Hypertension Associated with Fructose and High Salt: Renal and Sympathetic Mechanisms
Abstract
1. Introduction
2. Fructose Consumption, Hypertension and Mortality
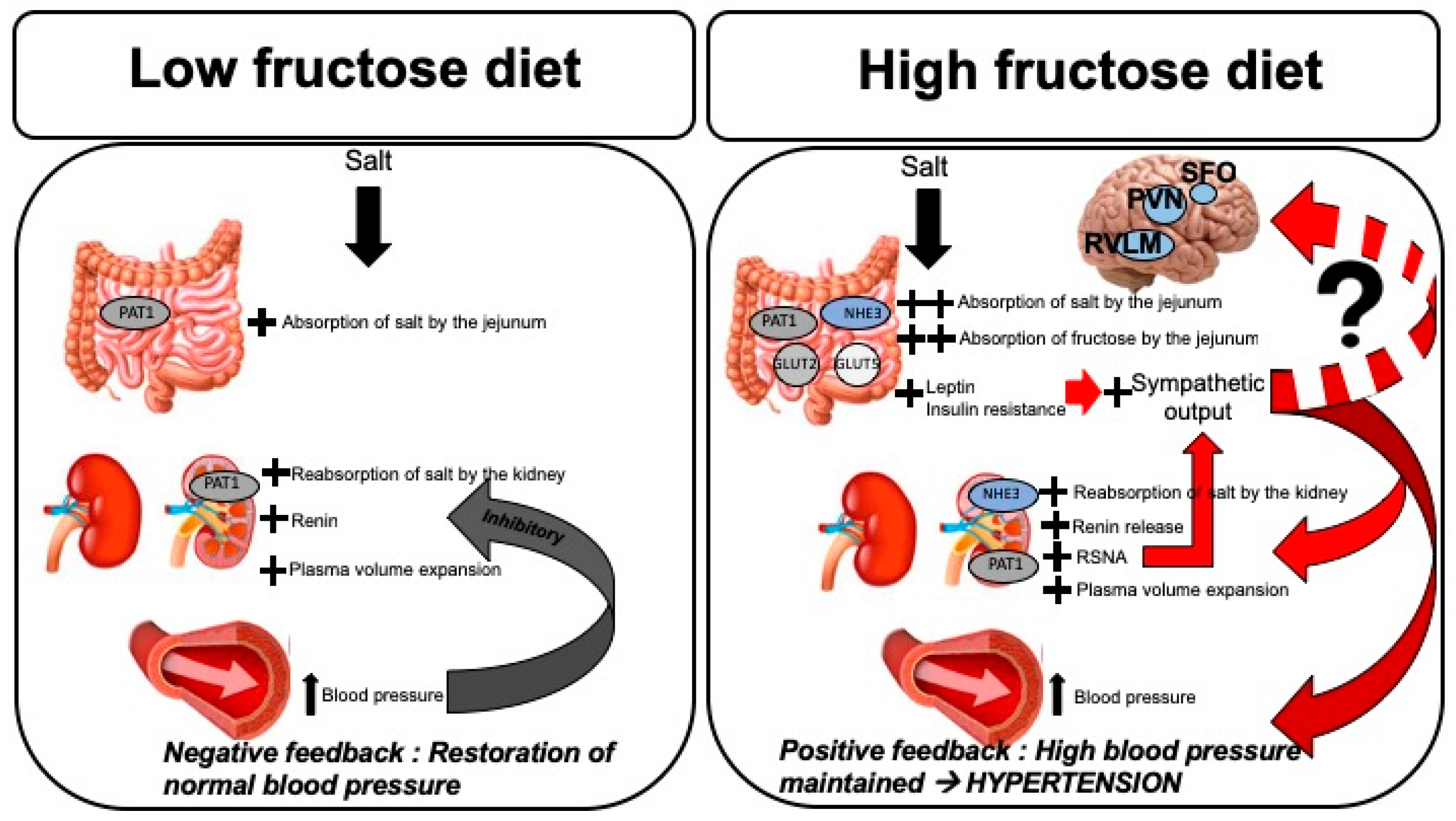
3. Fructose Influences Sodium Handling and Blood Pressure
3.1. Fructose Influences Gastrointestinal Sodium Absorption
3.2. Fructose Influences Renal Sodium Reabsorption and RAS
3.3. Fructose Influences the Renal Sympathetic Nervous System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ang II | angiotensin II |
| AT1 | angiotensin receptor type 1 |
| CARDIA | Coronary Artery Risk Development in Young Adults |
| GLUT | glucose transporter |
| HFCS | high fructose corn syrup |
| NHANES | National Health and Nutrition Examination Survey |
| NHE | sodium hydrogen exchanger |
| NKCC2 | sodium potassium chloride transporter |
| PAT1 | putative anion transporter 1 |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PRA | plasma renin activity |
| PVN | paraventricular nucleus |
| RAS | renin angiotensin system |
| RSNA | renal sympathetic nerve activity |
| RVLM | rostral ventrolateral medulla |
| SFO | subfornical organ. |
References
- Carretero, O.A.; Oparil, S. Essential hypertension. Part I: Definition and etiology. Circulation 2000, 101, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Williams, B.; Ritz, E. Essential hypertension. Lancet 2007, 370, 591–603. [Google Scholar] [CrossRef]
- Egan, B.M.; Zhao, Y.; Axon, R.N. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010, 303, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Reis, J.P.; Colangelo, L.A.; Shimbo, D.; Viera, A.J.; Allen, N.B.; Gidding, S.S.; Bress, A.P.; Greenland, P.; Muntner, P.; et al. Association of Blood Pressure Classification in Young Adults Using the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline with Cardiovascular Events Later in Life. JAMA 2018, 320, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Dorans, K.S.; Mills, K.T.; Liu, Y.; He, J. Trends in Prevalence and Control of Hypertension According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.C.; Wilt, T.J.; Welch, H.G. Impact of diastolic and systolic blood pressure on mortality: Implications for the definition of “normal”. J. Gen. Intern. Med. 2011, 26, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Li, T.; Mokdad, A.H.; Xian, H.; Yan, Y.; Maddukuri, G.; Al-Aly, Z. Changes in the US Burden of Chronic Kidney Disease From 2002 to 2016: An Analysis of the Global Burden of Disease Study. JAMA Netw. Open 2018, 1, e184412. [Google Scholar] [CrossRef] [PubMed]
- Lerman, L.O.; Chade, A.R.; Sica, V.; Napoli, C. Animal models of hypertension: An overview. J. Lab. Clin. Med. 2005, 146, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Warden, C.H.; Fisler, J.S. Comparisons of diets used in animal models of high-fat feeding. Cell Metab. 2008, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Le, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Jiao, R.Q.; Kong, L.D. High Dietary Fructose: Direct or Indirect Dangerous Factors Disturbing Tissue and Organ Functions. Nutrients 2017, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.O.; Kooi, E.R. Enzymatic conversion of d-glucose to d-fructose. Science 1957, 125, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Hanover, L.M.; White, J.S. Manufacturing, composition, and applications of fructose. Am. J. Clin. Nutr. 1993, 58, 724S–732S. [Google Scholar] [CrossRef] [PubMed]
- Marriott, B.P.; Cole, N.; Lee, E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 2009, 139, 1228S–1235S. [Google Scholar] [CrossRef] [PubMed]
- Jayalath, V.H.; Sievenpiper, J.L.; de Souza, R.J.; Ha, V.; Mirrahimi, A.; Santaren, I.D.; Blanco Mejia, S.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Total fructose intake and risk of hypertension: A systematic review and meta-analysis of prospective cohorts. J. Am. Coll. Nutr. 2014, 33, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Jayalath, V.H.; de Souza, R.J.; Ha, V.; Mirrahimi, A.; Blanco-Mejia, S.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; Wolever, T.M.; Beyene, J.; et al. Sugar-sweetened beverage consumption and incident hypertension: A systematic review and meta-analysis of prospective cohorts. Am. J. Clin. Nutr. 2015, 102, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Beunza, J.J.; Martinez-Gonzalez, M.A.; Ebrahim, S.; Bes-Rastrollo, M.; Nunez, J.; Martinez, J.A.; Alonso, A. Sedentary behaviors and the risk of incident hypertension: The SUN Cohort. Am. J. Hypertens. 2007, 20, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Bowman, T.S.; Gaziano, J.M.; Buring, J.E.; Sesso, H.D. A prospective study of cigarette smoking and risk of incident hypertension in women. J. Am. Coll. Cardiol. 2007, 50, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.P.; Stampfer, M.J.; Curhan, G.C. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA 2009, 302, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Manson, J.E.; Buring, J.E.; Sesso, H.D. Meat intake and the risk of hypertension in middle-aged and older women. J. Hypertens. 2008, 26, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.D.; Sims, M.; Higginbotham, J.C.; Crowther, M.R.; Wyatt, S.B.; Musani, S.K.; Payne, T.J.; Fox, E.R.; Parton, J.M. Psychosocial Factors Are Associated with Blood Pressure Progression among African Americans in the Jackson Heart Study. Am. J. Hypertens. 2016, 29, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. The kidney, hypertension, and obesity. Hypertension 2003, 41, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.W.; Kim, J.S.; Andrew, M.E.; Kim, S.J.; Hong, Y.P. Body mass index and blood pressure in Korean men and women: The Korean National Blood Pressure Survey. J. Hypertens. 1994, 12, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.; Frye, R.F.; Rivard, C.J.; Cheng, J.; McFann, K.K.; Segal, M.S.; Johnson, R.J.; Johnson, J.A. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism 2012, 61, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Perez-Pozo, S.E.; Sautin, Y.Y.; Manitius, J.; Sanchez-Lozada, L.G.; Feig, D.I.; Shafiu, M.; Segal, M.; Glassock, R.J.; Shimada, M.; et al. Hypothesis: Could excessive fructose intake and uric acid cause type 2 diabetes? Endocr. Rev. 2009, 30, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.S.; Ho, H.; Hoffman, B.B.; Reaven, G.M. Fructose-induced insulin resistance and hypertension in rats. Hypertension 1987, 10, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Rizza, R.A.; Romero, J.C. High-fructose feeding elicits insulin resistance, hyperinsulinism, and hypertension in normal mongrel dogs. Hypertension 1994, 23, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell. Biochem. 2009, 332, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M.; Dulloo, A.G.; Yepuri, G.; Montani, J.P. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R730–R737. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pozo, S.E.; Schold, J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Johnson, R.J.; Lillo, J.L. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: Role of uric acid in the hypertensive response. Int. J. Obes. (Lond.) 2010, 34, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Mcneill, J.H. Fructose-Induced Hypertension in Rats Is Concentration-Dependent and Duration-Dependent. J. Pharmacol. Toxicol. Methods 1995, 33, 101–107. [Google Scholar] [CrossRef]
- Glushakova, O.; Kosugi, T.; Roncal, C.; Mu, W.; Heinig, M.; Cirillo, P.; Sanchez-Lozada, L.G.; Johnson, R.J.; Nakagawa, T. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J. Am. Soc. Nephrol. 2008, 19, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.R.; Demarco, V.G.; Jia, G.; Sun, Z.; Nistala, R.; Meininger, G.A.; Sowers, J.R. The role of tissue Renin-Angiotensin-aldosterone system in the development of endothelial dysfunction and arterial stiffness. Front. Endocrinol. (Lausanne) 2013, 4, 161. [Google Scholar] [CrossRef] [PubMed]
- Katakam, P.V.; Ujhelyi, M.R.; Hoenig, M.E.; Miller, A.W. Endothelial dysfunction precedes hypertension in diet-induced insulin resistance. Am. J. Physiol. 1998, 275, R788–R792. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Amlal, H.; Haas, P.J.; Dringenberg, U.; Fussell, S.; Barone, S.L.; Engelhardt, R.; Zuo, J.; Seidler, U.; Soleimani, M. Fructose-induced hypertension: Essential role of chloride and fructose absorbing transporters PAT1 and Glut5. Kidney Int. 2008, 74, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Cabral, P.D.; Hong, N.J.; Hye Khan, M.A.; Ortiz, P.A.; Beierwaltes, W.H.; Imig, J.D.; Garvin, J.L. Fructose stimulates Na/H exchange activity and sensitizes the proximal tubule to angiotensin II. Hypertension 2014, 63, e68–e73. [Google Scholar] [CrossRef] [PubMed]
- Gordish, K.L.; Kassem, K.M.; Ortiz, P.A.; Beierwaltes, W.H. Moderate (20%) fructose-enriched diet stimulates salt-sensitive hypertension with increased salt retention and decreased renal nitric oxide. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Soncrant, T.; Komnenov, D.; Beierwaltes, W.H.; Chen, H.; Wu, M.; Rossi, N.F. Bilateral renal cryodenervation decreases arterial pressure and improves insulin sensitivity in fructose-fed Sprague-Dawley rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R529–R538. [Google Scholar] [CrossRef] [PubMed]
- De Wardener, H.E.; He, F.J.; MacGregor, G.A. Plasma sodium and hypertension. Kidney Int. 2004, 66, 2454–2466. [Google Scholar] [CrossRef] [PubMed]
- Haddy, F.J. Role of dietary salt in hypertension. Life Sci. 2006, 79, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Karppanen, H.; Mervaala, E. Sodium intake and hypertension. Prog. Cardiovasc. Dis. 2006, 49, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Meneton, P.; Jeunemaitre, X.; de Wardener, H.E.; MacGregor, G.A. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol. Rev. 2005, 85, 679–715. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, C.I.; Harley, B. Adaptation of glucose transport across rat enterocyte basolateral membrane in response to altered dietary carbohydrate intake. J. Physiol. 1991, 437, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Gould, G.W.; Thomas, H.M.; Jess, T.J.; Bell, G.I. Expression of human glucose transporters in Xenopus oocytes: Kinetic characterization and substrate specificities of the erythrocyte, liver, and brain isoforms. Biochemistry 1991, 30, 5139–5145. [Google Scholar] [CrossRef] [PubMed]
- Kane, S.; Seatter, M.J.; Gould, G.W. Functional studies of human GLUT5: Effect of pH on substrate selection and an analysis of substrate interactions. Biochem. Biophys. Res. Commun. 1997, 238, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Leturque, A.; Brot-Laroche, E.; Le Gall, M.; Stolarczyk, E.; Tobin, V. The role of GLUT2 in dietary sugar handling. J. Physiol. Biochem. 2005, 61, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Petrovic, S.; Mann, E.; Soleimani, M. Identification of an apical Cl(-)/HCO3(-) exchanger in the small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G573–G579. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Alborzi, P. The role of salt in the pathogenesis of fructose-induced hypertension. Int. J. Nephrol. 2011, 2011, 392708. [Google Scholar] [CrossRef] [PubMed]
- Dudeja, P.K.; Rao, D.D.; Syed, I.; Joshi, V.; Dahdal, R.Y.; Gardner, C.; Risk, M.C.; Schmidt, L.; Bavishi, D.; Kim, K.E.; et al. Intestinal distribution of human Na+/H+ exchanger isoforms NHE-1, NHE-2, and NHE-3 mRNA. Am. J. Physiol. 1996, 271, G483–G493. [Google Scholar] [CrossRef] [PubMed]
- Seidler, U.; Rottinghaus, I.; Hillesheim, J.; Chen, M.; Riederer, B.; Krabbenhoft, A.; Engelhardt, R.; Wiemann, M.; Wang, Z.; Barone, S.; et al. Sodium and chloride absorptive defects in the small intestine in Slc26a6 null mice. Pflugers Arch. 2008, 455, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Dantzler, W.H. Comparative Physiology of the Vertebrate Kidney; Springer: Berlin, Germany; New York, NY, USA, 1989; p. x. 198p. [Google Scholar]
- Boron, W.F.; Boulpaep, E.L. Medical Physiology: A Cellular and Molecular Approach, Updated 2nd ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2012; p. xiii. 1337p. [Google Scholar]
- Aldred, K.L.; Harris, P.J.; Eitle, E. Increased proximal tubule NHE-3 and H+-ATPase activities in spontaneously hypertensive rats. J. Hypertens. 2000, 18, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Tank, J.E.; Moe, O.W.; Henrich, W.L. Abnormal regulation of proximal tubule renin mRNA in the Dahl/Rapp salt-sensitive rat. Kidney Int. 1998, 54, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Alpern, R.J.; Cogan, M.G.; Rector, F.C., Jr. Effect of luminal bicarbonate concentration on proximal acidification in the rat. Am. J. Physiol. 1982, 243, F53–F59. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Leite, G.D.; Crajoinas, R.O.; Neri, E.A.; Bezerra, C.N.; Girardi, A.C.; Reboucas, N.A.; Malnic, G. Fructose acutely stimulates NHE3 activity in kidney proximal tubule. Kidney Blood Press. Res. 2012, 36, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vicente, A.; Cabral, P.D.; Hong, N.J.; Asirwatham, J.; Yang, N.; Berthiaume, J.M.; Dominici, F.P.; Garvin, J.L. Dietary Fructose Enhances the Ability of Low Concentrations of Angiotensin II to Stimulate Proximal Tubule Na(+) Reabsorption. Nutrients 2017, 9, 885. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vicente, A.; Hong, N.J.; Yang, N.; Cabral, P.D.; Berthiaume, J.M.; Dominici, F.P.; Garvin, J.L. Dietary Fructose Increases the Sensitivity of Proximal Tubules to Angiotensin II in Rats Fed High-Salt Diets. Nutrients 2018, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hu, X.; Shi, M.; Knepper, M.A.; Ecelbarger, C.A. Effects of dietary fat, NaCl, and fructose on renal sodium and water transporter abundances and systemic blood pressure. Am. J. Physiol. Ren. Physiol. 2004, 287, F1204–F1212. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.R.; Kassem, K.M.; Ortiz, P.A. Fructose acutely stimulates NKCC2 activity in rat thick ascending limbs (TALs) by increasing surface NKCC2 expression. Am. J. Physiol. Ren. Physiol. 2018. [Google Scholar] [CrossRef]
- Xu, C.; Lu, A.; Lu, X.; Zhang, L.; Fang, H.; Zhou, L.; Yang, T. Activation of Renal (Pro)Renin Receptor Contributes to High Fructose-Induced Salt Sensitivity. Hypertension 2017, 69, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Zenner, Z.P.; Gordish, K.L.; Beierwaltes, W.H. Free radical scavenging reverses fructose-induced salt-sensitive hypertension. Integr. Blood Press. Control 2018, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Giacchetti, G.; Sechi, L.A.; Griffin, C.A.; Don, B.R.; Mantero, F.; Schambelan, M. The tissue renin-angiotensin system in rats with fructose-induced hypertension: Overexpression of type 1 angiotensin II receptor in adipose tissue. J. Hypertens. 2000, 18, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Dhar, I.; Dhar, A.; Wu, L.; Desai, K.M. Increased methylglyoxal formation with upregulation of renin angiotensin system in fructose fed Sprague Dawley rats. PLoS ONE 2013, 8, e74212. [Google Scholar] [CrossRef] [PubMed]
- Yokota, R.; Ronchi, F.A.; Fernandes, F.B.; Jara, Z.P.; Rosa, R.M.; Leite, A.P.O.; Fiorino, P.; Farah, V.; do Nascimento, N.R.F.; Fonteles, M.C.; et al. Intra-Renal Angiotensin Levels Are Increased in High-Fructose Fed Rats in the Extracorporeal Renal Perfusion Model. Front. Physiol. 2018, 9, 1433. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.D.; Bohm, M.; Sievert, H.; Rump, C.L.; Schmieder, R.E.; Krum, H.; Mahfoud, F.; Schlaich, M.P. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur. Heart J. 2014, 35, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Krum, H.; Schlaich, M.P.; Sobotka, P.A.; Bohm, M.; Mahfoud, F.; Rocha-Singh, K.; Katholi, R.; Esler, M.D. Percutaneous renal denervation in patients with treatment-resistant hypertension: Final 3-year report of the Symplicity HTN-1 study. Lancet 2014, 383, 622–629. [Google Scholar] [CrossRef]
- Osborn, J.W.; Foss, J.D. Renal Nerves and Long-Term Control of Arterial Pressure. Compr Physiol 2017, 7, 263–320. [Google Scholar] [CrossRef] [PubMed]
- DiBona, G.F. Physiology in perspective: The Wisdom of the Body. Neural control of the kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R633–R641. [Google Scholar] [CrossRef] [PubMed]
- Teff, K.L.; Elliott, S.S.; Tschop, M.; Kieffer, T.J.; Rader, D.; Heiman, M.; Townsend, R.R.; Keim, N.L.; D’Alessio, D.; Havel, P.J. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J. Clin. Endocrinol. Metab. 2004, 89, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Pirzgalska, R.M.; Pereira, M.M.; Kubasova, N.; Barateiro, A.; Seixas, E.; Lu, Y.H.; Kozlova, A.; Voss, H.; Martins, G.G.; et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 2015, 163, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Le, K.A. Does fructose consumption contribute to non-alcoholic fatty liver disease? Clin. Res. Hepatol. Gastroenterol. 2012, 36, 554–560. [Google Scholar] [CrossRef] [PubMed]
- DiBona, G.F.; Kopp, U.C. Neural control of renal function. Physiol. Rev. 1997, 77, 75–197. [Google Scholar] [CrossRef] [PubMed]
- Campese, V.M.; Ye, S.; Zhong, H.; Yanamadala, V.; Ye, Z.; Chiu, J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H695–H703. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komnenov, D.; Levanovich, P.E.; Rossi, N.F. Hypertension Associated with Fructose and High Salt: Renal and Sympathetic Mechanisms. Nutrients 2019, 11, 569. https://doi.org/10.3390/nu11030569
Komnenov D, Levanovich PE, Rossi NF. Hypertension Associated with Fructose and High Salt: Renal and Sympathetic Mechanisms. Nutrients. 2019; 11(3):569. https://doi.org/10.3390/nu11030569
Chicago/Turabian StyleKomnenov, Dragana, Peter E. Levanovich, and Noreen F. Rossi. 2019. "Hypertension Associated with Fructose and High Salt: Renal and Sympathetic Mechanisms" Nutrients 11, no. 3: 569. https://doi.org/10.3390/nu11030569
APA StyleKomnenov, D., Levanovich, P. E., & Rossi, N. F. (2019). Hypertension Associated with Fructose and High Salt: Renal and Sympathetic Mechanisms. Nutrients, 11(3), 569. https://doi.org/10.3390/nu11030569



