Vitamin C Activates Osteoblastogenesis and Inhibits Osteoclastogenesis via Wnt/β-Catenin/ATF4 Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
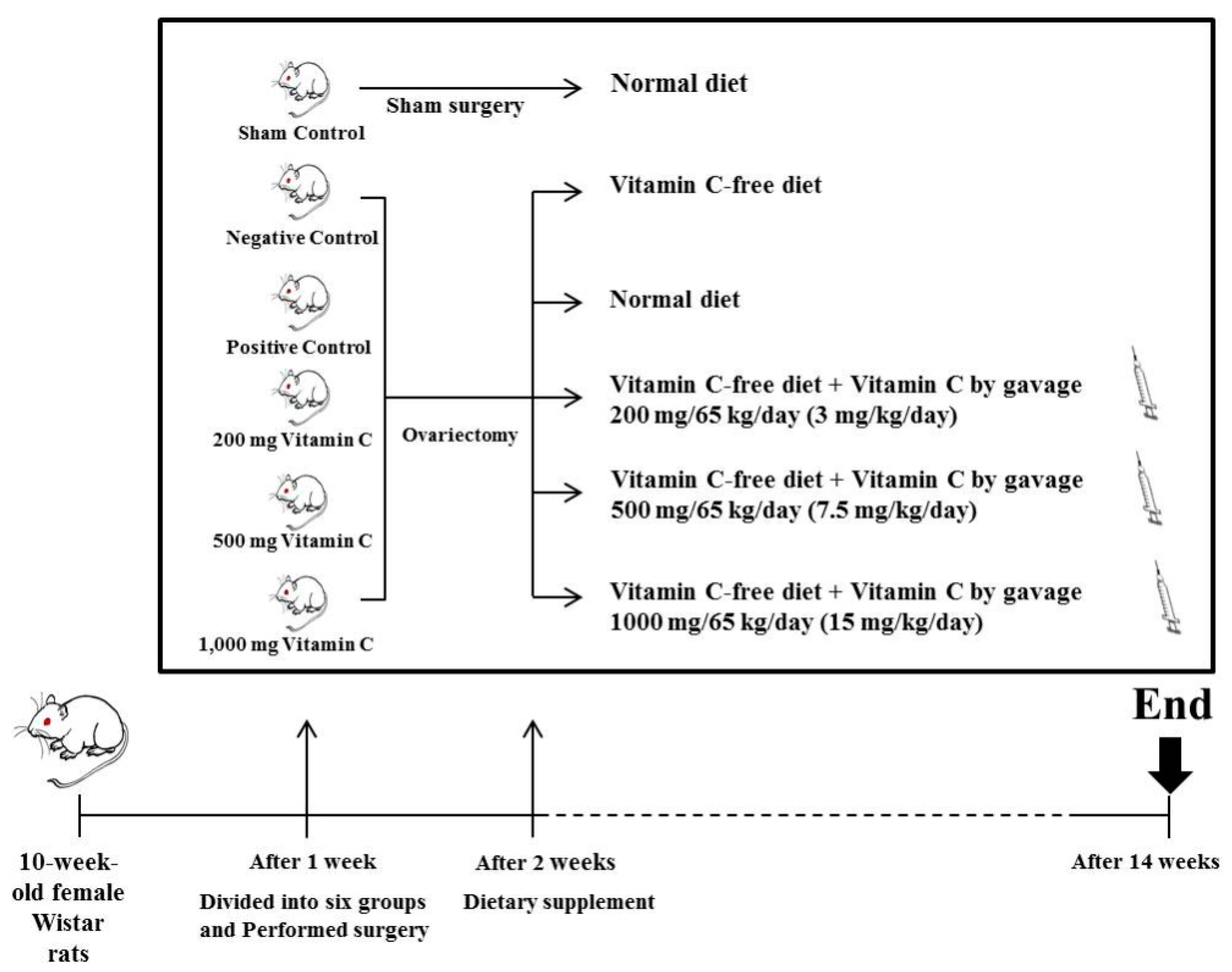
2.1. Animals and Diet
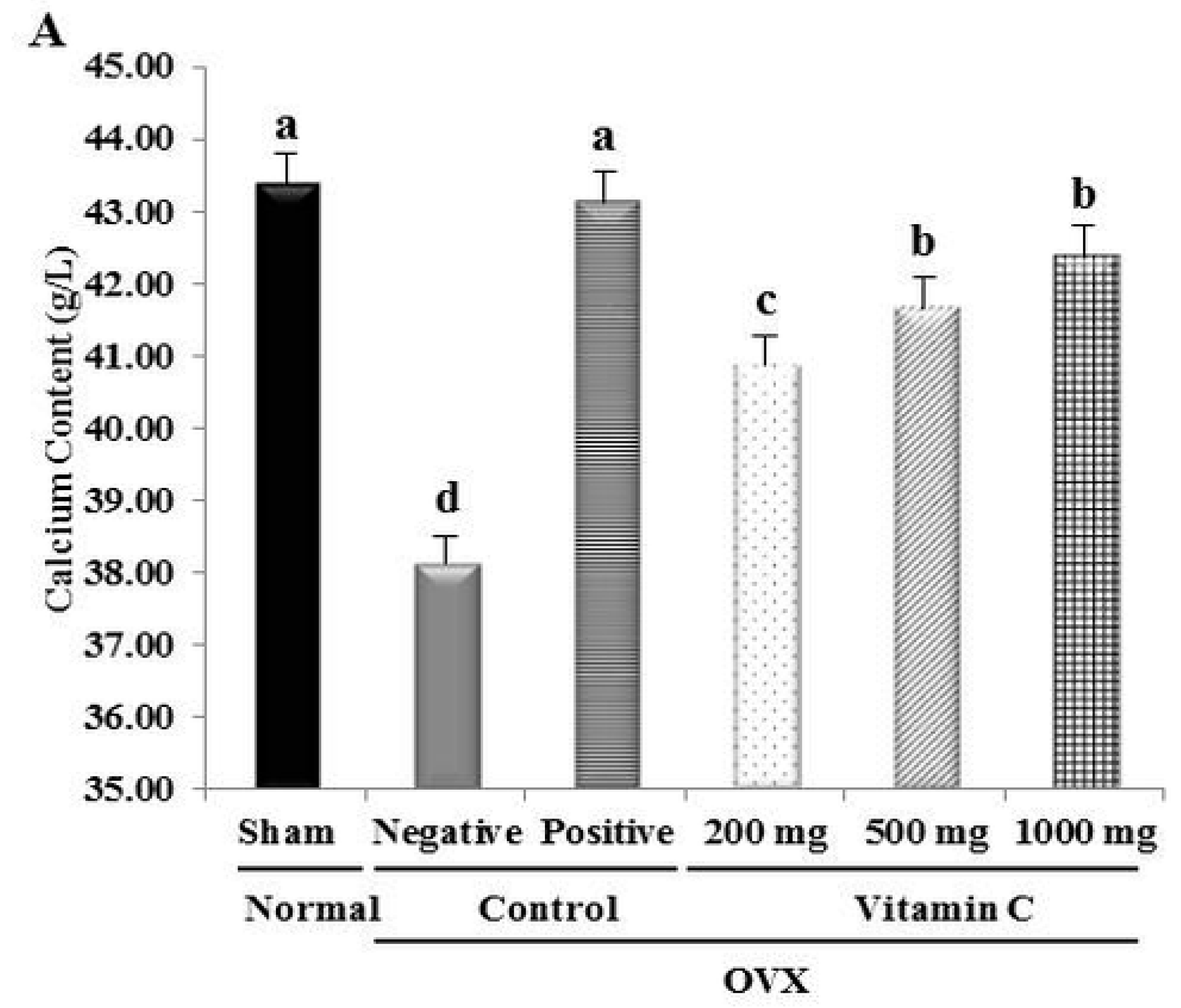
2.2. Tibia Bone Ca2+ Content
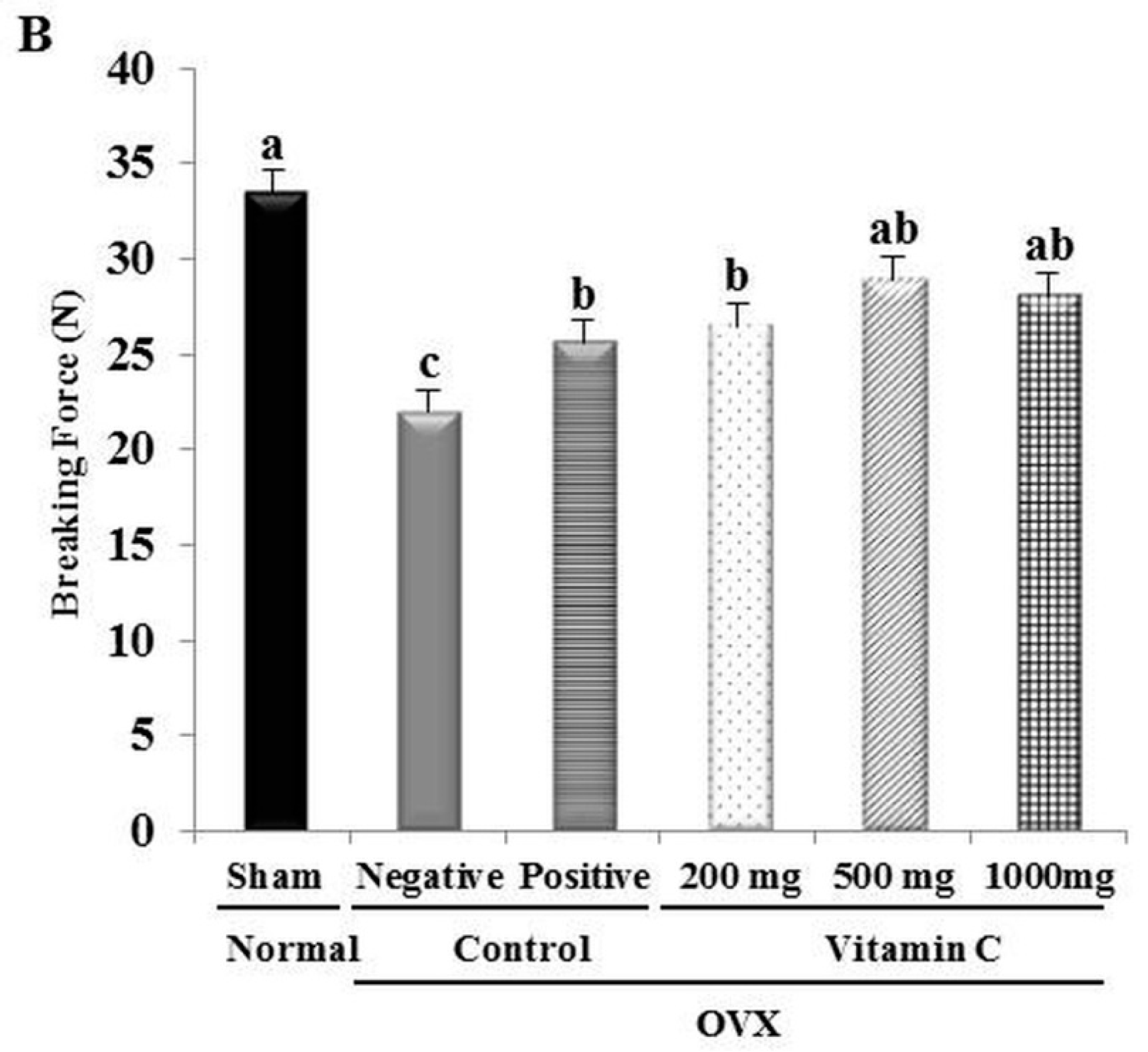
2.3. Determination of Tibial Bone Strength
2.4. Micro-Computed Tomography (Micro-CT) Analysis
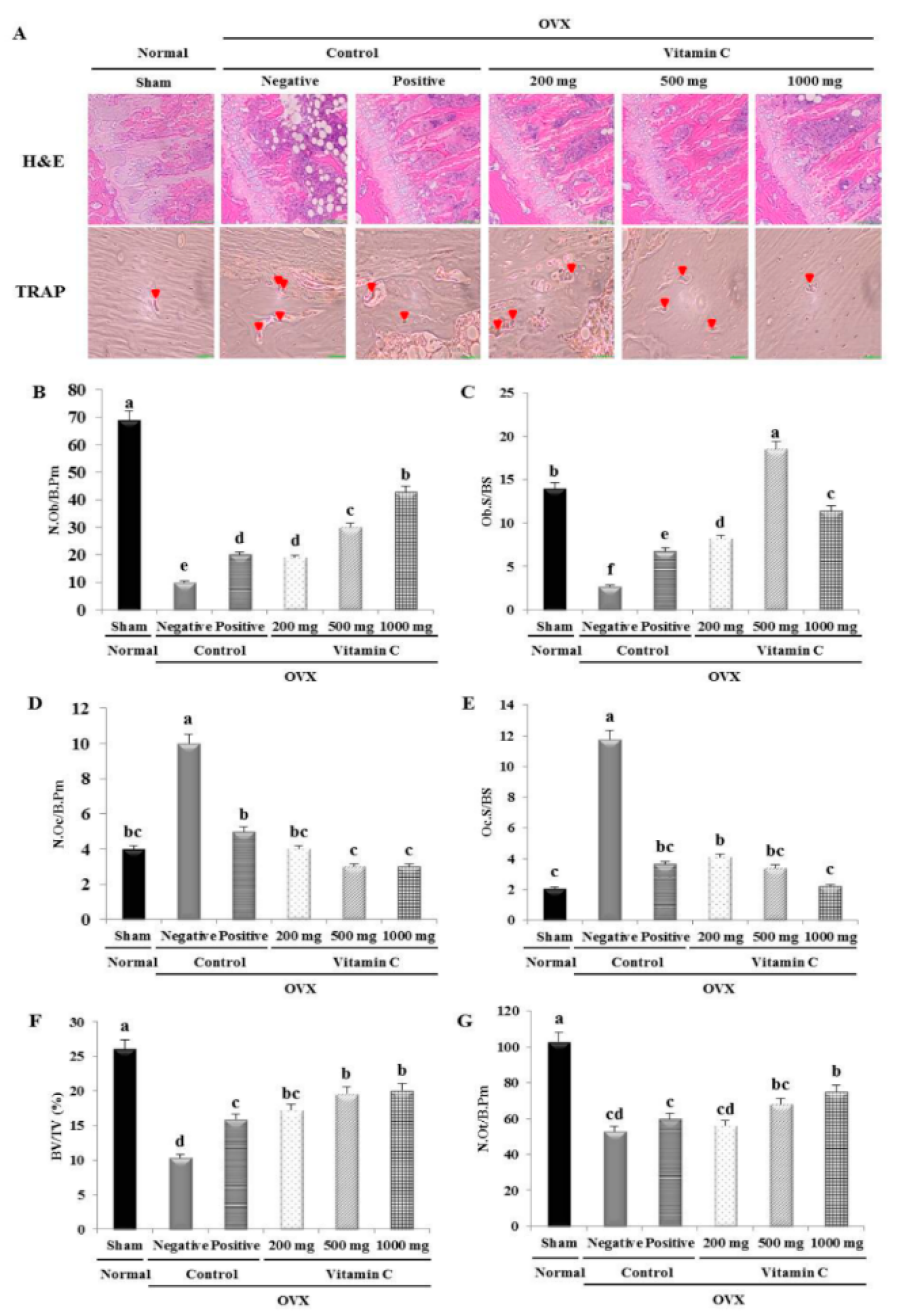
2.5. Histological and Histomorphometrical Analyses of Tibia
2.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Assay
2.7. Western Blotting Analysis
2.8. Statistical Analysis
3. Results
3.1. Food Efficiency Ratio
3.2. Ca2+ Content and Tibial Bone Strength are Increased in OVX Rats Treated with Vitamin C
3.3. Vitamin C Improves Bone Microarchitecture and Bone Formation Parameters, and Suppresses Bone Resorption Parameters
3.4. Vitamin C Enhances Bone Formation Parameters and Suppresses Bone Resorption Parameters
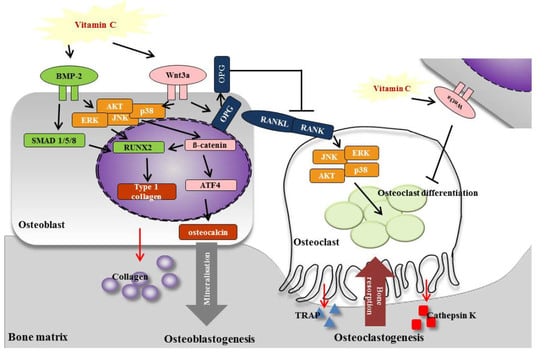
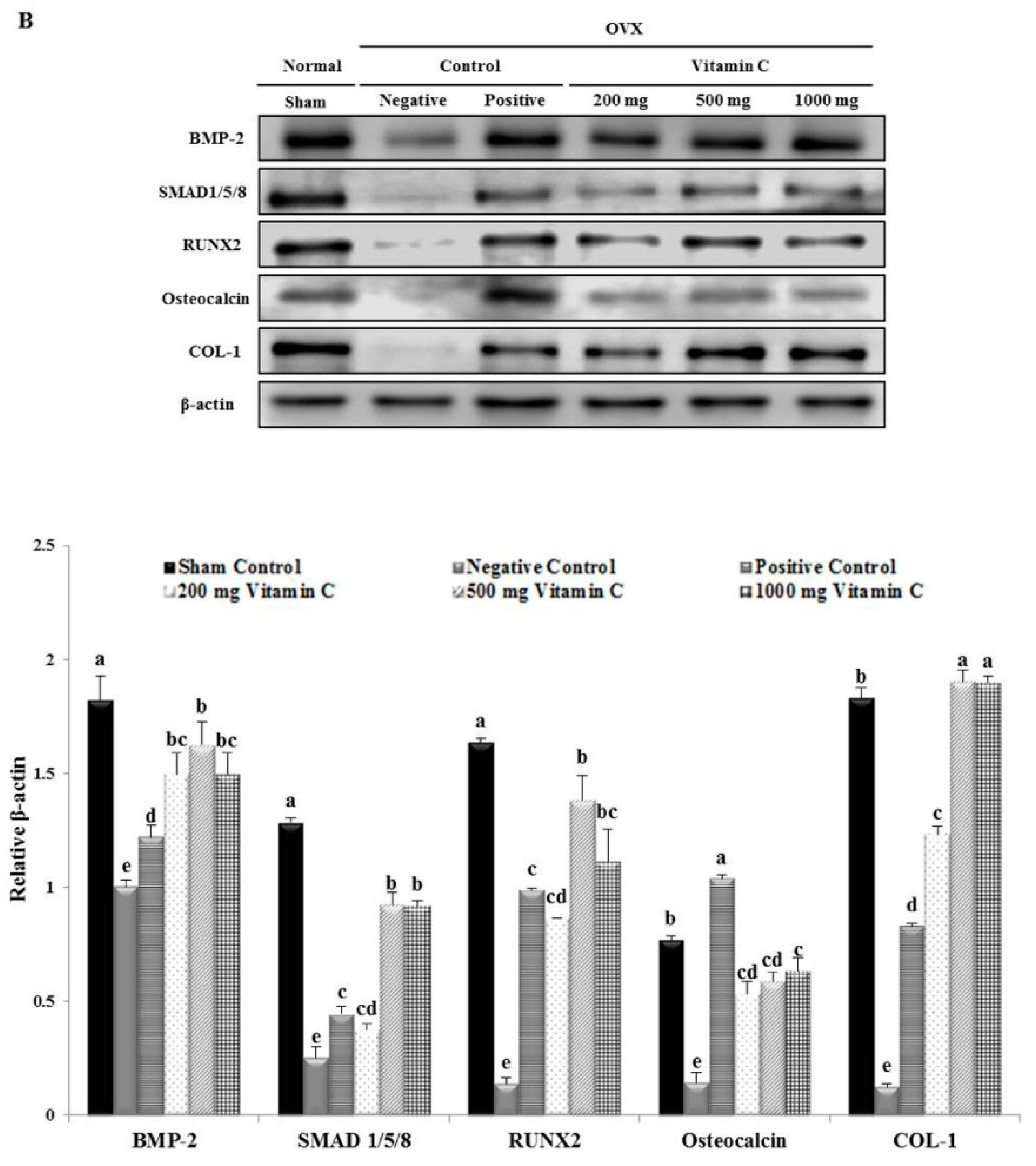
3.5. Vitamin C Promotes Expression of Osteoblastogenesis-Related Factors, Including Those in the BMP-2/SMAD1/5/8/RUNX2 Signaling Pathways
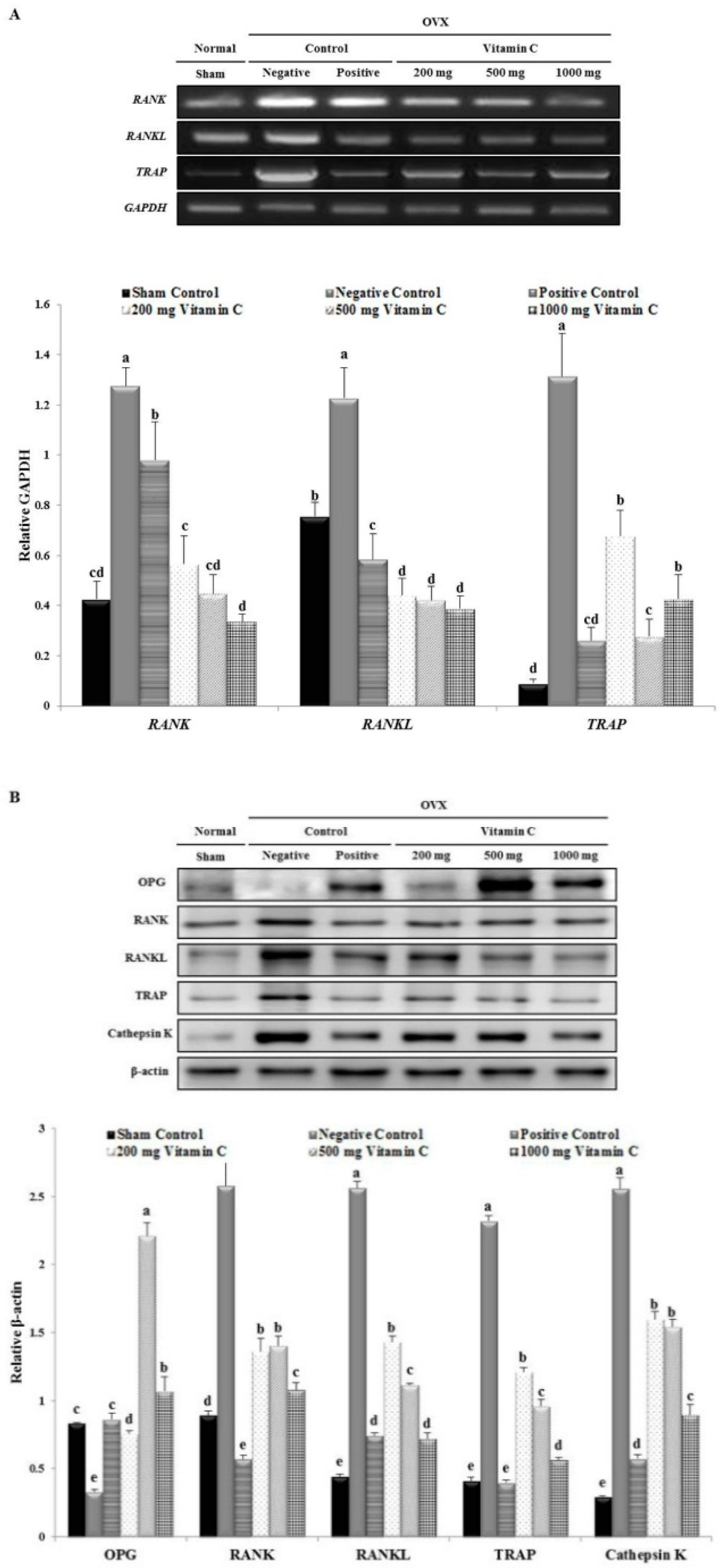
3.6. Vitamin C Inhibits Expression of Osteoclastogenesis-Related Factors, Including RANK and TRAP
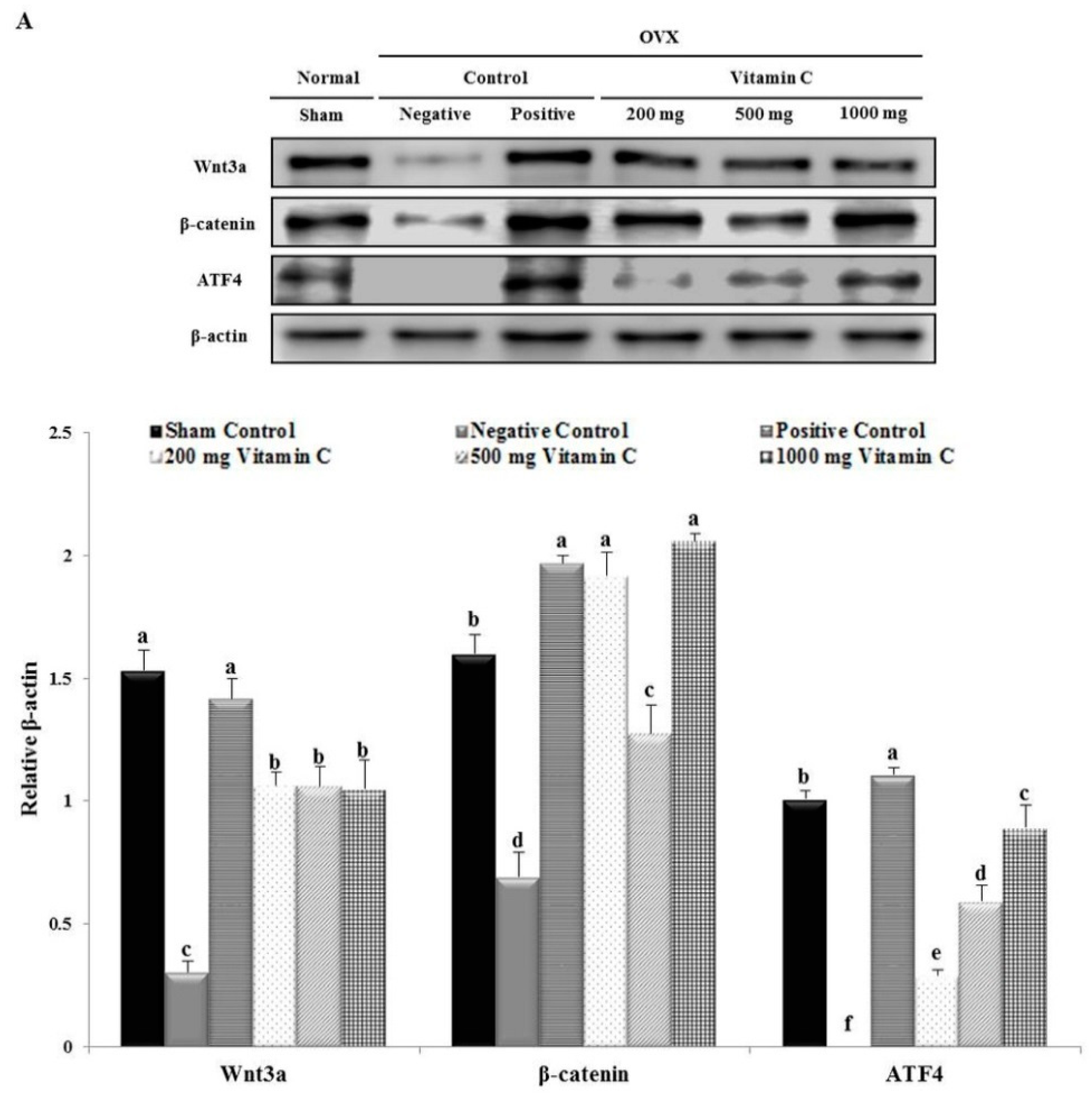
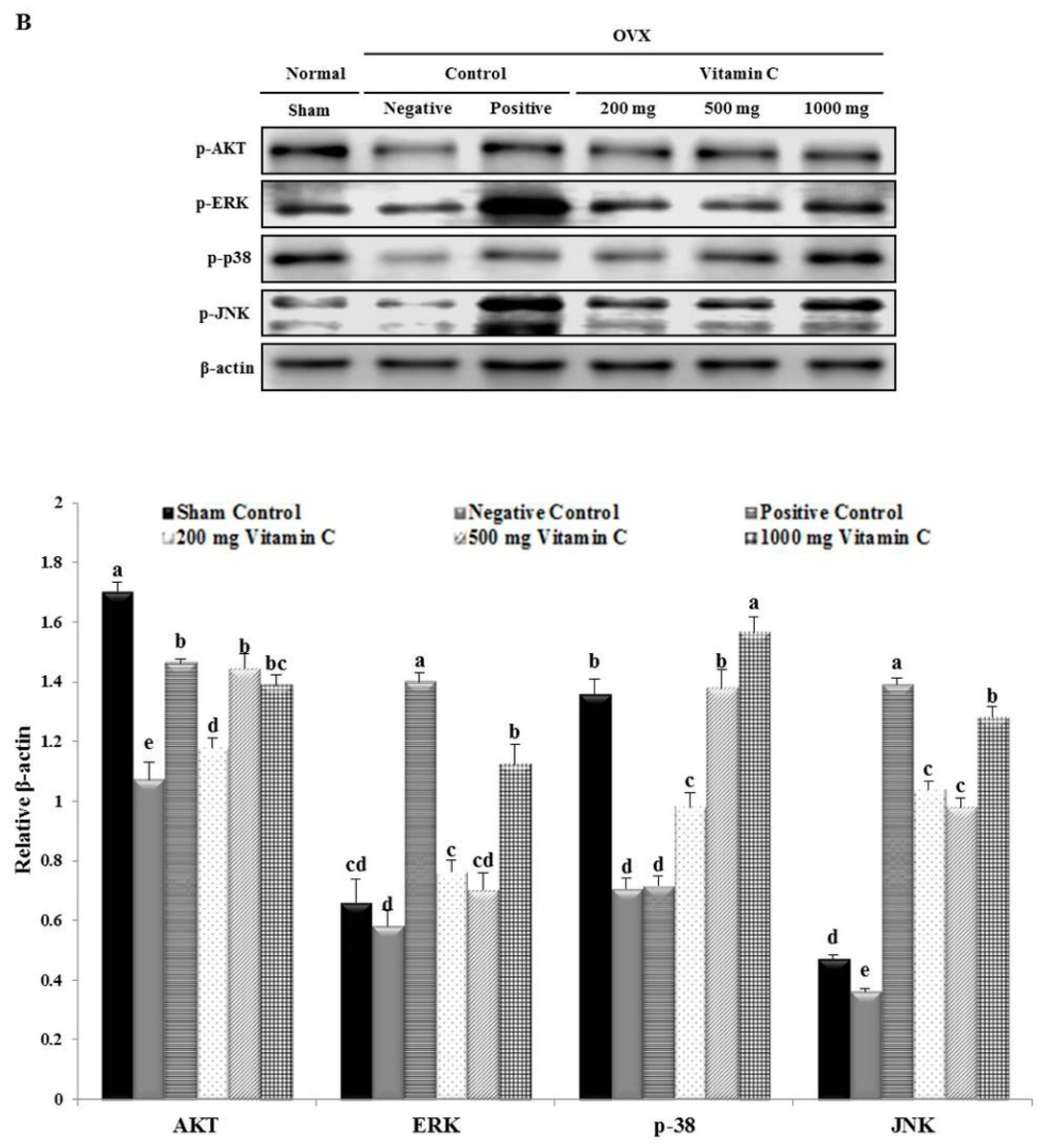
3.7. Vitamin C Regulates Wnt3a/β-catenin, AKT, and MAPK Signaling
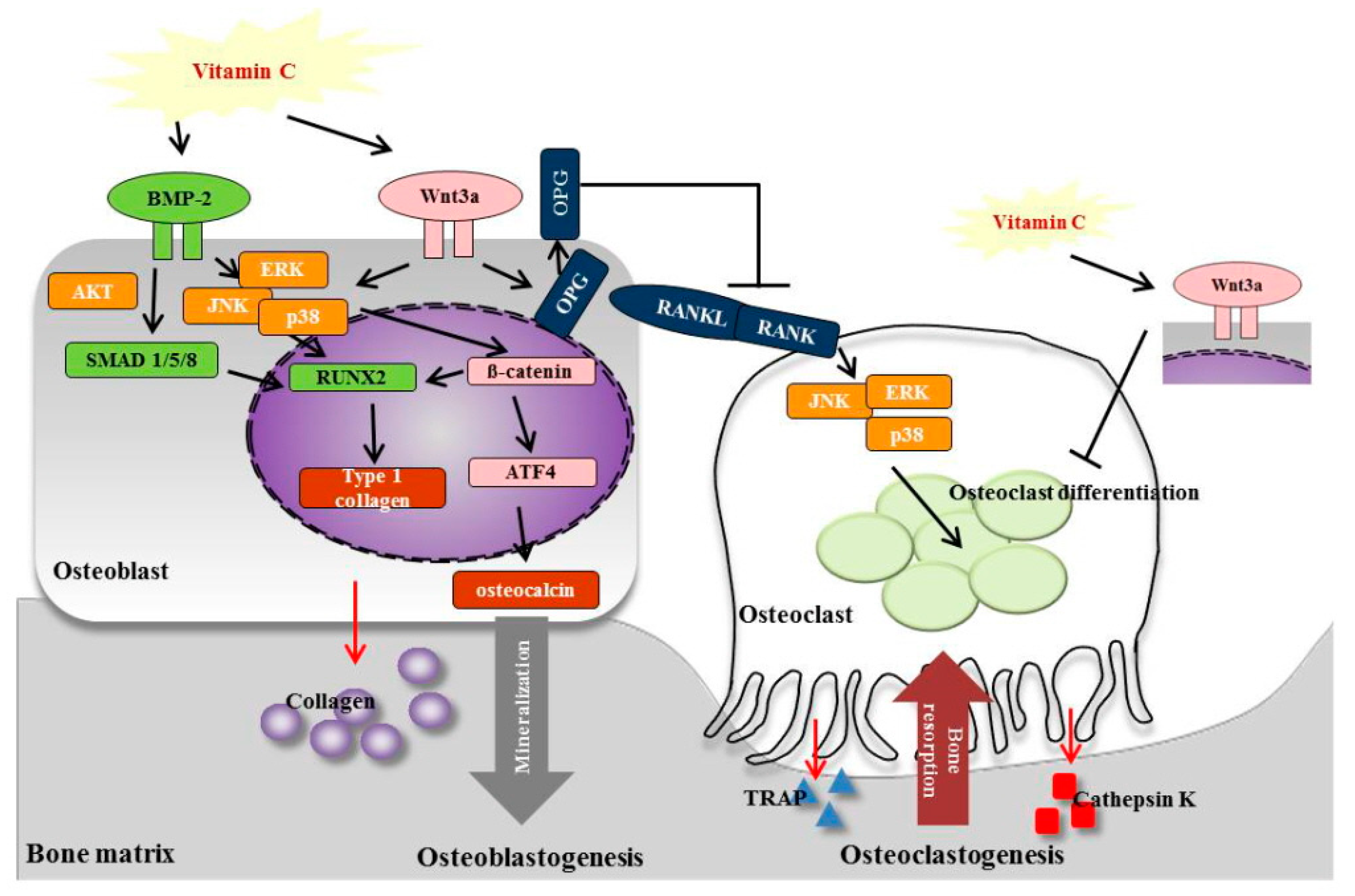
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Klibanski, A.; Adams-Campbell, L.; Bassford, T.; Blair, S.N.; Boden, S.D.; Dickersin, K.; Gifford, D.R.; Glasse, L.; Goldring, S.R.; Hruska, K.; et al. Osteoporosis prevention, diagnosis, and therapy. JAMA J. Am. Med. Assoc. 2001, 285, 785–795. [Google Scholar]
- Nakamura, Y.; Suzuki, T.; Kamimura, M.; Ikegami, S.; Murakami, K.; Uchiyama, S.; Taguchi, A.; Kato, H. Two-year clinical outcome of denosumab treatment alone and in combination with teriparatide in Japanese treatment-naive postmenopausal osteoporotic women. Bone Res. 2017, 5, 16055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett-Connor, E.; Siris, E.S.; Wehern, L.E.; Miller, P.D.; Abbott, T.A.; Berger, M.L.; Santora, A.C.; Sherwood, L.M. Osteoporosis and fracture risk in women of different ethnic groups. J. Bone Miner. Res. 2005, 20, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Oh, H.J.; Kim, D.J.; Lee, Y.; Chung, Y.S. The prevalence of osteoporosis in Korean adults aged 50 years or older and the higher diagnosis rates in women who were beneficiaries of a national screening program: The Korea National Health and Nutrition Examination Survey 2008–2009. J. Bone Miner. Res. 2012, 27, 1879–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.J.; Joo, I.W.; Jang, M.J.; Kim, Y.T.; Oh, K.; Oh, H.J. Prevalence of osteoporosis in the Korean population based on Korea national health and nutrition examination survey (KNHANES), 2008–2011. Yonsei Med. J. 2014, 55, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mukwaya, E.; Pan, H.; Li, X.M.; Yang, J.L.; Ge, J.; Wang, H.Y. Combination therapy of Chinese herbal medicine Fructus Ligustri Lucidi with high calcium diet on calcium imbalance induced by ovariectomy in mice. Pharm. Biol. 2015, 53, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Kim, H.Y.; Yang, W.M.; Jeong, H.J.; Kim, H.M. Glutamic acid ameliorates estrogen deficiency-induced menopausal-like symptoms in ovariectomized mice. Nutr. Res. 2015, 35, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Shab-Bidar, S.; Djafarian, K. Vitamin C intake in relation to bone mineral density and risk of hip fracture and osteoporosis: A systematic review and meta-analysis of observational studies. Br. J. Nutr. 2018, 119, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R. The pecking order of free radicals and antioxidants: Lipid peroxidation, α-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, A.; Fujita, T.; Handa, S.; Shirasawa, T.; Koseki, H.; Kitamura, T.; Enomoto, N.; Sato, N.; Shimosawa, T.; Maruyama, N. Senescence marker protein-30 knockout mouse liver is highly susceptible to tumor necrosis factor-α- and Fas-mediated apoptosis. Am. J. Pathol. 2002, 161, 1273–1281. [Google Scholar] [CrossRef]
- Schwarz, R.I. Procollagen secretion meets the minimum requirements for the rate-controlling step in the ascorbate induction of procollagen synthesis. J. Biol. Chem. 1985, 260, 3045–3049. [Google Scholar] [PubMed]
- Schwarz, R.I.; Kleinman, P.; Owens, N. Ascorbate can act as an inducer of the collagen pathway because most steps are tightly coupled. Ann. N. Y. Acad. Sci. 1987, 498, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.I.; Mandell, R.B.; Bissell, M.J. Ascorbate induction of collagen synthesis as a means for elucidating a mechanism of quantitative control of tissue-specific function. Mol. Cell. Biol. 1981, 1, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.J.; Barrett-Connor, E.L.; Schneider, D.L. Vitamin C supplement use and bone mineral density in postmenopausal women. J. Bone Miner. Res. 2001, 16, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Cao, J.; Sun, M.; Yuen, T.; Zhou, R.; Li, J.; Peng, Y.; Moonga, S.S.; Guo, L.; Mechanick, J.I.; et al. Vitamin C prevents hypogonadal bone loss. PLoS ONE 2012, 7, e47058. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R.T.; Iyer, B.S.; Cui, Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J. Bone Miner. Res. 1994, 9, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R.T.; Iyer, B.S. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J. Bone Miner. Res. 1992, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R.T.; Young, J. Regulation of alkaline phosphatase by 1,25-dihydroxyvitamin D3 and ascorbic acid in bone-derived cells. J. Bone Miner. Res. 1990, 5, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Cui, Y.; Ducy, P.; Karsenty, G.; Franceschi, R.T. Ascorbic acid-dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: Requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol. Endocrinol. 1997, 11, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Hadzir, S.N.; Ibrahim, S.N.; Abdul Wahab, R.M.; Zainol Abidin, I.Z.; Senafi, S.; Ariffin, Z.Z.; Abdul Razak, M.; Zainal Ariffin, S.H. Ascorbic acid induces osteoblast differentiation of human suspension mononuclear cells. Cytotherapy 2014, 16, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, P.; Hall, S.; Wongworawat, W.D.; Mohan, S. The Roles and Mechanisms of Actions of Vitamin C in Bone: New Developments. J. Bone Miner. Res. 2015, 30, 1945–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.K.; Lee, E.M.; Kim, A.Y.; Lee, E.J.; Min, C.W.; Kang, K.K.; Lee, M.M.; Jeong, K.S. Vitamin C deficiency accelerates bone loss inducing an increase in PPAR-γ expression in SMP30 knockout mice. Int. J. Exp. Pathol. 2012, 93, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Nong, M.N.; Zhao, J.M.; Peng, X.M.; Zong, S.H.; Zeng, G.F. Polygonatum sibiricum polysaccharide inhibits osteoporosis by promoting osteoblast formation and blocking osteoclastogenesis through Wnt/β-catenin signalling pathway. Sci. Rep. 2016, 6, 32261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Zhu, K.; Lai, Y.; Zhao, Z.; Fan, J.; Im, H.J.; Chen, D.; Xiao, G. ATF4 promotes β-catenin expression and osteoblastic differentiation of bone marrow mesenchymal stem cells. Int. J. Biol. Sci. 2013, 9, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Jiang, D.; Ge, C.; Zhao, Z.; Lai, Y.; Boules, H.; Phimphilai, M.; Yang, X.; Karsenty, G.; Franceschi, R.T. Cooperative Interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J. Biol. Chem. 2005, 280, 30689–30696. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Karsenty, G. ATF4, the osteoblast accumulation of which is determined post-translationally, can induce osteoblast-specific gene expression in non-osteoblastic cells. J. Biol. Chem. 2004, 279, 47109–47114. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yu, S.; Yao, Z.; Galson, D.L.; Jiang, Y.; Zhang, X.; Fan, J.; Lu, B.; Guan, Y.; Luo, M.; et al. Activating transcription factor 4 regulates osteoclast differentiation in mice. J. Clin. Investig. 2010, 120, 2755–2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R.; National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordin, B.E.; Horsman, A.; Marshall, D.H.; Simpson, M.; Waterhouse, G.M. Calcium requirement and calcium therapy. Clin. Orthop. Relat. Res. 1979, 140, 216–239. [Google Scholar] [CrossRef]
- Genant, H.K.; Cann, C.E.; Ettinger, B.; Gordan, G.S. Quantitative computed tomography of vertebral spongiosa: A sensitive method for detecting early bone loss after oophorectomy. Ann. Intern. Med. 1982, 97, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.F.; Ke, H.Z.; Jee, W.S. Prostaglandin E2 adds bone to a cancellous bone site with a closed growth plate and low bone turnover in ovariectomized rats. Bone 1994, 15, 137–146. [Google Scholar] [CrossRef]
- Lynch, S.R.; Berelowitz, I.; Seftel, H.C.; Miller, G.B.; Krawitz, P.; Charlton, R.W.; Bothwell, T.H. Osteoporosis in Johannesburg Bantu Males: Its Relationship to Siderosis and Ascorbic Acid Deficiency. Am. J. Clin. Nutr. 1967, 20, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Hasling, C.; Søndergaard, K.; Charles, P.; Mosekilde, L. Calcium metabolism in postmenopausal osteoporotic women is determined by dietary calcium and coffee intake. J. Nutr. 1992, 122, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Iki, M.; Akiba, T.; Matsumoto, T.; Nishino, H.; Kagamimori, S.; Kagawa, Y.; Yoneshima, H.; JPOS Study Group. Reference database of biochemical markers of bone turnover for the Japanese female population. Japanese Population-based Osteoporosis (JPOS) Study. Osteoporos. Int. 2004, 15, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Kuhlmann, M.K.; Seibert, E.; Kotanko, P.; Levin, N.W.; Handelman, G.J. Vitamin C deficiency and secondary hyperparathyroidism in chronic haemodialysis patients. Nephrol. Dial. Transplant. 2008, 23, 2058–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Wang, X.F.; Li, D.Y.; Chen, Y.C.; Zhao, L.J.; Liu, X.G.; Guo, Y.F.; Shen, J.; Lin, X.; Deng, J.; et al. The good, the bad, and the ugly of calcium supplementation: A review of calcium intake on human health. Clin. Interv. Aging 2018, 28, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Munger, R.G.; West, N.A.; Cutler, D.R.; Wengreen, H.J.; Corcoran, C.D. Antioxidant intake and risk of osteoporotic hip fracture in Utah: An effect modified by smoking status. Am. J. Epidemiol. 2006, 163, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, W.; Liu, Y.S.; Zhou, Y.S. Bone micro-architectural analysis of mandible and tibia in ovariectomised rats: A quantitative structural comparison between undecalcified histological sections and micro-CT. Bone Jt. Res. 2016, 5, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.Z.; Foley, G.L.; Simmons, H.A.; Shen, V.; Thompson, D.D. Long-term treatment of lasofoxifene preserves bone mass and bone strength and does not adversely affect the uterus in ovariectomized rats. Endocrinology 2004, 145, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.G.; Zhang, P.; Wu, Y.Q.; Ma, X.H.; Pang, J.; Jiang, L.Y.; Fang, B. Ovariectomy induces osteoporosis in the maxillary alveolar bone: An in vivo micro-CT and histomorphometric analysis in rats. Oral Dis. 2014, 20, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchia, A.; Sakamotoa, K.; Minamizatoab, T.; Katsubea, K.; Nakanishia, S. Regulation of osteoblast differentiation mediated by BMP, Notch, and CCN3/NOV. Jpn. Dent. Sci. Rev. 2008, 44, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, H.J.; Li, Q.L.; Chi, X.Z.; Ueta, C.; Komori, T.; Wozney, J.M.; Kim, E.G.; Choi, J.Y.; Ryoo, H.M.; et al. Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell. Biol. 2000, 20, 8783–8792. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, Y.J.; Kim, H.J.; Park, H.D.; Kang, A.R.; Kyung, H.M.; Sung, J.H.; Wozney, J.M.; Kim, H.J.; Ryoo, H.M. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGFβ1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J. Biol. Chem. 2003, 278, 34387–34394. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.; Beddington, R.S.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Lemonnier, J.; Ghayor, C.; Guicheux, J.; Caverzasio, J. Protein kinase C-independent activation of protein kinase D is involved in BMP-2-induced activation of stress mitogen-activated protein kinases JNK and p38 and osteoblastic cell differentiation. J. Biol. Chem. 2004, 279, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.T.; Zanatta, M.; Donatelli, L.; Viviano, G.; Cavallini, C.; Scupoli, M.T.; Dalle Carbonare, L. Ascorbic acid induces either differentiation or apoptosis in MG-63 osteosarcoma lineage. Anticancer Res. 2014, 34, 1617–1627. [Google Scholar] [PubMed]
- Koide, M.; Kobayashi, Y.; Yamashita, T.; Uehara, S.; Nakamura, M.; Hiraoka, B.Y.; Ozaki, Y.; Iimura, T.; Yasuda, H.; Takahashi, N.; et al. Bone Formation Is Coupled to Resorption Via Suppression of Sclerostin Expression by Osteoclasts. J. Bone Miner. Res. 2017, 32, 2074–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saftig, P.; Hunziker, E.; Wehmeyer, O.; Jones, S.; Boyde, A.; Rommerskirch, W.; Moritz, J.D.; Schu, P.; von Figura, K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 13453–13458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, H.S.; Kim, G.J.; Song, D.H.; Chung, K.H.; Lee, K.J.; Kim, D.H.; An, J.H. Calcium supplement derived from Gallus gallus domesticus promotes BMP-2/RUNX2/SMAD5 and suppresses TRAP/RANK expression through MAPK signaling activation. Nutrients 2017, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, E.Y.; Lee, B.; Min, J.H.; Song, D.U.; Lim, J.M.; Eom, J.W.; Yeom, M.; Jung, H.S.; Sohn, Y. The effects of Lycii Radicis Cortex on RANKL-induced osteoclast differentiation and activation in RAW 264.7 cells. Int. J. Mol. Med. 2016, 37, 649–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhao, L.; Ma, Q.; Wang, Q.; Chu, P.K.; Zhang, Y. The role of the Wnt/β-catenin pathway in the effect of implant topography on MG63 differentiation. Biomaterials 2012, 33, 7993–8002. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Uehara, S.; Koide, M.; Takahashi, N. The regulation of osteoclast differentiation by Wnt signals. Bonekey Rep. 2015, 4, 713. [Google Scholar] [CrossRef] [PubMed]
- Ghosh-Choudhury, N.; Abboud, S.L.; Nishimura, R.; Celeste, A.; Mahimainathan, L.; Choudhury, G.G. Requirement of BMP-2-induced Phosphatidylinositol 3-Kinase and Akt Serine/Threonine Kinase in Osteoblast Differentiation and Smad-dependent BMP-2 Gene Transcription. J. Biol. Chem. 2002, 277, 3361–3368. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.L.; Yuan, Y.; Tu, J.; Zou, G.M.; Li, Q. Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis. 2014, 17, e1187. [Google Scholar] [CrossRef] [PubMed]
- Bokui, N.; Otani, T.; Igarashi, K.; Kaku, J.; Oda, M.; Nagaoka, T.; Seno, M.; Tatematsu, K.; Okajima, T.; Matsuzaki, T.; et al. Involvement of MAPK signaling molecules and Runx2 in the NELL1-induced osteoblastic differentiation. FEBS Lett. 2008, 582, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Bikkavilli, R.K.; Feigin, M.E.; Malbon, C.C. G alpha o mediates WNT-JNK signaling through dishevelled 1 and 3, RhoA family members, and MEKK 1 and 4 in mammalian cells. J. Cell Sci. 2008, 15, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Caverzasio, J.; Manen, D. Essential role of Wnt3a-mediated activation of mitogen-activated protein kinase p38 for the stimulation of alkaline phosphatase activity and matrix mineralization in C3H10T1/2 mesenchymal cells. Endocrinology 2007, 148, 5323–5330. [Google Scholar] [CrossRef] [PubMed]
- Hildesheim, J.; Salvador, J.M.; Hollander, M.C.; Fornace, A.J., Jr. Casein kinase 2- and protein kinase A-regulated adenomatous polyposis coli and beta-catenin cellular localization is dependent on p38 MAPK. J. Biol. Chem. 2005, 280, 17221–17226. [Google Scholar] [CrossRef] [PubMed]
- Keren, A.; Bengal, E.; Frank, D. p38 MAP kinase regulates the expression of XMyf5 and affects distinct myogenic programs during Xenopus development. Dev. Biol. 2005, 288, 73–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikkavilli, R.K.; Feigin, M.E.; Malbon, C.C. p38 mitogen-activated protein kinase regulates canonical Wnt-β-catenin signaling by inactivation of GSK3β. J. Cell Sci. 2008, 121, 3598–3607. [Google Scholar] [CrossRef] [PubMed]
- Thornton, T.M.; Pedraza-Alva, G.; Deng, B.; Wood, C.D.; Aronshtam, A.; Clements, J.L.; Sabio, G.; Davis, R.J.; Matthews, D.E.; Doble, B.; et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science 2008, 320, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wanachewin, O.; Boonmaleerat, K.; Pothacharoen, P.; Reutrakul, V.; Kongtawelert, P. Sesamin stimulates osteoblast differentiation through p38 and ERK1/2 MAPK signaling pathways. BMC Complement. Altern. Med. 2012, 12, 71. [Google Scholar] [CrossRef]


| OVX | ||||||
|---|---|---|---|---|---|---|
| Normal | Control | Vitamin C | ||||
| Sham * | Negative ** | Positive * | 200 mg ** | 500 mg ** | 1000 mg ** | |
| Food intake (g/day) | 25.48 ± 3.13 a | 23.47 ± 3.04 a | 26.36 ± 4.43 a | 22.14 ± 4.93 a | 24.05 ± 2.44 a | 25.28 ± 4.68 a |
| Initial body weight (g) | 229.48 ± 14.28 a | 223.34 ± 13.27 a | 234.54 ± 14.46 a | 224.47 ± 14.63 a | 233.81 ± 15.35 a | 227.05 ± 11.48 a |
| Final body weight (g) | 302.58 ± 24.61 a | 311.99 ± 22.91 a | 314.09 ± 20.88 a | 336.39 ± 30.46 a | 328.96 ± 34.36 a | 327.12 ± 20.18 a |
| Body weight gain (g/week) | 6.09 ± 7.15 a | 7.38 ± 2.29 a | 6.62 ± 1.66 a | 9.18 ± 2.39 a | 7.92 ± 2.96 a | 8.33 ± 1.36 a |
| FER a | 0.039 ± 0.016 a | 0.049 ± 0.015 a | 0.039 ± 0.009 a | 0.053 ± 0.014 a | 0.051 ± 0.019 a | 0.053 ± 0.008 a |
| Composition | OVX | |||||
|---|---|---|---|---|---|---|
| Normal | Control | Vitamin C | ||||
| Sham * | Negative ** | Positive * | 200 mg ** | 500 mg ** | 1000 mg ** | |
| Casein (g/kg) | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 |
| L-Cystine (g/kg) | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Sucrose (g/kg) | 334.288 | 334.288 | 334.288 | 334.288 | 334.288 | 334.288 |
| Corn Starch (g/kg) | 313.0 | 313.0 | 313.0 | 313.0 | 313.0 | 313.0 |
| Soybean Oil (g/kg) | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 |
| Cellulose (g/kg) | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| Mineral Mix (g/kg) a | 13.37 | 13.37 | 13.37 | 13.37 | 13.37 | 13.37 |
| Potassium Phosphate, Monobasic (g/kg) | 11.43 | 11.43 | 11.43 | 11.43 | 11.43 | 11.43 |
| Calcium Carbonate (g/kg) | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Vitamin Mix (g/kg) b | 10.0 | 10.0 vitamin C - free | 10.0 | 10.0 vitamin C - free | 10.0 vitamin C - free | 10.0 vitamin C - free |
| Ethoxyquin, Antioxidant | 0.012 | 0.012 | 0.012 | 0.012 | 0.012 | 0.012 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.K.; Kim, G.-J.; Yoo, H.-S.; Song, D.H.; Chung, K.-H.; Lee, K.-J.; Koo, Y.T.; An, J.H. Vitamin C Activates Osteoblastogenesis and Inhibits Osteoclastogenesis via Wnt/β-Catenin/ATF4 Signaling Pathways. Nutrients 2019, 11, 506. https://doi.org/10.3390/nu11030506
Choi HK, Kim G-J, Yoo H-S, Song DH, Chung K-H, Lee K-J, Koo YT, An JH. Vitamin C Activates Osteoblastogenesis and Inhibits Osteoclastogenesis via Wnt/β-Catenin/ATF4 Signaling Pathways. Nutrients. 2019; 11(3):506. https://doi.org/10.3390/nu11030506
Chicago/Turabian StyleChoi, Hyeon Kyeong, Gyeong-Ji Kim, Han-Seok Yoo, Da Hye Song, Kang-Hyun Chung, Kwon-Jai Lee, Young Tae Koo, and Jeung Hee An. 2019. "Vitamin C Activates Osteoblastogenesis and Inhibits Osteoclastogenesis via Wnt/β-Catenin/ATF4 Signaling Pathways" Nutrients 11, no. 3: 506. https://doi.org/10.3390/nu11030506
APA StyleChoi, H. K., Kim, G.-J., Yoo, H.-S., Song, D. H., Chung, K.-H., Lee, K.-J., Koo, Y. T., & An, J. H. (2019). Vitamin C Activates Osteoblastogenesis and Inhibits Osteoclastogenesis via Wnt/β-Catenin/ATF4 Signaling Pathways. Nutrients, 11(3), 506. https://doi.org/10.3390/nu11030506