H.; Malik, S.; Ananthakrishnan, A.N.; Garber, J.J.; Higuchi, L.M.; Joshi, A.; Peloquin, J.; Richter, J.M.; Stewart, K.O.; Curhan, G.C.; et al. Identification and Characterization of a Novel Association between Dietary Potassium and Risk of Crohn’s Disease and Ulcerative Colitis. Front. Immunol. 2016, 7, 554. [Google Scholar] [CrossRef] [PubMed]
- Miyake, S.; Yamamura, T. Gut environmental factors and multiple sclerosis. J. Neuroimmunol. 2018. [Google Scholar] [CrossRef]
- Berer, K.; Martinez, I.; Walker, A.; Kunkel, B.; Schmitt-Kopplin, P.; Walter, J.; Krishnamoorthy, G. Dietary non-fermentable fiber prevents autoimmune neurological disease by changing gut metabolic and immune status. Sci. Rep. 2018, 8, 10431. [Google Scholar] [CrossRef]
- Lombardi, V.C.; De Meirleir, K.L.; Subramanian, K.; Nourani, S.M.; Dagda, R.K.; Delaney, S.L.; Palotás, A. Nutritional modulation of the intestinal microbiota: Future opportunities for the prevention and treatment of neuroimmune and neuroinflammatory disease. J. Nutr. Biochem. 2018, 61, 1–16. [Google Scholar] [CrossRef]
- Shahi, S.K.; Freedman, S.N.; Mangalam, A.K. Gut microbiome in multiple sclerosis: The players involved and the roles they play. Gut Microbes 2017, 8, 607–615. [Google Scholar] [CrossRef]
- Freedman, S.N.; Shahi, S.K.; Mangalam, A.K. The “Gut Feeling”: Breaking Down the Role of Gut Microbiome in Multiple Sclerosis. Neurotherapeutics 2018, 15, 109–125. [Google Scholar] [CrossRef]
- Ledikwe, J.H.; Blanck, H.M.; Khan, L.K.; Serdula, M.K.; Seymour, J.D.; Tohill, B.C.; Rolls, B.J. Dietary energy density determined by eight calculation methods in a nationally representative United States population. J. Nutr. 2005, 135, 273–278. [Google Scholar] [CrossRef]
- Pérez-Escamilla, R.; Obbagy, J.E.; Altman, J.M.; Essery, E.V.; McGrane, M.M.; Wong, Y.P.; Spahn, J.M.; Williams, C.L. Dietary Energy Density and Body Weight in Adults and Children: A Systematic Review. J. Acad. Nutr. Diet. 2012, 112, 671–684. [Google Scholar] [CrossRef]
- Vernarelli, J.A.; Mitchell, D.C.; Rolls, B.J.; Hartman, T.J. Dietary energy density and obesity: How consumption patterns differ by body weight status. Eur. J. Nutr. 2018, 57, 351–361. [Google Scholar] [CrossRef]
- Mokry, L.E.; Ross, S.; Timpson, N.J.; Sawcer, S.; Davey Smith, G.; Richards, J.B. Obesity and Multiple Sclerosis: A Mendelian Randomization Study. PLoS Med. 2016, 13, e1002053. [Google Scholar] [CrossRef]
- Marck, C.H.; Neate, S.L.; Taylor, K.L.; Weiland, T.J.; Jelinek, G.A. Prevalence of Comorbidities, Overweight and Obesity in an International Sample of People with Multiple Sclerosis and Associations with Modifiable Lifestyle Factors. PLoS ONE 2016, 11, e0148573. [Google Scholar] [CrossRef]
- Tettey, P.; Simpson, S.; Taylor, B.; Ponsonby, A.L.; Lucas, R.M.; Dwyer, T.; Kostner, K.; van der Mei, I.A. An adverse lipid profile and increased levels of adiposity significantly predict clinical course after a first demyelinating event. J. Neurol. Neurosurg. Psychiatry 2017, 88, 395–401. [Google Scholar] [CrossRef]
- Pasquinelli, S.; Solaro, C. Nutritional assessment and malnutrition in multiple sclerosis. Neurol.Sci. 2008, 29 (Suppl. 4), S367. [Google Scholar] [CrossRef]
- Edwards, N.C.; Munsell, M.; Menzin, J.; Phillips, A.L. Comorbidity in US patients with multiple sclerosis. Patient Relat. Outcome Meas. 2018, 9, 97–102. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute. DASH Eating Plan. Available online: https://www.nhlbi.nih.gov/health-topics/dash-eating-plan (accessed on 28 February 2019).
- USDA Center for Nutrition Policy and Promotion. Report Card on the Quality of Americans’ Diets. Available online: http://origin.www.cnpp.usda.gov/Publications/NutritionInsights/Insight28.pdf (accessed on 1 September 2018).
| Saturated Fat | ≤15 g |
| Foods Recommended | 2+ cup-eq 1 (~45–250 g) fruit per day, fresh preferred 2 |
| 2+ cup-eq (~20–250 g) vegetables 2 per day | |
| 4 servings grains per day, whole preferred 2 | |
| 2 cups (~490 g) dairy products with <1% fat per day | |
| Protein foods, daily | |
| Egg whites | |
| Poultry, white meat, no skin | |
| White fish and shellfish | |
| Nuts and seeds 3 | |
| 4 to 10 teaspoons (20–50 g) oil 4 per day | |
| Foods Limited | Fatty fish ≤ 50 g (1.75 ounce) per day 3 |
| Whole eggs ≤ 1 per day, ≤ 3 per week | |
| Caffeinated beverages ≤ 3 cups (237–246 g) per day | |
| Alcoholic beverages, maximum one drink per day | |
| Foods Not Recommended | Beef, pork, dark meat poultry 5 |
| Processed food containing saturated fat | |
| Butter, animal fats | |
| High fat dairy productsHigh-sugar products | |
| Coconut oil, palm oil, margarine, lard, shortening, hydrogenated oil | |
| Coconut, chocolate, cocoa butter | |
| Supplements | 1 teaspoon (5 g) cod liver oil |
| 1 multivitamin/mineral | |
| 1000 mg vitamin C 2 | |
| 400 IU vitamin E 2 |
| Food Group | Mean Servings |
|---|---|
| Fruits and Vegetables, Total (cup-equivalents 1) | 4.2 |
| Fruits, Total (cup-equivalents 1) | 1.9 |
| Juice | 0.4 |
| Whole Fruit | 1.4 |
| Vegetables, Total (cup-equivalents 1) | 2.3 |
| Dark-green Vegetables | 1.0 |
| Deep-yellow Vegetables | 0.2 |
| Tomato | 0.3 |
| White Potatoes | 0.5 |
| Other Starchy Vegetables | 0.1 |
| Other Vegetables | 0.5 |
| Grains, Total (servings 2) | 5.8 |
| Whole Grain | 4.0 |
| Some Whole Grain | 0.0 |
| Refined Grain | 1.8 |
| Meat/Fish/Eggs/Nuts/Seeds, Total (servings 3) | 5.5 |
| Beef/Pork/Lamb | 0.0 |
| Poultry | 1.4 |
| Fish and Shellfish | 2.1 |
| Cold Cuts and Sausage | 0.0 |
| Organ Meats | 0.0 |
| Eggs | 0.6 |
| Nuts and Seeds including Butters | 1.4 |
| Dairy and Nondairy, Total (cup-equivalents) | 2.3 |
| Milk, dairy, low fat and fat free | 2.3 |
| Yogurt, dairy, fat free | 0.1 |
| Milk, non-dairy | 0.0 |
| Fats, Total (servings 4) | 4.2 |
| Oil 5 | 3.5 |
| Butter and Other Animal Fats | 0.0 |
| Salad Dressing | 0.8 |
| Sweets, Total (servings 6) | 6.7 |
| Nutrient | Mean | SD 2 |
|---|---|---|
| Energy (kcal) | 1719 | 234 |
| Energy (kJ) | 7192 | 979 |
| Total Protein (g) | 79.8 | 12.9 |
| Total Carbohydrate (g) | 256.6 | 38.6 |
| Total Dietary Fiber (g) | 25.8 | 3.5 |
| Soluble Dietary Fiber (g) | 5.7 | 0.8 |
| Insoluble Dietary Fiber (g) | 20.0 | 2.9 |
| Total Sugars (g) | 124.8 | 23.5 |
| Added Sugars (by Total Sugars) (g) | 51.2 | 25.7 |
| Gluten (g) | 11.4 | 3.4 |
| Glycemic Index (glucose reference) | 56.0 | 3.6 |
| Glycemic Load (glucose reference) | 128.9 | 20.2 |
| Total Fat (g) | 47.7 | 10.4 |
| Total Saturated Fatty Acids (SFA) (g) | 8.1 | 2.0 |
| Total Trans-Fatty Acids (TRANS) (g) | 0.2 | 0.1 |
| Total Monounsaturated Fatty Acids (MUFA) (g) | 16.6 | 3.6 |
| Total Polyunsaturated Fatty Acids (PUFA) (g) | 18.8 | 5.1 |
| Total Conjugated Linoleic Acid (CLA 18:2) (g) | 0.0 | 0.0 |
| PUFA 18:2 (linoleic acid) (g) | 16.1 | 4.6 |
| PUFA 18:3 n-3 (alpha-linolenic acid [ALA]) (g) | 2.0 | 0.6 |
| Omega 6 Fatty Acids (g) 3 | 16.3 | 4.6 |
| Omega-3 Fatty Acids (g) | 2.5 | 1.0 |
| Omega 6:3 ratio | 6.9 | 1.7 |
| PUFA 20:5 (eicosapentaenoic acid [EPA]) (g) | 0.1 | 0.2 |
| PUFA 22:5 (docosapentaenoic acid [DPA]) (g) | 0.1 | 0.1 |
| PUFA 22:6 (docosahexaenoic acid [DHA]) (g) | 0.4 | 0.7 |
| Cholesterol (mg) | 196 | 141 |
| % Calories from Protein | 18.4 | 2.3 |
| % Calories from Fat | 24.1 | 3.2 |
| % Calories from SFA | 4.1 | 0.7 |
| % Calories from TRANS | 0.1 | 0.0 |
| % Calories from MUFA | 8.3 | 1.2 |
| % Calories from PUFA | 9.5 | 1.8 |
| % Calories from 18:2 linoleic acid 4 | 8.1 | 1.7 |
| % Calories from 18:3n3 alpha-linolenic acid 4 | 1.0 | 0.2 |
| % Calories from Carbohydrate | 57.5 | 4.8 |
| % Calories from added sugar 4 | 9.7 | 4.5 |
| Total Grams (g) | 1766 | 217 |
| Kcal/Gram | 1.0 | 0.1 |
| kJ/Gram | 4.2 | 0.4 |
| Water (g) | 1370 | 168 |
| sodium:potassium ratio | 0.70 | 0.29 |
| calcium:phosphorus ratio | 0.72 | 0.19 |
| calcium:magnesium ratio | 2.91 | 0.86 |
| Phytic Acid (mg/1000 kcal) | 577 | 186 |
| Oxalic Acid (mg/1000 kcal) | 200 | 94 |
| Pantothenic Acid (mg/1000 kcal) | 4 | 0 |
| Betaine (mg/1000 kcal) | 111 | 38 |
| Sex | Females | Males | ||||||
|---|---|---|---|---|---|---|---|---|
| Age, Years | 19–30 | 31–50 | 51–70 | >70 | 19–30 | 31–50 | 51–70 | >70 |
| Energy, kcal 1 | 2000 | 1800 | 1600 | 1600 | 2600 | 2200 | 2000 | 2000 |
| Energy, kJ | 8368 | 7531 | 6694 | 6694 | 10,878 | 9205 | 8368 | 8368 |
| Protein, grams 2 | 93 | 84 | 74 | 74 | 121 | 102 | 93 | 93 |
| Carbohydrate, grams 3 | 299 | 269 | 239 | 239 | 388 | 328 | 299 | 299 |
| Fat, grams 4 | 55 | 50 | 44 | 44 | 72 | 61 | 55 | 55 |
| Saturated Fat, grams 5 | 9 | 8 | 8 | 8 | 12 | 10 | 9 | 9 |
| Percent Recommended Dietary Allowance (RDA) | ||||||||
| Vitamin A, %RDA | 161 | 145 | 129 | 129 | 162 | 137 | 125 | 125 |
| Vitamin C, %RDA | 186 | 167 | 148 | 148 | 201 | 170 | 155 | 155 |
| Vitamin D, %RDA | 91 6 | 82 | 73 | 55 | 119 | 100 | 91 | 68 |
| Vitamin E, %RDA | 78 | 71 | 63 | 63 | 102 | 86 | 78 | 78 |
| Vitamin B1, %RDA | 165 | 149 | 132 | 132 | 197 | 167 | 152 | 152 |
| Vitamin B2, %RDA | 247 | 224 | 202 | 202 | 247 | 228 | 209 | 209 |
| Vitamin B3, %RDA | 313 | 282 | 251 | 251 | 356 | 301 | 274 | 274 |
| Vitamin B6, %RDA | 184 | 165 | 127 | 127 | 239 | 202 | 140 | 140 |
| Folate, %RDA | 120 | 108 | 96 | 96 | 156 | 132 | 120 | 120 |
| Vitamin B12, %RDA | 226 | 204 | 181 | 181 | 294 | 249 | 226 | 226 |
| Calcium, %RDA | 131 | 118 | 87 | 87 | 170 | 144 | 131 | 109 |
| Copper, %RDA | 178 | 160 | 142 | 142 | 231 | 196 | 178 | 178 |
| Iron, %RDA | 79 | 71 | 143 | 143 | 232 | 196 | 178 | 178 |
| Magnesium, %RDA | 144 | 126 | 112 | 112 | 145 | 117 | 106 | 106 |
| Phosphorus, %RDA | 255 | 230 | 204 | 204 | 332 | 281 | 255 | 255 |
| Selenium, %RDA | 264 | 237 | 211 | 211 | 343 | 290 | 264 | 264 |
| Zinc, %RDA | 139 | 125 | 112 | 112 | 132 | 112 | 101 | 101 |
| Average % RDA | 174 | 157 | 142 | 141 | 215 | 183 | 164 | 161 |
| MAR, % 7 | 97 | 96 | 95 | 94 | 100 | 99 | 98 | 97 |
| Percent Adequate Intake (AI) | ||||||||
| Dietary Fiber, %AI | 128 | 108 | 114 | 114 | 103 | 87 | 100 | 100 |
| Linoleic Acid, %AI | 156 | 141 | 136 | 136 | 143 | 121 | 134 | 134 |
| α-Linolenic Acid, %AI | 207 | 186 | 165 | 165 | 185 | 156 | 142 | 142 |
| Vitamin K, %AI | 309 | 278 | 248 | 248 | 302 | 255 | 232 | 232 |
| Manganese, %AI | 338 | 304 | 271 | 271 | 344 | 291 | 265 | 265 |
| Choline, %AI | 107 | 92 | 81 | 81 | 102 | 86 | 79 | 79 |
| Potassium, %AI | 87 | 78 | 70 | 70 | 113 | 96 | 87 | 87 |
| Percent Tolerable Upper Intake Level (UL) | ||||||||
| Sodium, %UL | 118 | 106 | 94 | 94 | 153 | 130 | 118 | 118 |
| Category | Females 31–50 Years | Males 31–50 Years | ||
|---|---|---|---|---|
| Swank | HEP 1 | Swank | HEP | |
| Energy, kcal | 1800 | 1797 | 2200 | 2198 |
| Energy, kJ | 7531 | 7519 | 9205 | 9196 |
| Protein, grams | 80 | 87 | 97 | 100 |
| Protein, %kcal | 18 | 19 | 18 | 18 |
| Fat, grams | 56 | 61 | 68 | 78 |
| Fat, %kcal | 28 | 31 | 28 | 32 |
| Saturated Fat, grams | 11 | 15 | 13 | 20 |
| Saturated Fat, %kcal | 5 | 8 | 5 | 8 |
| EPA 2, grams | 0.1 | 0.1 | 0.1 | 0.1 |
| DHA 3, grams | 0.2 | 0.2 | 0.2 | 0.2 |
| Carbohydrate, grams | 254 | 233 | 311 | 286 |
| Carbohydrate, %kcal | 57 | 52 | 57 | 52 |
| Dietary Fiber, grams | 26 | 29 | 32 | 35 |
| Percent Recommended Dietary Allowance (RDA) | ||||
| Vitamin A, %RDA | 145 | 125 | 138 | 109 |
| Vitamin C, %RDA | 195 | 133 | 198 | 141 |
| Vitamin D, %RDA | 42 4 | 45 | 52 | 47 |
| Vitamin E, %RDA | 74 | 61 | 90 | 74 |
| Vitamin B1, %RDA | 142 | 153 | 160 | 165 |
| Vitamin B2, %RDA | 179 | 185 | 185 | 175 |
| Vitamin B3, %RDA | 162 | 160 | 173 | 166 |
| Vitamin B6, %RDA | 178 | 274 | 218 | 201 |
| Folate, %RDA | 161 | 143 | 197 | 172 |
| Vitamin B12, %RDA | 288 | 274 | 352 | 304 |
| Calcium, %RDA | 115 | 126 | 140 | 134 |
| Copper, %RDA | 145 | 146 | 177 | 173 |
| Iron, %RDA | 95 | 91 | 261 | 242 |
| Magnesium, %RDA | 120 | 105 | 112 | 94 |
| Phosphorus, %RDA | 228 | 239 | 279 | 266 |
| Selenium, %RDA | 195 | 193 | 238 | 221 |
| Zinc, %RDA | 169 | 171 | 150 | 143 |
| Average %RDA | 155 | 154 | 183 | 166 |
| MAR, % 5 | 95 | 94 | 97 | 95 |
| Percent Adequate Intake (AI) | ||||
| Dietary Fiber, %AI | 103 | 114 | 83 | 114 |
| Linoleic Acid, %AI | 150 | 143 | 129 | 125 |
| α-Linolenic Acid, %AI | 195 | 185 | 164 | 157 |
| Vitamin K, %AI | 388 | 147 | 356 | 142 |
| Manganese, %AI | 266 | 213 | 254 | 199 |
| Choline, %AI | 81 | 77 | 77 | 69 |
| Potassium, %AI | 67 | 67 | 82 | 79 |
| Percent Tolerable Upper Intake Level (UL) | ||||
| Sodium, %UL | 66 | 75 | 80 | 84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chenard, C.A.; Rubenstein, L.M.; Snetselaar, L.G.; Wahls, T.L. Nutrient Composition Comparison between the Low Saturated Fat Swank Diet for Multiple Sclerosis and Healthy U.S.-Style Eating Pattern. Nutrients 2019, 11, 616. https://doi.org/10.3390/nu11030616
Chenard CA, Rubenstein LM, Snetselaar LG, Wahls TL. Nutrient Composition Comparison between the Low Saturated Fat Swank Diet for Multiple Sclerosis and Healthy U.S.-Style Eating Pattern. Nutrients. 2019; 11(3):616. https://doi.org/10.3390/nu11030616
Chicago/Turabian StyleChenard, Catherine A., Linda M. Rubenstein, Linda G. Snetselaar, and Terry L. Wahls. 2019. "Nutrient Composition Comparison between the Low Saturated Fat Swank Diet for Multiple Sclerosis and Healthy U.S.-Style Eating Pattern" Nutrients 11, no. 3: 616. https://doi.org/10.3390/nu11030616
APA StyleChenard, C. A., Rubenstein, L. M., Snetselaar, L. G., & Wahls, T. L. (2019). Nutrient Composition Comparison between the Low Saturated Fat Swank Diet for Multiple Sclerosis and Healthy U.S.-Style Eating Pattern. Nutrients, 11(3), 616. https://doi.org/10.3390/nu11030616





