Sorting out the Value of Cruciferous Sprouts as Sources of Bioactive Compounds for Nutrition and Health
Abstract
1. Introduction
2. Bioactive Secondary Metabolites in Edible Cruciferous Sprouts
2.1. Phenolic Compounds in Cruciferous Sprouts
2.2. Glucosinolates in Cruciferous Sprouts
3. Elicitation of Brassicaceae Sprouts to Enhance the Content of Bioactive (Poly)phenols and Glucosinolates
4. The Challenges of Including Cruciferous Sprouts in Balanced Diets and Personalized Nutrition
4.1. Effect of Cruciferous Sprouts on Type 2 Diabetes Mellitus
4.2. Anti-Inflammatory Activity of Cruciferous Sprouts
4.3. Capacity of Bioactive Molecules to Modulate Oxidative Stress (OS)
4.4. Enhancing the Consumption of Cruciferous Sprouts to Reduce Carcinogenesis
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Gan, R.-Y.; Lui, W.-Y.; Wu, K.; Chan, C.-L.; Dai, S.-H.; Sui, Z.-Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Moreno, D.A.; Perez-Balibrea, S.; Garcia-Viguera, C. Phytochemical quality and bioactivity of edible sprouts. Nat. Prod. Commun. 2006, 11, 1037–1048. [Google Scholar]
- Baenas, N.; Ferreres, F.; García-Viguera, C.; Moreno, D.A. Radish sprouts—Characterization and elicitation of novel varieties rich in anthocyanins. Food Res. Int. 2015, 69, 305–312. [Google Scholar] [CrossRef]
- Conzatti, A.; Telles da Silva Fróes, F.C.; Schweigert Perry, I.D.; Guerini de Souza, C. Clinical and molecular evidence of the consumption of broccoli, glucoraphanin and sulforaphane in humans. Nutr. Hosp. 2015, 31, 559–569. [Google Scholar]
- Baenas, N.; Gómez-Jodar, I.; Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Boil. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, A.D. Elicitation: A Tool for Enriching the Bioactive Composition of Foods. Molecules 2014, 19, 13541. [Google Scholar] [CrossRef] [PubMed]
- Gagné, F. Chapter 6—Oxidative Stress. In Biochemical Ecotoxicology; Gagné, F., Ed.; Academic Press: Oxford, UK, 2014; pp. 103–115. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C. Anti-nociceptive and anti-inflammatory actions of sulforaphane in chronic constriction injury-induced neuropathic pain mice. Inflammopharmacology 2016, 25, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhu, Y. The primary active components, antioxidant properties, and differential metabolite profiles of radish sprouts (Raphanus sativus L.) upon domestic storage: Analysis of nutritional quality. J. Sci. Food Agric. 2018, 98, 5853–5860. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J.K.; Kim, H.; Kim, Y.J.; Park, Y.J.; Kim, S.J.; Kim, C.; Park, S.U. Transcriptome analysis and metabolic profiling of green and red kale (Brassica oleracea var. acephala) seedlings. Food Chem. 2018, 241, 7–13. [Google Scholar] [CrossRef]
- Liang, X.; Lee, H.W.; Li, Z.; Lu, Y.; Zou, L.; Ong, C.N. Simultaneous Quantification of 22 Glucosinolates in 12 Brassicaceae Vegetables by Hydrophilic Interaction Chromatography–Tandem Mass Spectrometry. ACS Omega 2018, 3, 15546–15553. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- De Camargo, C.A.; Schwember, R.A.; Parada, R.; Garcia, S.; Maróstica Júnior, R.M.; Franchin, M.; Regitano-d’Arce, A.M.; Shahidi, F. Opinion on the Hurdles and Potential Health Benefits in Value-Added Use of Plant Food Processing By-Products as Sources of Phenolic Compounds. Int. J. Mol. Sci. 2018, 19, 3498. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.; Moreno, D.A.; Cartea, M.E.; Ferreres, F.; García-Viguera, C.; Velasco, P. Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J. Chromatogr. A 2009, 1216, 6611–6619. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; García-Viguera, C.; Gil-Izquierdo, Á.; Moreno, D.A.; Pérez-Balibrea, S. Acylated anthocyanins in broccoli sprouts. Food Chem. 2010, 123, 358–363. [Google Scholar]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Fernández-Castillejo, S.; Rubió, L.; Macià, A.; Solà, R. Anthocyanin Tissue Bioavailability in Animals: Possible Implications for Human Health. A Systematic Review. J. Agric. Food Chem. 2018, 66, 11531–11543. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Wagner, A.; Maria Terschluesen, A.; Rimbach, G. Health Promoting Effects of Brassica-Derived Phytochemicals: From Chemopreventive and Anti-Inflammatory Activities to Epigenetic Regulation. Oxid. Med. Cell. Longev. 2013, 2013, 964539. [Google Scholar] [CrossRef]
- Xu, Y.; Szép, S.; Lu, Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc. Natl. Acad. Sci. USA 2009, 106, 20515–20519. [Google Scholar] [CrossRef]
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008, 47, 73–88. [Google Scholar] [CrossRef]
- Sita, G.; Hrelia, P.; Graziosi, A.; Morroni, F. Sulforaphane from Cruciferous Vegetables: Recent Advances to Improve Glioblastoma Treatment. Nutrients 2018, 10, 1755. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.S.; Jo, S.J.; Lee, G.J. Comparison of Glucosinolate Profiles in Different Tissues of Nine Brassica Crops. Molecules 2015, 20, 15827–15841. [Google Scholar] [CrossRef] [PubMed]
- Adwas, A.A.; Elkhoely, A.A.; Kabel, A.M.; Abdel-Rahman, M.N.; Eissa, A.A. Anti-cancer and cardioprotective effects of indol-3-carbinol in doxorubicin-treated mice. J. Infect. Chemother. 2016, 22, 36–43. [Google Scholar] [CrossRef]
- Frede, K.; Schreiner, M.; Zrenner, R.; Graefe, J.; Baldermann, S. Carotenoid biosynthesis of pak choi (Brassica rapa ssp. chinensis) sprouts grown under different light-emitting diodes during the diurnal course. Photochem. Photobiol. Sci. 2018, 17, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Villaño, D.; García-Viguera, C.; Moreno, D.A. Optimizing elicitation and seed priming to enrich broccoli and radish sprouts in glucosinolates. Food Chem. 2016, 204, 314–319. [Google Scholar] [CrossRef]
- Przybysz, A.; Wrochna, M.; Małecka-Przybysz, M.; Gawrońska, H.; Gawroński, S.W. The effects of Mg enrichment of vegetable sprouts on Mg concentration, yield and ROS generation. J. Sci. Food Agric. 2016, 96, 3469–3476. [Google Scholar] [CrossRef]
- Guo, R.; Yuan, G.; Wang, Q. Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chem. 2011, 129, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011, 129, 35–44. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Arasu, M.V.; Kim, S.J.; RomijUddin, M.; Park, W.T.; Lee, S.Y.; Park, S.U. Methyl Jasmonate- and Light-Induced Glucosinolate and Anthocyanin Biosynthesis in Radish Seedlings. Nat. Prod. Commun. 2015, 10, 1211–1214. [Google Scholar]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Folta, K.M. Sequential light programs shape kale (Brassica napus) sprout appearance and alter metabolic and nutrient content. Hortic. Res. 2014, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Miao, H.; Wang, Q. Effect of glucose on glucosinolates, antioxidants and metabolic enzymes in Brassica sprouts. Sci. Hortic. 2011, 129, 535–540. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Biotic Elicitors Effectively Increase the Glucosinolates Content in Brassicaceae Sprouts. J. Agric. Food Chem. 2014, 62, 1881–1889. [Google Scholar] [CrossRef]
- Park, W.T.; Kim, Y.B.; Seo, J.M.; Kim, S.-J.; Chung, E.; Lee, J.-H.; Park, S.U. Accumulation of Anthocyanin and Associated Gene Expression in Radish Sprouts Exposed to Light and Methyl Jasmonate. J. Agric. Food Chem. 2013, 61, 4127–4132. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Yuan, G.; Wang, Q. Effect of sucrose and mannitol on the accumulation of health-promoting compounds and the activity of metabolic enzymes in broccoli sprouts. Sci. Hortic. 2011, 128, 159–165. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010, 29, 405–434. [Google Scholar] [CrossRef]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Banihani, S.A. Radish (Raphanus sativus) and Diabetes. Nutrients 2017, 9, 1014. [Google Scholar] [CrossRef]
- Taniguchi, H.; Kobayashi-Hattori, K.; Tenmyo, C.; Kamei, T.; Uda, Y.; Sugita-Konishi, Y.; Oishi, Y.; Takita, T. Effect of Japanese radish (Raphanus sativus) sprout (Kaiware-daikon) on carbohydrate and lipid metabolisms in normal and streptozotocin-induced diabetic rats. Phytother. Res. 2006, 20, 274–278. [Google Scholar] [CrossRef]
- Rescigno, T.; Tecce, M.F.; Capasso, A. Protective and restorative effects of nutrients and phytochemicals. Open Biochem. J. 2018, 12, 46–64. [Google Scholar] [CrossRef]
- Housley, L.; Magana, A.A.; Hsu, A.; Beaver, L.M.; Wong, C.P.; Stevens, J.F.; Choi, J.; Jiang, Y.; Bella, D.; Williams, D.E.; et al. Untargeted Metabolomic Screen Reveals Changes in Human Plasma Metabolite Profiles Following Consumption of Fresh Broccoli Sprouts. Mol. Nutr. Food Res. 2018, 62, 1700665. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Piegholdt, S.; Schloesser, A.; Moreno, D.A.; García-Viguera, C.; Rimbach, G.; Wagner, A.E. Metabolic Activity of Radish Sprouts Derived Isothiocyanates in Drosophila melanogaster. Int. J. Mol. Sci. 2016, 17, 251. [Google Scholar] [CrossRef] [PubMed]
- Black, A.M.; Armstrong, E.A.; Scott, O.; Juurlink, B.J.H.; Yager, J.Y. Broccoli sprout supplementation during pregnancy prevents brain injury in the newborn rat following placental insufficiency. Behav. Brain Res. 2015, 291, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C.; Ferreres, F.; Gil, J.I.; Gil-Izquierdo, Á. The intake of broccoli sprouts modulates the inflammatory and vascular prostanoids but not the oxidative stress-related isoprostanes in healthy humans. Food Chem. 2015, 173, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Chillon, M.T.; Carazo-Diaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villano, D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Hosseinpanah, F.; Keyzad, A.; Azizi, F. Effects of broccoli sprout with high sulforaphane concentration on inflammatory markers in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. J. Funct. Foods 2012, 4, 837–841. [Google Scholar] [CrossRef]
- Christiansen, B.; Bellostas Muguerza, N.; Petersen, A.M.; Kveiborg, B.; Madsen, C.R.; Thomas, H.; Ihlemann, N.; Sorensen, J.C.; Kober, L.; Sorensen, H.; et al. Ingestion of broccoli sprouts does not improve endothelial function in humans with hypertension. PLoS ONE 2010, 5, e12461. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Noyan Ashraf, M.H.; Facci, M.; Wang, R.; Paterson, P.G.; Ferrie, A.; Juurlink, B.H.J. Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proc. Natl. Acad. Sci. USA 2004, 101, 7094–7099. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Okoń, K.; Krośniak, M.; Prochownik, E.; Żmudzki, P.; Kryczyk-Kozioł, J.; Zagrodzki, P. Interaction between iodine and glucosinolates in rutabaga sprouts and selected biomarkers of thyroid function in male rats. J. Trace Elem. Med. Boil. 2018, 46, 110–116. [Google Scholar] [CrossRef]
- Sharma, D.; Sangha, G.K. Antioxidative effects of aqueous extract of broccoli sprouts against Triazophos induced hepatic and renal toxicity in female Wistar rats. J. Appl. Biomed. 2018, 16, 100–110. [Google Scholar] [CrossRef]
- Yanaka, A. Daily intake of broccoli sprouts normalizes bowel habits in human healthy subjects. J. Clin. Biochem. Nutr. 2018, 62, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Gonzalez-Trujano, M.E.; Guadarrama-Enriquez, O.; Pellicer, F.; Garcia-Viguera, C.; Moreno, D.A. Broccoli sprouts in analgesia—Preclinical in vivo studies. Food Funct. 2017, 8, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Melega, S.; Canistro, D.; Pagnotta, E.; Iori, R.; Sapone, A.; Paolini, M. Effect of sprout extract from Tuscan black cabbage on xenobiotic-metabolizing and antioxidant enzymes in rat liver. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 751, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.T.; Fayed, S.A.; Ahmed, A.M.; El Rahim, E.A. Effect of Egyptian Radish and Clover Sprouts on Blood Sugar and Lipid Metabolisms in Diabetic Rats. Glob. J. Biotechnol. Biochem. 2015, 10, 16–21. [Google Scholar]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Wade, K.L.; Jenkins, S.N.; Shapiro, T.A.; Fuchs, E.J.; Kerns, M.L.; Talalay, P. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol. Biomark. Prev. 2007, 16, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; Fahey, J.W.; Healy, Z.R.; Wehage, S.L.; Benedict, A.L.; Min, C.; Dinkova-Kostova, A.T. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc. Natl. Acad. Sci. USA 2007, 104, 17500–17505. [Google Scholar] [CrossRef]
- Riedl, M.A.; Saxon, A.; Diaz-Sanchez, D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin. Immunol. 2009, 130, 244–251. [Google Scholar] [CrossRef]
- Meyer, M.; Kesic, M.J.; Clarke, J.; Ho, E.; Simmen, R.C.; Diaz-Sanchez, D.; Noah, T.L.; Jaspers, I. Sulforaphane induces SLPI secretion in the nasal mucosa. Respir. Med. 2013, 107, 472–475. [Google Scholar] [CrossRef]
- Brown, R.H.; Reynolds, C.; Brooker, A.; Talalay, P.; Fahey, J.W. Sulforaphane improves the bronchoprotective response in asthmatics through Nrf2-mediated gene pathways. Respir. Res. 2015, 16, 106. [Google Scholar] [CrossRef]
- Kikuchi, M.; Ushida, Y.; Shiozawa, H.; Umeda, R.; Tsuruya, K.; Aoki, Y.; Suganuma, H.; Nishizaki, Y. Sulforaphane-rich broccoli sprout extract improves hepatic abnormalities in male subjects. World J. Gastroenterol. 2015, 21, 12457–12467. [Google Scholar] [CrossRef]
- Ushida, Y.S.; Suganuma, H.; Yanaka, A. Low-Dose of the Sulforaphane Precursor Glucoraphanin as a Dietary Supplement Induces Chemoprotective Enzymes in Humans. Food Nutr. Sci. 2015, 6, 1603–1612. [Google Scholar] [CrossRef]
- Bauman, J.E.; Zang, Y.; Sen, M.; Li, C.; Wang, L.; Egner, P.A.; Fahey, J.W.; Normolle, D.P.; Grandis, J.R.; Kensler, T.W.; et al. Prevention of Carcinogen-Induced Oral Cancer by Sulforaphane. Cancer Prev. Res. 2016, 9, 547–557. [Google Scholar] [CrossRef]
- Doss, J.F.; Jonassaint, J.C.; Garrett, M.E.; Ashley-Koch, A.E.; Telen, M.J.; Chi, J.T. Phase 1 Study of a Sulforaphane-Containing Broccoli Sprout Homogenate for Sickle Cell Disease. PLoS ONE 2016, 11, e0152895. [Google Scholar] [CrossRef] [PubMed]
- Murashima, M.; Watanabe, S.; Zhuo, X.G.; Uehara, M.; Kurashige, A. Phase 1 study of multiple biomarkers for metabolism and oxidative stress after one-week intake of broccoli sprouts. Biofactors 2004, 22, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Myzak, M.C.; Tong, P.; Dashwood, W.M.; Dashwood, R.H.; Ho, E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp. Biol. Med. 2007, 232, 227–234. [Google Scholar]
- Clarke, J.D.; Riedl, K.; Bella, D.; Schwartz, S.J.; Stevens, J.F.; Ho, E. Comparison of isothiocyanate metabolite levels and histone deacetylase activity in human subjects consuming broccoli sprouts or broccoli supplement. J. Agric. Food Chem. 2011, 59, 10955–10963. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, A.; Fahey, J.W.; Fukumoto, A.; Nakayama, M.; Inoue, S.; Zhang, S.; Tauchi, M.; Suzuki, H.; Hyodo, I.; Yamamoto, M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev. Res. 2009, 2, 353–360. [Google Scholar] [CrossRef]
- Heber, D.; Li, Z.; Garcia-Lloret, M.; Wong, A.M.; Lee, T.Y.; Thames, G.; Krak, M.; Zhang, Y.; Nel, A. Sulforaphane-rich broccoli sprout extract attenuates nasal allergic response to diesel exhaust particles. Food Funct. 2014, 5, 35–41. [Google Scholar] [CrossRef]
- Alumkal, J.J.; Slottke, R.; Schwartzman, J.; Cherala, G.; Munar, M.; Graff, J.N.; Beer, T.M.; Ryan, C.W.; Koop, D.R.; Gibbs, A.; et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Investig. New Drugs 2015, 33, 480–489. [Google Scholar] [CrossRef]
- Atwell, L.L.; Zhang, Z.; Mori, M.; Farris, P.; Vetto, J.T.; Naik, A.M.; Oh, K.Y.; Thuillier, P.; Ho, E.; Shannon, J. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev. Res. 2015, 8, 1184–1191. [Google Scholar] [CrossRef]
- Shiina, A.; Kanahara, N.; Sasaki, T.; Oda, Y.; Hashimoto, T.; Hasegawa, T.; Yoshida, T.; Iyo, M.; Hashimoto, K. An Open Study of Sulforaphane-rich Broccoli Sprout Extract in Patients with Schizophrenia. Clin. Psychopharmacol. Neurosci. 2015, 13, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.M.J.; Wollheim, C.B.; Wierup, N.; et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 2017, 9, eaah4477. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Connors, S.L.; Macklin, E.A.; Smith, K.D.; Fahey, J.W.; Talalay, P.; Zimmerman, A.W. Sulforaphane treatment of autism spectrum disorder (ASD). Proc. Natl. Acad. Sci. USA 2014, 111, 15550–15555. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Meyer, M.; Bauer, R.N.; Zhou, H.; Zhang, H.; Jones, S.; Robinette, C.; Noah, T.L.; Jaspers, I. Effect of Broccoli Sprouts and Live Attenuated Influenza Virus on Peripheral Blood Natural Killer Cells: A Randomized, Double-Blind Study. PLoS ONE 2016, 11, e0147742. [Google Scholar] [CrossRef] [PubMed]
- Musselman, D.L.; Betan, E.; Larsen, H.; Phillips, L.S. Relationship of depression to diabetes types 1 and 2: Epidemiology, biology, and treatment. Biol. Psychiatry 2003, 54, 317–329. [Google Scholar] [CrossRef]
- Bessesen, D.H. The role of carbohydrates in insulin resistance. J. Nutr. 2001, 131, 2782s–2786s. [Google Scholar] [CrossRef] [PubMed]
- Zaklos-Szyda, M.; Majewska, I.; Redzynia, M.; Koziolkiewicz, M. Antidiabetic effect of polyphenolic extracts from selected edible plants as alpha-amylase, alpha -glucosidase and PTP1B inhibitors, and beta pancreatic cells cytoprotective agents—A comparative study. Curr. Top. Med. Chem. 2015, 15, 2431–2444. [Google Scholar] [CrossRef]
- Amer, M.; El-Habibi el, S.; El-Gendy, A. Effects of Trifolium alexandrinum extracts on streptozotocin-induced diabetes in male rats. Ann. Nutr. Metab. 2004, 48, 343–347. [Google Scholar] [CrossRef]
- Wu, L.Y.; Juan, C.C.; Ho, L.T.; Hsu, Y.P.; Hwang, L.S. Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J. Agric. Food Chem. 2004, 52, 643–648. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Matsui, T. Protective role of sulphoraphane against vascular complications in diabetes. Pharm. Boil. 2016, 54, 2329–2339. [Google Scholar] [CrossRef]
- Sourris, K.C.; Forbes, J.M. Interactions between advanced glycation end-products (AGE) and their receptors in the development and progression of diabetic nephropathy—Are these receptors valid therapeutic targets. Curr. Drug Targets 2009, 10, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Matsui, T. Soluble form of a receptor for advanced glycation end products (sRAGE) as a biomarker. Front. Biosci. 2010, 2, 1184–1195. [Google Scholar] [CrossRef]
- Maeda, S.; Matsui, T.; Ojima, A.; Takeuchi, M.; Yamagishi, S. Sulforaphane inhibits advanced glycation end product-induced pericyte damage by reducing expression of receptor for advanced glycation end products. Nutr. Res. 2014, 34, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Tohidi, M.; Nazeri, P.; Mehran, M.; Azizi, F.; Mirmiran, P. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: A randomized double-blind clinical trial. Int. J. Food Sci. Nutr. 2012, 63, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Jenkins, S.N.; Fahey, J.W.; Ye, L.; Wehage, S.L.; Liby, K.T.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Protection against UV-light-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts. Cancer Lett. 2006, 240, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Dodd, G.; Kunsch, C. Sulforaphane inhibits TNF-alpha-induced activation of p38 MAP kinase and VCAM-1 and MCP-1 expression in endothelial cells. Inflamm. Res. 2009, 58, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Du Clos, T.W. Function of C-reactive protein. Ann. Med. 2000, 32, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.T.; Tortosa, M.; Martínez-Ballesta, M.C.; Velasco, P.; García-Viguera, C.; Moreno, D.A. Nutritional and phytochemical value of Brassica crops from the agri-food perspective. Anal. Appl. Boil. 2016, 170, 273–285. [Google Scholar] [CrossRef]
- Turpaev, K.T. Keap1-Nrf2 signaling pathway: Mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles. Biochemistry 2013, 78, 111–126. [Google Scholar] [CrossRef]
- Vomhof-Dekrey, E.E.; Picklo, M.J., Sr. The Nrf2-antioxidant response element pathway: A target for regulating energy metabolism. J. Nutr. Biochem. 2012, 23, 1201–1206. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Hedayati, M.; Hosseinpour-Niazi, S.; Azizi, F. Broccoli sprouts reduce oxidative stress in type 2 diabetes: A randomized double-blind clinical trial. Eur. J. Clin. Nutr. 2011, 65, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, Z.; Strobl, J.S.; Ehrich, M.; Misra, H.P.; Li, Y. Potent induction of total cellular and mitochondrial antioxidants and phase 2 enzymes by cruciferous sulforaphane in rat aortic smooth muscle cells: Cytoprotection against oxidative and electrophilic stress. Cardiovasc. Toxicol. 2008, 8, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.A.; Moon, S.Y.; Kim, W.Y.; Paek, S.M.; Park, H.H.; Lee, C.S. Structure-Based Classification and Anti-Cancer Effects of Plant Metabolites. Int. J. Mol. Sci. 2018, 19, 2651. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Akhtar, N.; Khan, M.S.; Hameed, A.; Irfan, M.; Arshad, M.A.; Ali, S.; Asrar, M. Plant derived anticancer agents: A green approach towards skin cancers. Biomed. Pharm. 2018, 103, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.K.; Gallicchio, L.; Lindsley, K.; Shiels, M.; Hammond, E.; Tao, X.G.; Chen, L.; Robinson, K.A.; Caulfield, L.E.; Herman, J.G.; et al. Cruciferous vegetable consumption and lung cancer risk: A systematic review. Cancer Epidemiol. Biomark. Prev. 2009, 18, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Oganesian, A.; Hendricks, J.D.; Pereira, C.B.; Orner, G.A.; Bailey, G.S.; Williams, D.E. Potency of dietary indole-3-carbinol as a promoter of aflatoxin B1-initiated hepatocarcinogenesis: Results from a 9000 animal tumor study. Carcinogenesis 1999, 20, 453–458. [Google Scholar] [CrossRef]
- Baenas, N.; Silván, J.M.; Medina, S.; de Pascual-Teresa, S.; García-Viguera, C.; Moreno, D.A. Metabolism and antiproliferative effects of sulforaphane and broccoli sprouts in human intestinal (Caco-2) and hepatic (HepG2) cells. Phytochem. Rev. 2015, 14, 1035–1044. [Google Scholar] [CrossRef]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of glucosinolates in vegetable crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef]
- Munday, R.; Mhawech-Fauceglia, P.; Munday, C.M.; Paonessa, J.D.; Tang, L.; Munday, J.S.; Lister, C.; Wilson, P.; Fahey, J.W.; Davis, W.; et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008, 68, 1593–1600. [Google Scholar] [CrossRef]
- Zhang, Y.; Munday, R.; Jobson, H.E.; Munday, C.M.; Lister, C.; Wilson, P.; Fahey, J.W.; Mhawech-Fauceglia, P. Induction of GST and NQO1 in cultured bladder cells and in the urinary bladders of rats by an extract of broccoli (Brassica oleracea italica) sprouts. J. Agric. Food Chem. 2006, 54, 9370–9376. [Google Scholar] [CrossRef]
- Bertl, E.; Bartsch, H.; Gerhauser, C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol. Cancer 2006, 5, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.J.; Singletary, K.W.; Venema, R.C. Sulforaphane suppresses angiogenesis and disrupts endothelial mitotic progression and microtubule polymerization. Vasc. Pharm. 2007, 46, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, Y. Mitochondria are the primary target in isothiocyanate-induced apoptosis in human bladder cancer cells. Mol. Cancer 2005, 4, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, Y.; Jobson, H.E.; Li, J.; Stephenson, K.K.; Wade, K.L.; Fahey, J.W. Potent activation of mitochondria-mediated apoptosis and arrest in S and M phases of cancer cells by a broccoli sprout extract. Mol. Cancer 2006, 5, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Thejass, P.; Kuttan, G. Antimetastatic activity of Sulforaphane. Life Sci. 2006, 78, 3043–3050. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Benedict, A.L.; Jenkins, S.N.; Ye, L.; Wehage, S.L.; Talalay, P. Dietary glucoraphanin-rich broccoli sprout extracts protect against UV radiation-induced skin carcinogenesis in SKH-1 hairless mice. Photochem. Photobiol. Sci. 2010, 9, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Knatko, E.V.; Higgins, M.; Fahey, J.W.; Dinkova-Kostova, A.T. Loss of Nrf2 abrogates the protective effect of Keap1 downregulation in a preclinical model of cutaneous squamous cell carcinoma. Sci. Rep. 2016, 6, 25804. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, T.; Wang, H.; Tao, S.; Lau, A.; Fang, D.; Zhang, D.D. Does Nrf2 contribute to p53-mediated control of cell survival and death? Antioxid. Redox Signal. 2012, 17, 1670–1675. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawai, Y.; Kitamoto, N.; Osawa, T.; Kato, Y. Covalent modification of lysine residues by allyl isothiocyanate in physiological conditions: Plausible transformation of isothiocyanate from thiol to amine. Chem. Res. Toxicol. 2009, 22, 536–542. [Google Scholar] [CrossRef]
- Cheung, K.L.; Kong, A.N. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010, 12, 87–97. [Google Scholar] [CrossRef]
- Hong, F.; Freeman, M.L.; Liebler, D.C. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem. Res. Toxicol. 2005, 18, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Thomas, N.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: A two-site interaction model for the Nrf2-Keap1 complex. J. Biol. Chem. 2006, 281, 24756–24768. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, Q.H.; Xu, K. Are isothiocyanates potential anti-cancer drugs? Acta Pharm. Sin. 2009, 30, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Cho, M.K. Phenethyl isothiocyanate induced apoptosis via down regulation of Bcl-2/XIAP and triggering of the mitochondrial pathway in MCF-7 cells. Arch. Pharm. Res. 2008, 31, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Powolny, A.A.; Singh, S.V. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells. J. Biol. Chem. 2008, 283, 30151–30163. [Google Scholar] [CrossRef]
- Sahu, R.P.; Zhang, R.; Batra, S.; Shi, Y.; Srivastava, S.K. Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis 2009, 30, 1744–1753. [Google Scholar] [CrossRef]
- Gong, A.; He, M.; Krishna Vanaja, D.; Yin, P.; Karnes, R.J.; Young, C.Y. Phenethyl isothiocyanate inhibits STAT3 activation in prostate cancer cells. Mol. Nutr. Food Res. 2009, 53, 878–886. [Google Scholar] [CrossRef]
- Prawan, A.; Saw, C.L.; Khor, T.O.; Keum, Y.S.; Yu, S.; Hu, L.; Kong, A.N. Anti-NF-kappaB and anti-inflammatory activities of synthetic isothiocyanates: Effect of chemical structures and cellular signaling. Chem. Biol. Interact. 2009, 179, 202–211. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dey, S.; Bhattacharya, R.K.; Roy, M. Isothiocyanates sensitize the effect of chemotherapeutic drugs via modulation of protein kinase C and telomerase in cervical cancer cells. Mol. Cell. Biochem. 2009, 330, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Menegon, S.; Columbano, A.; Giordano, S. The Dual Roles of NRF2 in Cancer. Trends Mol. Med. 2016, 22, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Royston, K.J.; Tollefsbol, T.O. The Epigenetic Impact of Cruciferous Vegetables on Cancer Prevention. Curr. Pharm. Rep. 2015, 1, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, S.M.; Royce, S.G.; Licciardi, P.V.; Karagiannis, T.C. Dietary Sulforaphane in Cancer Chemoprevention: The Role of Epigenetic Regulation and HDAC Inhibition. Antioxid. Redox Signal. 2015, 22, 1382–1424. [Google Scholar] [CrossRef]
- Clarke, J.D.; Hsu, A.; Yu, Z.; Dashwood, R.H.; Ho, E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol. Nutr. Food Res. 2011, 55, 999–1009. [Google Scholar] [CrossRef] [PubMed]
| Edible Sprout | Main Bioactive Compounds | Main Bioactivities Associated with Sprout Consumption | References |
|---|---|---|---|
| Broccoli (Brassica oleracea var. Italica) | Flavonoids Quercetin, kaempferol, and flavonol glycosides | Cancer risk (↓) Degenerative diseases (↓) Obesity-related metabolic disorders (↓) Allergic nasal symptoms (↓) Inflammation (↓) Pain (↓) Antioxidant capacity (↑) | [5,8] |
| Phenolic acids Chlorogenic, sinapic, and ferulic acid derivatives | |||
| Glucosinolates Glucoraphanin, glucoiberin, glucoraphenin, glucobrassicin, 4-hydroxyglucobrassicin, 4-methoxyglucobrassicin, and neoglucobrassicin | |||
| Isothiocyanates Sulphoraphane, iberin, and indole-3-carbinol | |||
| Radish (Raphanus sativus L.) | Flavonoids Quercetin | Risk of cancer (↓) Heart disease (↓) Diabetes (↓) Antioxidant capacity (↑) | [9] |
| Phenolic acids Ferulic, caffeic and p-coumaric acids, and derivatives | |||
| Glucosinolates Glucoraphenin, dehydroerucin, glucobrassicin, and 4-methoxyglucobrassicin | |||
| Isothiocyanates Sulforaphene, sulforaphane, and indole-3-carbinol | |||
| Kale (Brassica oleracea var. acephala) | Flavonoids Quercetin and cyanidin | Risk of cancer (↓) Heart disease (↓) Diabetes (↓) Antioxidant capacity (↑) | [10] |
| Phenolic acids Chlorogenic and ferulic acids | |||
| Glucosinolates Glucoraphanin, glucoiberin, gluconapin, gluconasturtin, progoitrin, gluconapin, gluconapoleiferin, sinigrin, glucobrassicin, 4-hydroxyglucobrassicin, 4-methoxyglucobrassicin, and neoglucobrassicin | |||
| Pak choi (Brassica rapa var. chinensis) | Flavonoids Kaempferol, quercetin, and isorhamnetin glucosides | Risk of cancer (↓) Heart disease (↓) Diabetes (↓) Antioxidant capacity (↑) | [10,11] |
| Phenolic acids Ferulic, sinapic, caffeic, and p-coumaric acids, and derivatives | |||
| Glucosinolates Gluconapin, glucoalyssin, gluconasturtin, progoitrin, glucobrassicin, 4-hydroxyglucobrassicin, 4-methoxyglucobrassicin, and neoglucobrassicin |
| Raw Edible Sprout | Elicitor Treatment | Elicitor Classification | Application | Target Compound and Increase | Reference |
|---|---|---|---|---|---|
| Broccoli sprouts (Brassica oleracea) (7 days of growth) | Sucrose, fructose, and glucose (146 mM) | Biotic elicitor | In 0.5% agar media for 5 days after sowing seeds | Total anthocyanins (10.0%) | [28] |
| Broccoli sprouts (Brassica oleracea) (7 days of growth) | Sucrose and mannitol (176 mM) | Biotic elicitor | Hydroponic system for 5 days after sowing seeds | Total anthocyanins (40.0%) and phenolics (60.0%) Total glucosinolates (50.0%) | [28] |
| Broccoli (Brassica oleracea) (7 days of growth) | Met (5 mM) Trp (10 mM) SA (100 μM) MeJA (25 μM) | Biotic elicitors (Met, Trp, and plant hormones—SA and MeJA) | Daily exogenous spraying during 3, 5, and 7 days | Met: glucoiberin, glucoraphanin, and glucoerucin (30.0%) Trp: 4-hydroxyglucobrassicin, glucobrassicin, 4-Methoxyglucobrassicin, and neoglucobrassicin (80.0%) SA: 4-hydroxyglucobrassicin, glucobrassicin, 4-Methoxyglucobrassicin, and neoglucobrassicin (30.0%) MeJA: 4-hydroxyglucobrassicin, glucobrassicin, 4-Methoxyglucobrassicin, neoglucobrassicin (50.0%) | [29] |
| Broccoli sprouts (Brassica oleracea) | Sucrose (146 mM) | Biotic elicitor | In 0.5% agar media for 5 days after sowing | Total GLS (2.0-fold) | [28] |
| Broccoli sprouts (Brassica oleracea) (7 days of growth) | Mg (300 mg L−1) | Abiotic elicitor | Suplementation with MgSO4 | Increase of total ascorbic acid contain (29.1–44.5%) | [27] |
| Radish sprouts (raphanistrum subsp. sativus) (12 days of growth) | MeJA (100 μM) | Biotic elicitor (plant hormones—MeJA) | Treatment with MeJA in growth chamber under dark conditions | Glucoalyssin (1.4-fold) Glucoerucin (2.0-fold) Glucotropaeolin (1.8-fold) Glucoraphasatin (1.4-fold) | [30] |
| Radish sprouts (raphanistrum subsp. sativus) (12 days of growth) | MeJA (100 μM) Light | Biotic elicitor (plant hormones—MeJA-) Abiotic elicitor | Treatment with MeJA in growth chamber under light | Glucoraphanin (1.5-fold) Glucoerucin (1.6-fold) Glucotropaeolin (1.3-fold) 4-hydroxyglucobrassicin (4.4-fold) Pergonidin (1.7-fold) Cyanidin (2.0-fold) | [30] |
| Radish sprouts (raphanistrum subsp. sativus) (7 days of growth) | Mg (300 mg L−1) | Abiotic elicitor | Supplementation with MgSO4 | Phenolic compounds (13.9–21.7%) | [27] |
| Radish sprouts (raphanistrum subsp. sativus) | NaCl (100 mM) | Abiotic elicitor | In 0.5% agar media for 3.5 and 7.0 days after sowing | Total phenolics (30 and 50% in 5 and 7 day-old sprouts, respectively) Total GLS (50% and 120% in 5 and 7 day-old sprouts, respectively) | [31] |
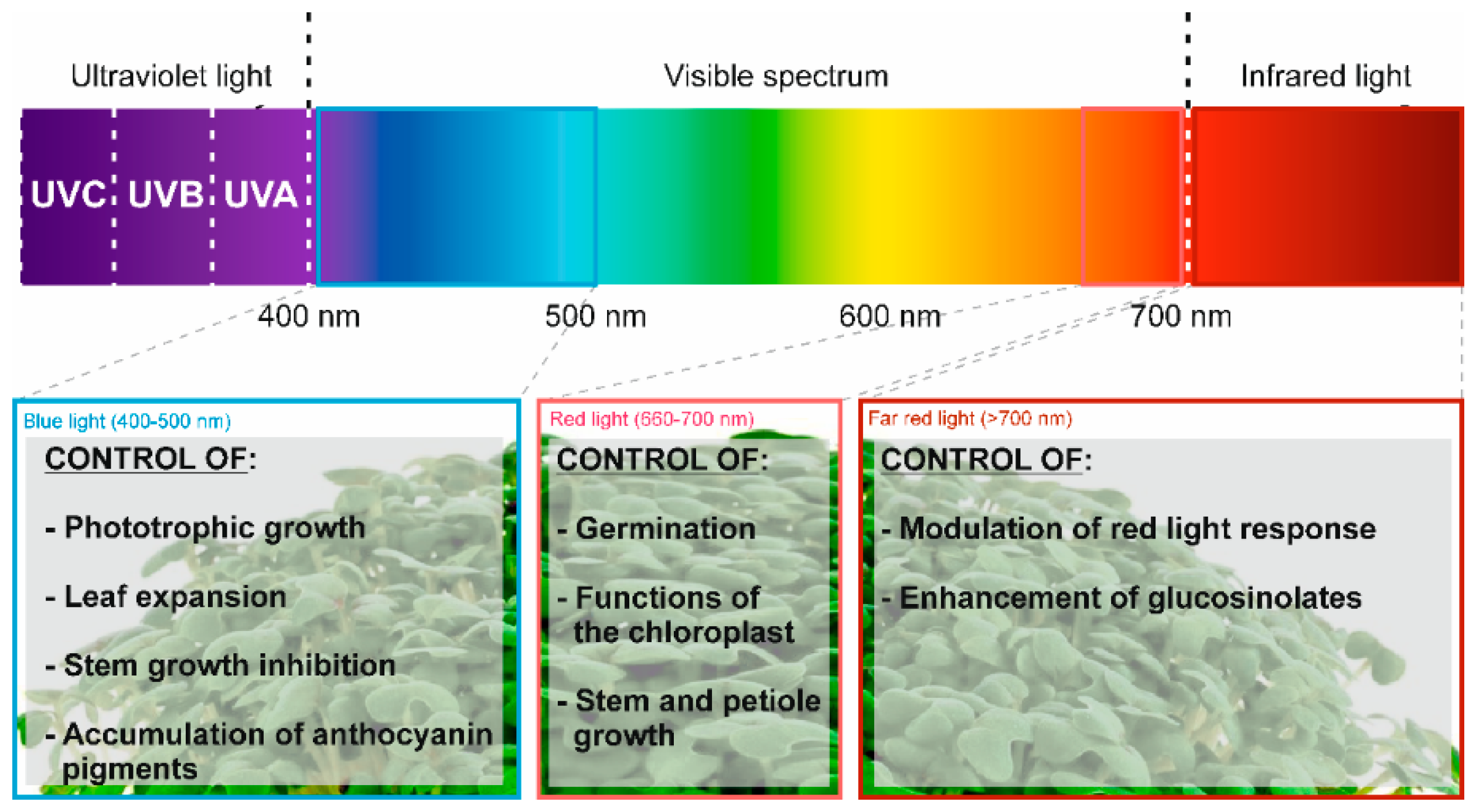
| Pak Choi sprouts (rapa subsp. chinensis) | Application of different wavelengths of LED light (white, blue, and red) | Abiotic elicitor | Medium of perlite for 5 days in darkness and 18 h at the different wavelengths | Total carotenoid content (12.1% and 9.2% with white light (respect to blue and red light, respectively) | [25] |
| Pak Choi sprouts (rapa subsp. chinensis) | Application of different wavelengths of LED light (white, blue, and red) | Abiotic elicitor | Medium of perlite for 5 days in darkness and 18 h at the different wavelengths | Enhanced transcription of genes involved in carotenoid biosynthesis (CYP97A3, CYP97C1, βLCY, εLCY, β-OHASE1, PDS, PSY, VDE, ZEP) | [25] |
| Kale Sprouts (oleracea var. sabellica) | Application of different light wavelengths (470, 660, and 730 nm) | Abiotic elicitor | Seeds stratified for 2 days, exposed to light for 1 h, exposed to darkness for between 1 and 3 days and later, the specific light treatment | Total GLS content (31.7%) | [32] |
| Radish, Chinese kale and pak choi sprouts (3 days of growth) | Glucose (5 g 100 mL−1) | Biotic elicitor | Hydroponic system for 3 days after sowing seeds | Total phenolics (20.0%), gluconapin (150.0% and 60.0% in Chinese kale and pak choi, respectively), glucobrassicanapin (110-fold in pak choi) | [33] |
| Different Brassica sprouts (broccoli, turnip, and rutabaga) | MeJA (25 μM) JA (150 μM) Sucrose (146 mM) | Biotic elicitors (Sucrose and plant hormones—MeJA and JA) | Sprayed for 5 days before harvest | Total GLS (>50%, broccoli; >20.0% turnip; >100.0% rutabaga) | [34] |
| Radish sprouts (raphanistrum subsp. sativus) (8 days of growth) | MeJA (25 μM) SA (100 μM) Glucose (277 mM) | Biotic elicitors (glucose and plant hormones—MeJA and JA) | Sprayed for 5 days before harvest | Total GLS (20.0%) | [34] |
| Matrix | Pathophysiological Condition | Effect | Model | Action Mechanism Z | Ref. |
|---|---|---|---|---|---|
| Broccoli sprouts | Metabolic profile | No specific effect monitored | Humans | FA 14:1, FA 16:1, FA 18:1, FA 14:0, FA 16:0, FA 18:0, dehydroepiandrosterone, glutathione, cysteine, and glutamine (↑) Deoxy-uridin monophosohate (↓) | [42] |
| Radish sprouts | Energy metabolism | Decrease glucose level | Drosophila melanogaster | Expression of spargel (↑) | [43] |
| Broccoli sprouts | Pregnancy | Prevention of brain injury in newborns | Rats | Not determined | [44] |
| Broccoli sprouts | Inflammation and oxidative stress | Modulation of inflammation and vascular events | Humans | Not determined | [45] |
| Broccoli sprouts | Inflammation in overweight population | Anti-inflammatory activity | Humans | IL-6 and C-reactive protein (↓) | [46] |
| Broccoli sprout powder | Diabetes | Anti-inflammatory effect | Humans | C-reactive protein (↓) | [47] |
| Broccoli sprouts | Hypertension | Does not improve endothelial function of hypertension in humans | Humans | Not determined | [48] |
| Broccoli sprouts | Hypertension | Attenuation of oxidative stress, hypertension, and inflammation | Rats | Not determined | [49] |
| Rutabaga sprouts | Thyroid function and iodine deficiency. Role as goitrogenic foods | Protective effect against thyroid damage Goitrogenic activity not discarded | Male rats | Dietary source of iodine GPX1, GPX3, and FRAP (↓) | [50] |
| Broccoli sprouts | Hepatic and renal toxicity | Antioxidant activity | Female rats | Phase-II enzymes (↑) Lipid peroxidation and apoptosis (↓) | [51] |
| Broccoli sprouts | Bowel habits | Decrease in the constipation scoring system Decrease of Bifidobacterium | Humans | Not determined | [52] |
| Broccoli sprouts | Pain assessment and analgesia | Dose-dependent nociceptive activity | Rats | Agonists of central and peripheral opioid receptors | [53] |
| Tuscan black cabbage sprout extract | Xenobiotic metabolism and antioxidant defense | Improvement of the detoxification of xenebiotics | Rats | Induction of phase-II enzymes and boosting of the enzymatic activity of catalase, NAD(P)H:quinone reductase, glutathione reductase, and glutathione peroxidase | [54] |
| Japanese Radish Sprout | Diabetes | Decrease in plasma fructosamine, glucose, and insulin in diabetic rats | Rats | Not determined | [40] |
| Radish sprouts | Diabetes | Increase in blood glucose, triglycerides, total cholesterol, low-density lipoproteins, and very low density lipoproteins | Rats | Not determined | [55] |
| Broccoli sprout extracts | Skin disorders | Induction of phase-II response | Mice and humans | NQO1 enzyme activity (↑) | [56] |
| Broccoli sprout extracts | Skin disorders | Protection against inflammation, edema, and carcinogens in humans | Humans | Phase-II enzymes (↑) NQO1 enzyme activity (↑) | [57] |
| Broccoli sprout homogenate | Physiological upper airway | No specific effect monitored | Humans | Phase-II enzymes (↑) | [58] |
| Broccoli sprouts | Physiological upper airway | No specific effect monitored | Humans | Nrf2 activity (↑) Secretory leukocyte protease inhibitor (↑) | [59] |
| Broccoli sprout extract | Asthma | Blocking the bronchoconstrictor hyperresponsiveness of some asthmatic phenotypes | Humans | Activity of Nrf2 regulated antioxidant and anti-inflammatory genes (↓) | [60] |
| Broccoli sprout extract | Hepatic disturbances | Improvement of liver functions and reduction of oxidative stress | Rats | Not determined | [61] |
| Broccoli sprout-based supplements | General carcinogenic processes | Chemopreventive effect | Humans | Not determined | [62] |
| Broccoli sprout extract | Head and neck squamous cell carcinoma | Chemopreventive activity of sulforaphane against carcinogen-induced oral cancer | Mice | Time and dose dependent induction of Nrf2 and Nrf2 target genes (NQO1 and GCLC) Dephosphorilation of pSTAT3 | [63] |
| Broccoli sprouts homogenate | Sickle cell disease (hemoglobinopathy) | Change in the gene expression levels | Humans | Expression of Nrf2 targets (HMOX1 and HBG1) (↑) | [64] |
| Broccoli sprouts | Oxidative stress | Improvement in cholesterol metabolism and decrease in oxidative stress | Humans | Not determined | [65] |
| Broccoli sprouts | General carcinogenic processes | Chemopreventive agent | Humans | Histone deacetylase activity (↓) | [66] |
| Broccoli sprouts | Unspecific frame | Not determined | Humans | Histone deacetylase activity (↓) | [67] |
| Broccoli sprouts | Antimicrobial activity against Helicobacter pylori | Reduction of Helicobacter pylori colonization in mice Enhancement of sequelae of Helicobacter pylori infection in mice and humans | Mice and humans | Not determined | [68] |
| Broccoli sprout extract | Allergic response | Broccoli sprouts reduce the impact of particulate pollution of allergic disease and asthma | Humans | Not determined | [69] |
| Broccoli sprout extract | Prostate cancer | Inconclusive | Humans | Not determined | [70] |
| Broccoli sprout and myrosinase-treated broccoli sprout extracts | Chemoprevention of carcinogenesis processes | Inconclusive | Humans | No dose response was observed for molecular targets | [71] |
| Broccoli sprout extract | Psychiatric disorders | Improvement of the cognitive function in patients affected by schizophrenia | Humans | Not determined | [72] |
| Broccoli sprout extract | Type II diabetes | Reduction of fasting blood glucose and glycated hemoglobin | Mice | (↑) Nuclear translocation of Nrf2 (↓) Glucose production and intolerance | [73] |
| Broccoli sprout extract | Neurological disorder | Inconclusive improvement of Autism symptoms | Humans | (↑) Gene transcription in multiple cell signaling pathways | [74] |
| Broccoli sprout homogenate | Viral infections | Enhancement of antiviral defense response | Humans | Modulation of natural killer cell activation Production of granzyme B by natural killer cells (↑) | [75] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abellán, Á.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Sorting out the Value of Cruciferous Sprouts as Sources of Bioactive Compounds for Nutrition and Health. Nutrients 2019, 11, 429. https://doi.org/10.3390/nu11020429
Abellán Á, Domínguez-Perles R, Moreno DA, García-Viguera C. Sorting out the Value of Cruciferous Sprouts as Sources of Bioactive Compounds for Nutrition and Health. Nutrients. 2019; 11(2):429. https://doi.org/10.3390/nu11020429
Chicago/Turabian StyleAbellán, Ángel, Raúl Domínguez-Perles, Diego A. Moreno, and Cristina García-Viguera. 2019. "Sorting out the Value of Cruciferous Sprouts as Sources of Bioactive Compounds for Nutrition and Health" Nutrients 11, no. 2: 429. https://doi.org/10.3390/nu11020429
APA StyleAbellán, Á., Domínguez-Perles, R., Moreno, D. A., & García-Viguera, C. (2019). Sorting out the Value of Cruciferous Sprouts as Sources of Bioactive Compounds for Nutrition and Health. Nutrients, 11(2), 429. https://doi.org/10.3390/nu11020429






