Does Dietary-Induced Obesity in Old Age Impair the Contractile Performance of Isolated Mouse Soleus, Extensor Digitorum Longus and Diaphragm Skeletal Muscles?
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Muscle Preparation
2.3. Assessment of Contractile Performance
2.4. Muscle Morphology and Calculations
2.5. Statistical Analysis of Data
3. Results
3.1. Morphology
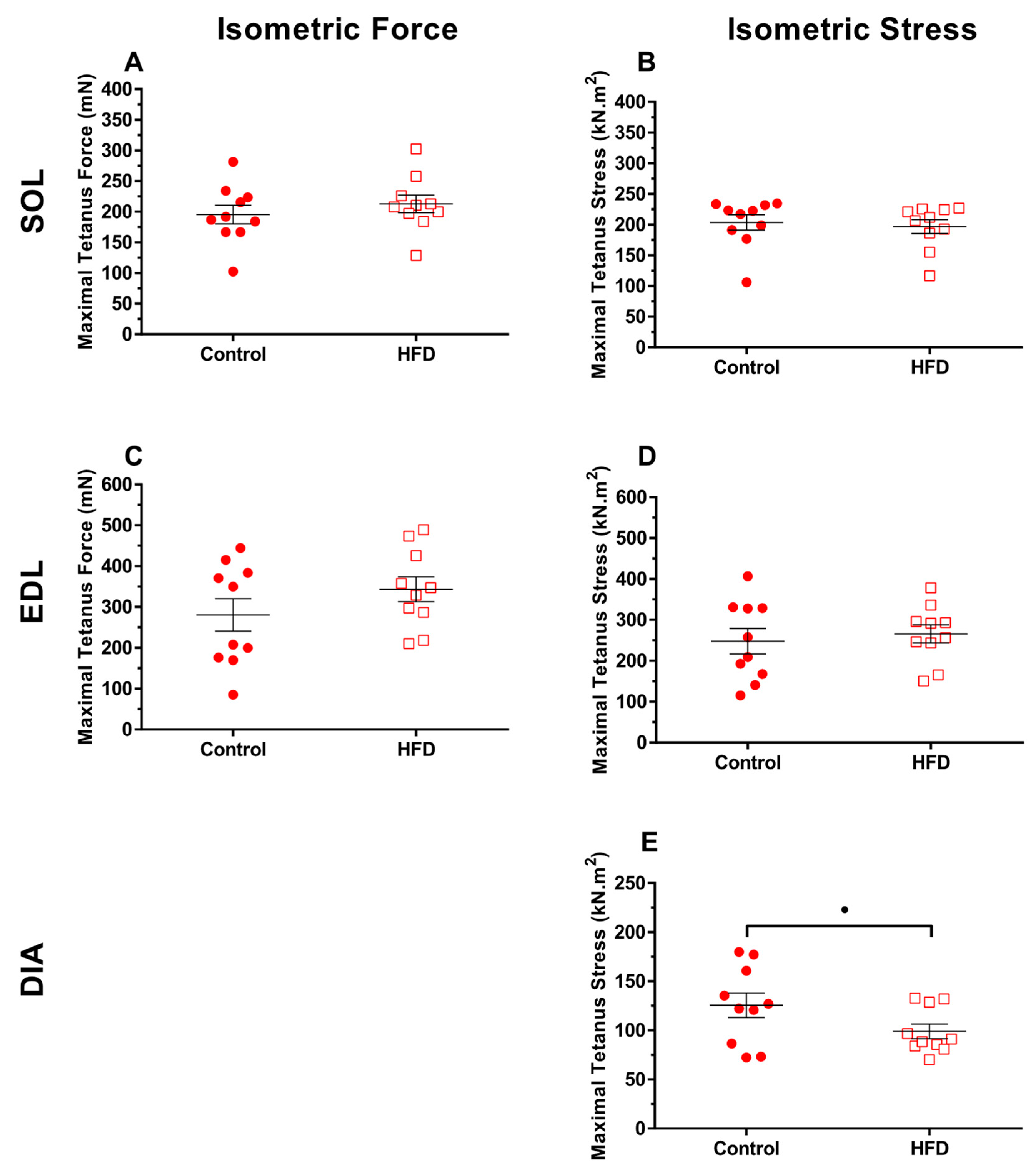
3.2. Isometric Properties
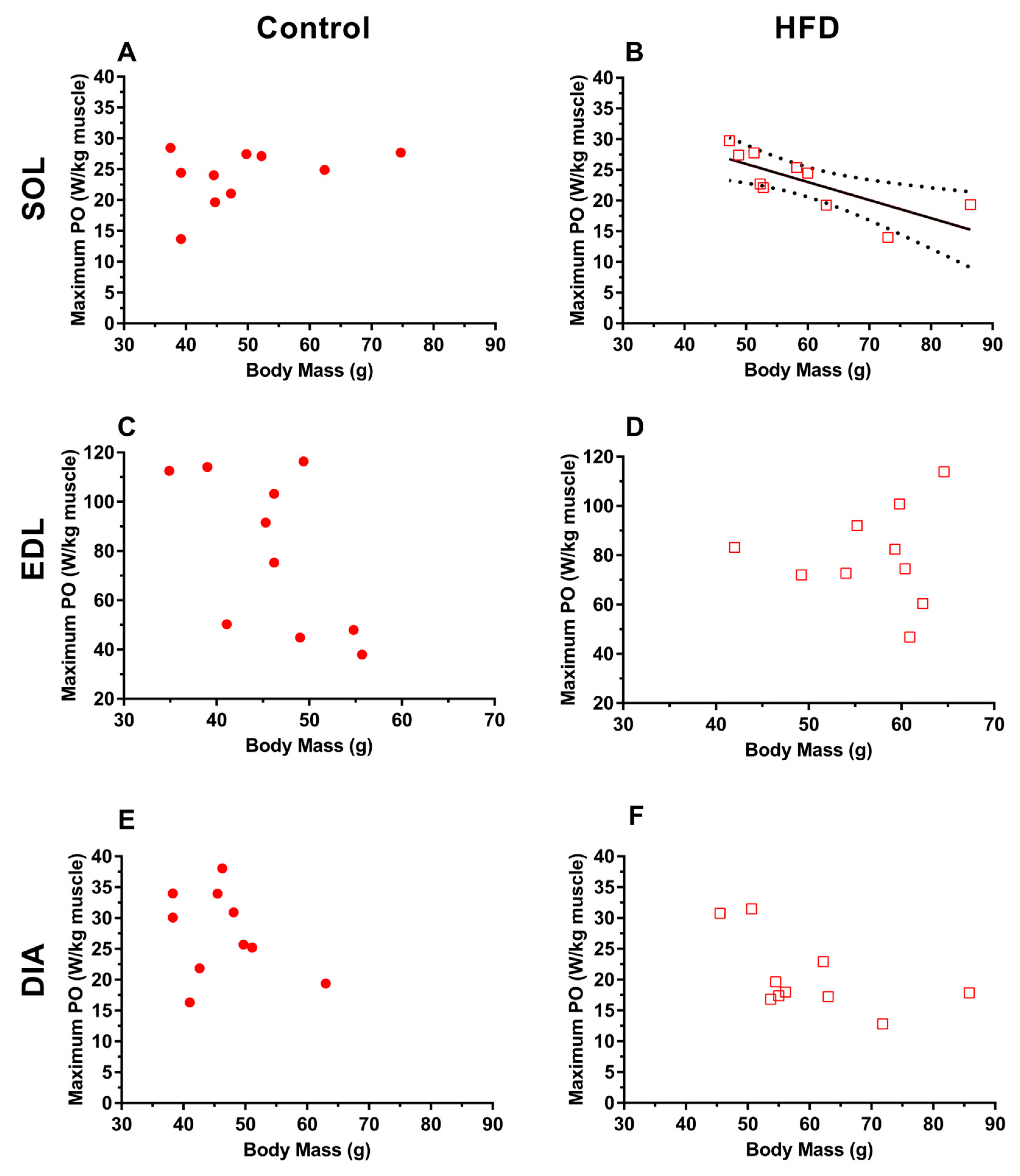
3.3. Work Loop Power Output
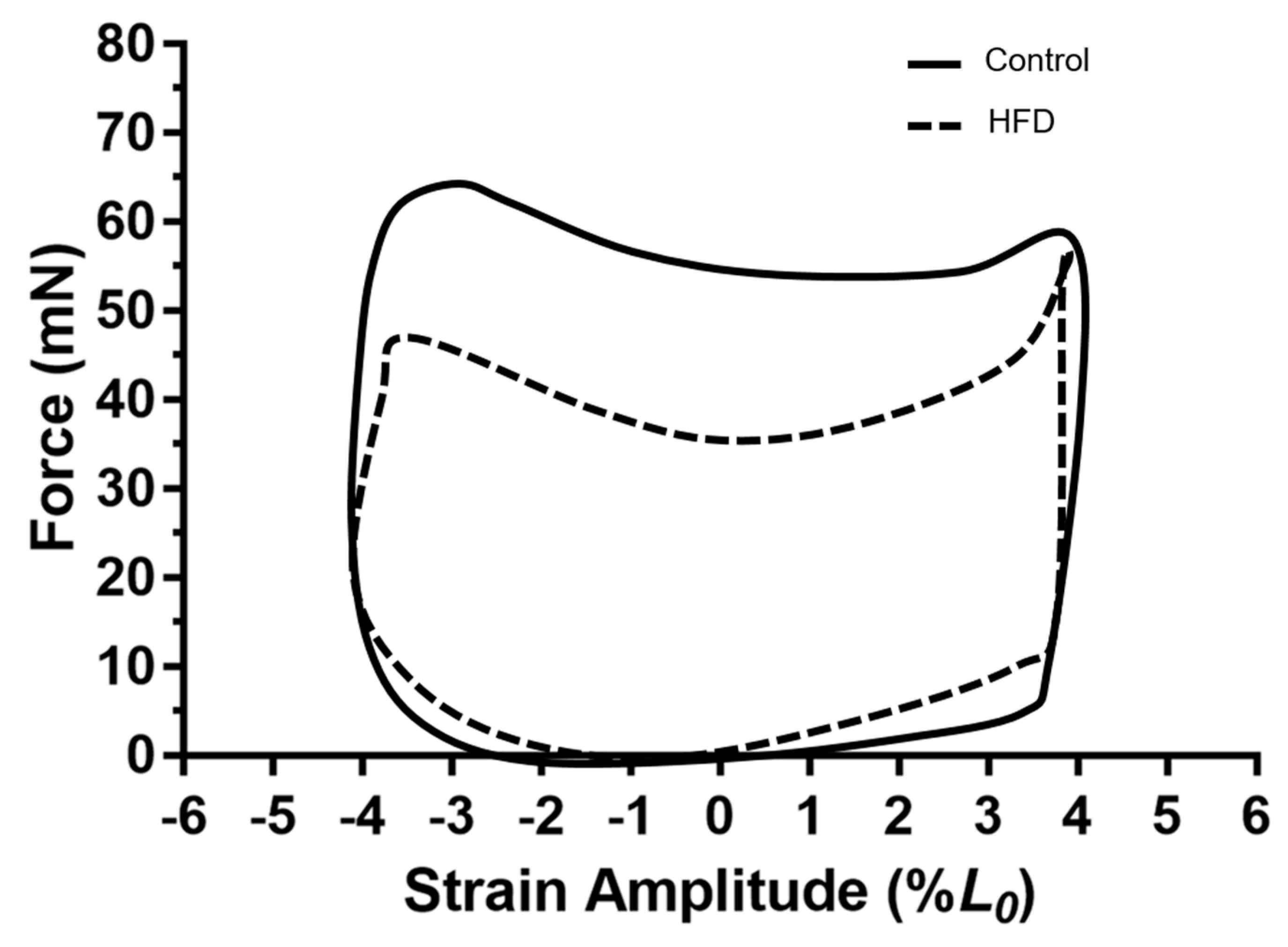
3.4. Work Loop Shapes for the Diaphragm
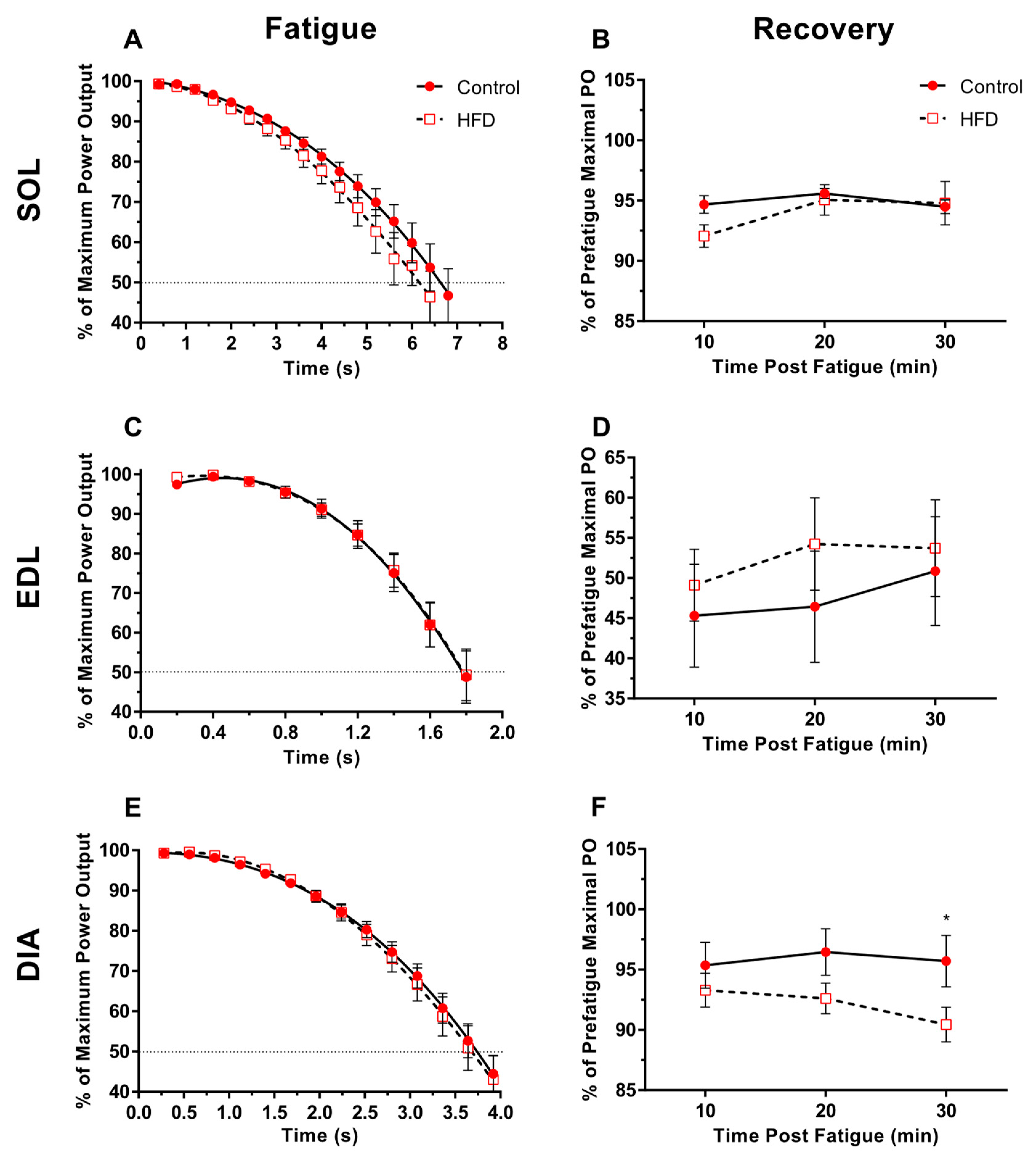
3.5. Fatigability and Recovery
4. Discussion
4.1. Age-Related Changes in Skeletal Muscle Contractile Function
4.2. Effects of Dietary-Induced Obesity on Animal and Muscle Morphology in Old Age
4.3. Effect of Dietary-Induced Obesity on Isometric Force and Work Loop Power in Old Age
4.4. Fatigability and Recovery
4.5. Differences in Contractile Performance between Young and Old Models of Obesity
4.6. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Dos Santos, L.; Cyrino, E.S.; Antunes, M.; Santos, D.A.; Sardinha, L.B. Sarcopenia and physical independence in older adults: The independent and synergic role of muscle mass and muscle function. J. Cachexia Sarcopenia Muscle 2017, 8, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.; James, R.S.; Seebacher, F. The effects of obesity on skeletal muscle contractile function. J. Exp. Biol. 2018, 221, jeb163840. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.J.; Erskine, R.M.; Morse, C.I.; Winwood, K.; Onambélé-Pearson, G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology 2016, 17, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, N.; Fujii, M.; Nishikawa, H.; Wada, J.; Shikata, K.; Makino, H.; Kimura, I. Clinical evaluation of muscle strength in 20-79-years-old obese Japanese. Diabetes Res. Clin. Pract. 2000, 48, 15–21. [Google Scholar] [CrossRef]
- Garcia-Vicencio, S.; Coudeyre, E.; Kluka, V.; Cardenoux, C.; Jegu, A.-G.; Fourot, A.-V.; Ratel, S.; Martin, V. The bigger, the stronger? Insights from muscle architecture and nervous characteristics in obese adolescent girls. Int. J. Obes. 2016, 40, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Zoico, E.; Di Francesco, V.; Guralnik, J.M.; Mazzali, G.; Bortolani, A.; Guariento, S.; Sergi, G.; Bosello, O.; Zamboni, M. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Lauwers-Cances, V.; Pahor, M.; Fillaux, J.; Grandjean, H.; Vellas, B. Muscle strength in obese elderly women: effect of recreational physical activity in a cross-sectional study. Am. J. Clin. Nutr. 2004, 79, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.J.; Erskine, R.M.; Morse, C.I.; Winwood, K.; Onambélé-Pearson, G.L. Combined effects of body composition and ageing on joint torque, muscle activation and co-contraction in sedentary women. Age 2014, 36, 9652. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.; Hill, C.; James, R.S.; Cox, V.M.; Seebacher, F. The effect of obesity on the contractile performance of isolated mouse soleus, EDL, and diaphragm muscles. J. Appl. Physiol. 2017, 122, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Kenny, A.M.; Kuchel, G.A. Muscle quality in aging: a multi-dimensional approach to muscle functioning with applications for treatment. Sports Med. 2015, 45, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Stenholm, S.; Alley, D.; Bandinelli, S.; Griswold, M.E.; Koskinen, S.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. The effect of obesity combined with low muscle strength on decline in mobility in older persons: Results from the InCHIANTI study. Int. J. Obes. 2009, 33, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Hilton, T.N.; Tuttle, L.J.; Bohnert, K.L.; Mueller, M.J.; Sinacore, D.R. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: Association with performance and function. Phys. Ther. 2008, 88, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Funai, K.; Song, H.; Yin, L.; Lodhi, I.J.; Wei, X.; Yoshino, J.; Coleman, T.; Semenkovich, C.F. Muscle lipogenesis balances insulin sensitivity and strength through calcium signaling. J. Clin. Investig. 2013, 123, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- DeNies, M.S.; Johnson, J.; Maliphol, A.B.; Bruno, M.; Kim, A.; Rizvi, A.; Rustici, K.; Medler, S. Diet-induced obesity alters skeletal muscle fiber types of male but not female mice. Physiol. Rep. 2014, 2, e00204. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, D.; Berdeaux, R. The effects of obesity on skeletal muscle regeneration. Front. Physiol. 2013, 4, 371. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.; James, R.S.; Little, A.G.; Cox, V.M.; Duncan, M.J.; Seebacher, F. Early effects of ageing on the mechanical performance of isolated locomotory (EDL) and respiratory (diaphragm) skeletal muscle using the work-loop technique. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R670–R684. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; James, R.S.; Cox, V.M.; Tallis, J. The Effect of Increasing Age on the Concentric and Eccentric Contractile Properties of Isolated Mouse Soleus and Extensor Digitorum Longus Muscles. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.L.; Warren, G.L.; Lowe, D.A. Soleus and EDL muscle contractility across the lifespan of female C57BL/6 mice. Exp. Gerontol. 2005, 40, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Head, S.I. Age- and gender-related changes in contractile properties of non-atrophied EDL muscle. PLoS ONE 2010, 5, e12345. [Google Scholar] [CrossRef] [PubMed]
- Graber, T.G.; Kim, J.H.; Grange, R.W.; McLoon, L.K.; Thompson, L.V. C57BL/6 life span study: Age-related declines in muscle power production and contractile velocity. Age 2015, 37, 9773. [Google Scholar] [CrossRef] [PubMed]
- Ciapaite, J.; van den Berg, S.A.; Houten, S.M.; Nicolay, K.; van Dijk, K.W.; Jeneson, J.A. Fiber-type-specific sensitivities and phenotypic adaptations to dietary fat overload differentially impact fast- versus slow-twitch muscle contractile function in C57BL/6J mice. J. Nutr. Biochem. 2015, 26, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, A.; Prosdocimo, D.A.; Mitchell, R.; Collins-Hooper, H.; Giallourou, N.; Swann, J.R.; Potter, P.; Epting, T.; Jain, M.K.; Patel, K. Investigating mechanisms underpinning the detrimental impact of a high-fat diet in the developing and adult hypermuscular myostatin null mouse. Skelet. Muscle 2015, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Bott, K.N.; Gittings, W.; Fajardo, V.A.; Baranowski, B.J.; Vandenboom, R.; LeBlanc, P.J.; Ward, W.E.; Peters, S.J. Musculoskeletal Structure and Function in Response to the Combined Effect of An Obesogenic Diet and Age in Male C57bl/6j Mice. Mol. Nutr. Food Res. 2017, 61. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/mnfr.201700137 (accessed on 25 November 2018). [CrossRef] [PubMed]
- Josephson, R.K. Mechanical power output from striated muscle during cyclic contraction. J. Exp. Biol. 1985, 114, 493–512. [Google Scholar]
- James, R.S.; Young, I.S.; Cox, V.M.; Goldspink, D.F.; Altringham, J.D. Isometric and isotonic muscle properties as determinants of work loop power output. Pflügers Arch. 1996, 432, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.; James, R.S.; Cox, V.M.; Hill, C.; Tallis, J. Investigating a dose-response relationship between high-fat diet consumption and the contractile performance of isolated mouse soleus, EDL and diaphragm muscles. Eur. J. Appl. Physiol. 2019, 119, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Sánchez Del Pino, M.J.; Gómez, C.; Peralta, J.L.; Boveris, A. Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002, 282, R985–R992. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, K.; Hellberg, N.; Bohlooly-Y, M.; Savendahl, L.; Johansson, M.S.; Berglindh, T.; Bosaeus, I.; Ohlsson, C. Body fat content can be predicted in vivo in mice using a modified dual-energy X-ray absorptiometry technique. J. Nutr. 2001, 131, 2963–2966. [Google Scholar] [CrossRef] [PubMed]
- Bernardis, L.L.; Patterson, B.D. Correlation between “Lee index” and carcass fat content in weanling and adult female rats with hypothalamic lesions. J. Endocrinol. 1968, 40, 527–528. [Google Scholar] [CrossRef] [PubMed]
- James, R.S.; Tallis, J.A.; Seebacher, F.; Storey, K. Daily torpor reduces mass and changes stress and power output of soleus and EDL muscles in the Djungarian hamster, Phodopus sungorus. J. Exp. Biol. 2011, 214, 2896–2902. [Google Scholar] [CrossRef] [PubMed]
- James, R.S.; Altringham, J.D.; Goldspink, D.F. The mechanical properties of fast and slow skeletal muscles of the mouse in relation to their locomotory function. J. Exp. Biol. 1995, 198, 491–502. [Google Scholar] [PubMed]
- Méndez, J.; Keys, A. Density and composition of mammalian muscle. Metabolism 1960, 9, 184–188. [Google Scholar]
- James, R.S.; Tallis, J.; Angilletta, M.J., Jr. Regional thermal specialisation in a mammal: temperature affects power output of core muscle more than that of peripheral muscle in adult mice (Mus musculus). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2015, 185, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.; Higgins, M.F.; Seebacher, F.; Cox, V.M.; Duncan, M.J.; James, R.S. The effects of 8 weeks voluntary wheel running on the contractile performance of isolated locomotory (soleus) and respiratory (diaphragm) skeletal muscle during early ageing. J. Exp. Biol. 2017, 220, 3733–3741. [Google Scholar] [CrossRef] [PubMed]
- Lynch, G.S.; Rafael, J.A.; Hinkle, R.T.; Cole, N.M.; Chamberlain, J.S.; Faulkner, J.A. Contractile properties of diaphragm muscle segments from old mdx and old transgenic mdx mice. Am. J. Physiol. 1997, 272, C2063–C2068. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V. Distribution Theory for Glass’s Estimator of Effect size and Related Estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Zaykin, D.V.; Zhivotovsky, L.A.; Westfall, P.H.; Weir, B.S. Truncated product method for combining p-values. Genet. Epidemiol. 2002, 22, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.; James, R.S.; Cox, V.M.; Duncan, M.J. The effect of physiological concentrations of caffeine on the power output of maximally and submaximally stimulated mouse EDL (fast) and soleus (slow) muscle. J. Appl. Physiol. 2012, 112, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.; James, R.S.; Cox, V.M.; Duncan, M.J. The effect of a physiological concentration of caffeine on the endurance of maximally and submaximally stimulated mouse soleus muscle. J. Physiol. Sci. 2013, 63, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.; Higgins, M.F.; Cox, V.M.; Duncan, M.J.; James, R.S. Does a physiological concentration of taurine increase acute muscle power output, time to fatigue, and recovery in isolated mouse soleus (slow) muscle with or without the presence of caffeine? Can. J. Physiol. Pharmacol. 2014, 92, 42–49. [Google Scholar] [CrossRef] [PubMed]
- James, R.S.; Wilson, R.S.; Askew, G.N. Effects of caffeine on mouse skeletal muscle power output during recovery from fatigue. J. Appl. Physiol. 2004, 96, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Addison, O.; Marcus, R.L.; Lastayo, P.C.; Ryan, A.S. Intermuscular fat: a review of the consequences and causes. Int. J. Endocrinol. 2014, 2014, 309570. [Google Scholar] [CrossRef] [PubMed]
- Maffiuletti, N.A.; Ratel, S.; Sartorio, A.; Martin, V. The Impact of Obesity on In vivo Human Skeletal Muscle Function. Curr. Obes. Rep. 2013, 2, 251–260. [Google Scholar] [CrossRef]
- Hirani, V.; Naganathan, V.; Blyth, F.; Couteur, L.G.D.; Seibel, M.J.; Waite, L.M.; Handelsman, D.J.; Cumming, R.G. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. Age Ageing 2017, 46, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.; Sparrow, D.; Weiss, S.T. Effects of obesity and fat distribution on ventilatory function: The normative aging study. Chest 1997, 111, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.T.; Druz, W.S.; Kondragunta, V.R. Diaphragmatic responses to body position changes in obese patients with obstructive sleep apnea. Am. Rev. Respir. Dis 1986, 133, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.Y.; Hsu, Y.Y.; Chen, R.C.; Liu, W.L.; Pan, W.H. Abdominal Obesity and Low Skeletal Muscle Mass Jointly Predict Total Mortality and Cardiovascular Mortality in an Elderly Asian Population. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Barclay, C.J. Modelling diffusive O(2) supply to isolated preparations of mammalian skeletal and cardiac muscle. J. Muscle Res. Cell Motil. 2005, 26, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Häkkinen, K.; Anton, A.; Garrues, M.; Ibañez, J.; Ruesta, M.; Gorostiaga, E.M. Maximal strength and power, endurance performance, and serum hormones in middle-aged and elderly men. Med. Sci. Sports Exerc. 2001, 33, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.R.; Suriyaarachchi, P.; Gomez, F.; Curcio, C.L.; Boersma, D.; Gunawardene, P.; Demontiero, O.; Duque, G. Phenotype of sarcopenic obesity in older individuals with a history of falling. Arch. Gerontol. Geriatr. 2016, 65, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Machann, J.; Bachmann, O.P.; Brechtel, K.; Dahl, D.B.; Wietek, B.; Klumpp, B.; Häring, H.-U.; Claussen, C.D.; Jacob, S.; Schick, F. Lipid content in the musculature of the lower leg assessed by fat selective MRI: Intra- and interindividual differences and correlation with anthropometric and metabolic data. J. Magn. Reson. Imaging 2003, 17, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Hepple, R.T.; Rice, C.L. Innervation and neuromuscular control in ageing skeletal muscle. J. Physiol. 2016, 594, 1965–1978. [Google Scholar] [CrossRef] [PubMed]

| Control | HFD | p-Value | Effect Size | |
|---|---|---|---|---|
| Body Mass (g) | 47.2 ± 3.0 | 58.6 ± 3.6 | <0.001 | 1.19 |
| Nasoanal Length (cm) | 11.8 ± 0.2 | 12.4 ± 0.2 | <0.001 | 0.91 |
| Body Circumference (cm) | 8.4 ± 0.4 | 10.6 ± 0.6 | <0.001 | 1.26 |
| Body Mass Index (kg∙m2) | 0.34 ± 0.01 | 0.38 ± 0.02 | <0.001 | 0.87 |
| Lee Index of Obesity | 305 ± 5 | 313 ± 5 | 0.04 | 0.54 |
| Fat Pad Mass (g) | 3.6 ± 0.9 | 7.9 ± 1.2 | <0.001 | 1.23 |
| Fat Pad Mass:Body Mass (%) | 7.0 ± 0.8 | 12.8 ± 0.8 | <0.001 | 1.32 |
| Soleus | EDL | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | HFD | p-Value | Effect Size | Control | HFD | p-Value | Effect Size | |
| Muscle Mass (mg) | 9.4 ± 0.5 | 11.0 ± 0.6 | 0.04 | 1.02 | 10.6 ± 0.6 | 12.6 ± 0.5 | 0.014 | 1.19 |
| Muscle Length (mm) | 9.3 ± 0.1 | 9.5 ± 0.2 | 0.30 | 0.38 | 9.1 ± 0.2 | 9.1 ± 0.2 | 0.76 | 0.00 |
| Muscle CSA (m2) | 1.0 × 10−6 ± 4.5 × 10−8 | 1.1 × 10−6 ± 3.9 × 10−8 | 0.05 | 0.89 | 1.1 × 10−6 ± 5.9 × 10−8 | 1.3 × 10−6 ± 4.2 × 10−8 | 0.02 | 1.12 |
| Muscle Mass:Body Mass (%) | 0.52 ± 0.04 | 0.55 ± 0.03 | 0.67 | 0.25 | 0.45 ± 0.04 | 0.45 ± 0.02 | 0.98 | 0.00 |
| THPT (ms) | LSHR (ms) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | HFD | p-Value | Effect Size | Control | HFD | p-Value | Effect Size | |
| Soleus | 37.6 ± 2.3 | 40.1 ± 2.2 | 0.38 | 0.38 | 52.3 ± 3.2 | 48.0 ± 1.9 | 0.21 | 0.55 |
| EDL | 16.0 ± 1.1 | 16.3 ± 0.9 | 0.81 | 0.10 | 17.4 ± 1.2 | 17.6 ± 1.1 | 0.89 | 0.06 |
| Diaphragm | 24.4 ± 1.0 | 26.5 ± 1.2 | 0.21 | 0.60 | 25.0 ± 1.0 | 26.8 ± 0.8 | 0.13 | 0.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, C.; James, R.S.; Cox, V.M.; Tallis, J. Does Dietary-Induced Obesity in Old Age Impair the Contractile Performance of Isolated Mouse Soleus, Extensor Digitorum Longus and Diaphragm Skeletal Muscles? Nutrients 2019, 11, 505. https://doi.org/10.3390/nu11030505
Hill C, James RS, Cox VM, Tallis J. Does Dietary-Induced Obesity in Old Age Impair the Contractile Performance of Isolated Mouse Soleus, Extensor Digitorum Longus and Diaphragm Skeletal Muscles? Nutrients. 2019; 11(3):505. https://doi.org/10.3390/nu11030505
Chicago/Turabian StyleHill, Cameron, Rob S. James, Val M. Cox, and Jason Tallis. 2019. "Does Dietary-Induced Obesity in Old Age Impair the Contractile Performance of Isolated Mouse Soleus, Extensor Digitorum Longus and Diaphragm Skeletal Muscles?" Nutrients 11, no. 3: 505. https://doi.org/10.3390/nu11030505
APA StyleHill, C., James, R. S., Cox, V. M., & Tallis, J. (2019). Does Dietary-Induced Obesity in Old Age Impair the Contractile Performance of Isolated Mouse Soleus, Extensor Digitorum Longus and Diaphragm Skeletal Muscles? Nutrients, 11(3), 505. https://doi.org/10.3390/nu11030505





