New Insights on the Nutrition Status and Antioxidant Capacity in Multiple Sclerosis Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. MS Patients Display Significant Lower Intake of Many Nutritional Components
3.2. Routine Blood Tests Show Lower Iron in MS Patients
3.3. MS Patients Present Unique Fatty Acid Profiles on Cell Membranes
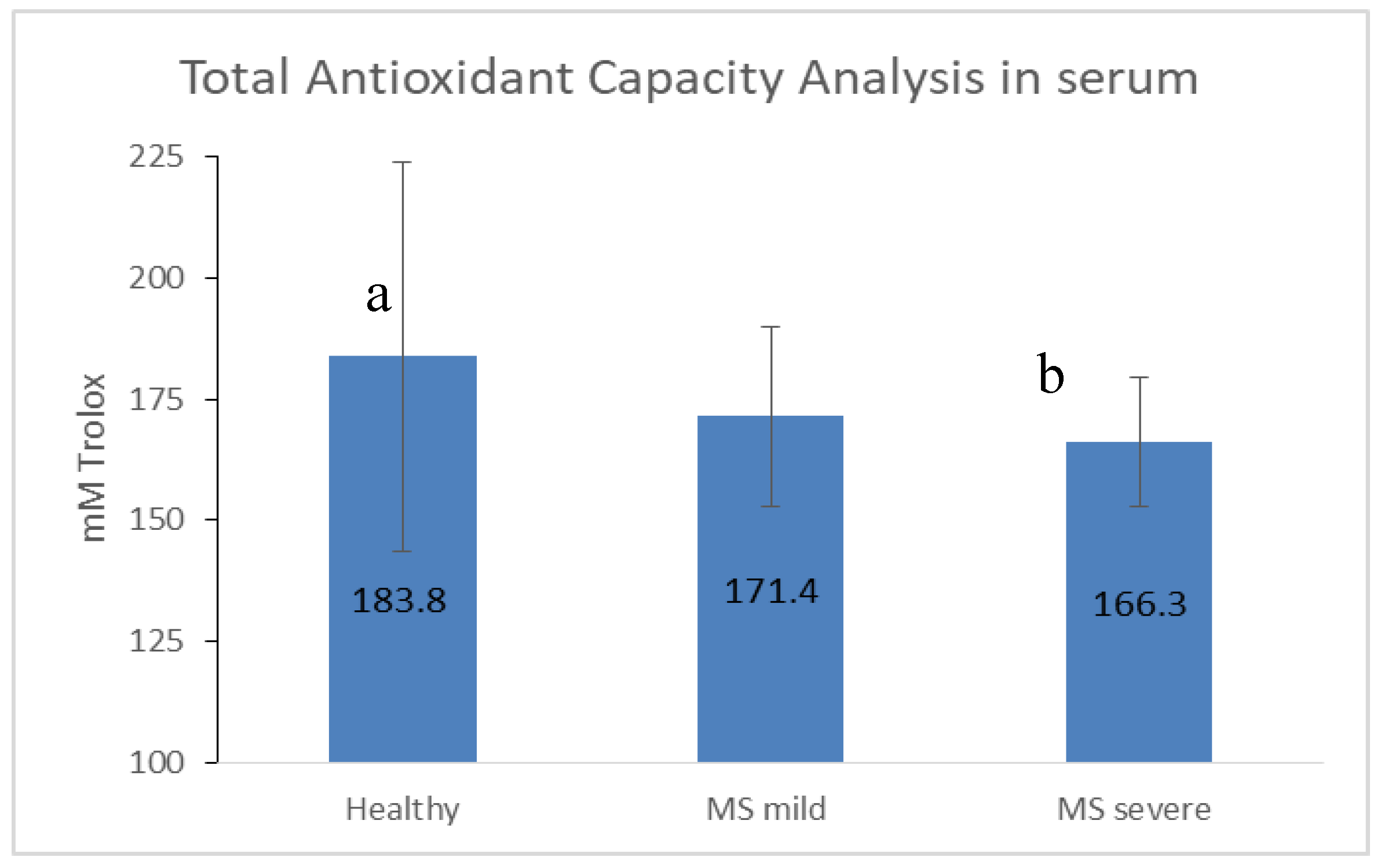
3.4. Antioxidant Capacity Is Limited in MS, Especially in the Severe State
4. Discussion
4.1. Differences in Consumption Levels of Nutritional Components Are Found between MS Patients and Healthy Controls
4.2. MS Patients Display Low Levels of Essential Minerals
4.3. MS Patients Display Low Intake Levels of B Vitamins
4.4. The Fatty Acid Profiles on Cell Membranes in MS Patients Are Different from Controls
4.5. Decreased Antioxidant Capacity Is Associated with MS Disease Severity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munger, K.L. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann. Neurol. 2007, 61, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Riccio, P.; Rossano, R. Nutrition facts in multiple sclerosis. ASN Neuro 2015, 7, 1759091414568185. [Google Scholar] [CrossRef] [PubMed]
- Bagur, M.J.; Murcia, M.A.; Jimenez-Monreal, A.M.; Tur, J.A.; Bibiloni, M.M.; Alonso, G.L.; Martinez-Tome, M. Influence of Diet in Multiple Sclerosis: A Systematic Review. Adv. Nutr. 2017, 8, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Balto, J.M.; Ensari, I.; Hubbard, E.A.; Khan, N.; Barnes, J.L.; Motl, R.W. Individual and Co-occurring SNAP Risk Factors: Smoking, Nutrition, Alcohol Consumption, and Physical Activity in People with Multiple Sclerosis. Int. J. MS Care 2016, 18, 298–304. [Google Scholar] [CrossRef]
- Swank, R.L.; Dugan, B.B. Effect of low saturated fat diet in early and late cases of multiple sclerosis. Lancet 1990, 336, 37–39. [Google Scholar] [CrossRef]
- Haase, S.; Haghikia, A.; Gold, R.; Linker, R.A. Dietary fatty acids and susceptibility to multiple sclerosis. Mult. Scler. J. 2018, 24, 12–16. [Google Scholar] [CrossRef]
- Besler, H.T.; Comoglu, S.; Okcu, Z. Serum levels of antioxidant vitamins and lipid peroxidation in multiple sclerosis. Nutr. Neurosci. 2002, 5, 215–220. [Google Scholar] [CrossRef]
- Choi, I.Y.; Lee, P.; Adany, P.; Hughes, A.J.; Belliston, S.; Denney, D.R.; Lynch, S.G. In vivo evidence of oxidative stress in brains of patients with progressive multiple sclerosis. Mult. Scler. J. 2018, 24, 1029–1038. [Google Scholar] [CrossRef]
- Thompson, F.E.; Byers, T. Dietary assessment resource manual. J. Nutr. 1994, 124 (Suppl. 11), 2245S–2317S. [Google Scholar] [PubMed]
- Kaluski, D.N.; Goldsmith, R.; Arie, O.M.; Mayer, C.; Green, M. The first Israeli national health and nutrition survey (MABAT) as a policy maker. Public Health Rev. 2000, 28, 23–26. [Google Scholar] [PubMed]
- von Schacky, C. Omega-3 index and cardiovascular health. Nutrients 2014, 6, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.E.; Clausen, J. Glutathione peroxidase activity, associated enzymes and substrates in blood cells from patients with multiple sclerosis—Effects of antioxidant supplementation. Acta Pharmacol. Et Toxicol. 1986, 59 (Suppl. 7), 450–453. [Google Scholar] [CrossRef]
- Alschuler, K.N.; Gibbons, L.E.; Rosenberg, D.E.; Ehde, D.M.; Verrall, A.M.; Bamer, A.M.; Jensen, M.P. Body mass index and waist circumference in persons aging with muscular dystrophy, multiple sclerosis, post-polio syndrome, and spinal cord injury. Disabil. Health J. 2012, 5, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sorgun, M.H.; Yucesan, C.; Tegin, C. Is malnutrition a problem for multiple sclerosis patients? J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2014, 21, 1603–1605. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.F.; Frischer, J.M.; Webb, S.M.; Tham, M.; Adiele, R.C.; Robinson, C.A.; Fitz-Gibbon, P.D.; Weigand, S.D.; Metz, I.; Nehzati, S.; et al. Pathogenic implications of distinct patterns of iron and zinc in chronic MS lesions. Acta Neuropathol. 2017, 134, 45–64. [Google Scholar] [CrossRef]
- Karimi, A.; Bahrampour, K.; Momeni Moghaddam, M.A.; Asadikaram, G.; Ebrahimi, G.; Torkzadeh-Mahani, M.; Esmaeili Tarzi, M.; Nematollahi, M.H. Evaluation of lithium serum level in multiple sclerosis patients: A neuroprotective element. Mult. Scler. Relat. Disord. 2017, 17, 244–248. [Google Scholar] [CrossRef]
- Alizadeh, A.; Mehrpour, O.; Nikkhah, K.; Bayat, G.; Espandani, M.; Golzari, A.; Jarahi, L.; Foroughipour, M. Comparison of serum Concentration of Se, Pb, Mg, Cu, Zn, between MS patients and healthy controls. Electron. Physician 2016, 8, 2759–2764. [Google Scholar] [CrossRef]
- Pawlitzki, M.; Uebelhor, J.; Sweeney-Reed, C.M.; Stephanik, H.; Hoffmann, J.; Lux, A.; Reinhold, D. Lower Serum Zinc Levels in Patients with Multiple Sclerosis Compared to Healthy Controls. Nutrients 2018, 10, 967. [Google Scholar] [CrossRef]
- Sheykhansari, S.; Kozielski, K.; Bill, J.; Sitti, M.; Gemmati, D.; Zamboni, P.; Singh, A.V. Redox metals homeostasis in multiple sclerosis and amyotrophic lateral sclerosis: A review. Cell Death Dis. 2018, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Bredholt, M.; Frederiksen, J.L. Zinc in Multiple Sclerosis: A Systematic Review and Meta-Analysis. ASN Neuro 2016, 8, 1759091416651511. [Google Scholar] [CrossRef] [PubMed]
- Ramsaransing, G.S.; Mellema, S.A.; De Keyser, J. Dietary patterns in clinical subtypes of multiple sclerosis: An exploratory study. Nutr. J. 2009, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Lees, K.R.; Ford, I.; Davis, S. Magnesium for acute stroke (Intravenous Magnesium Efficacy in Stroke trial): Randomised controlled trial. Lancet 2004, 363, 439–445. [Google Scholar] [PubMed]
- Yasui, M.; Yase, Y.; Ando, K.; Adachi, K.; Mukoyama, M.; Ohsugi, K. Magnesium concentration in brains from multiple sclerosis patients. Acta Neurol. Scand. 1990, 81, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Herbert, E.; Engel-Hills, P.; Hattingh, C.; Fouche, J.P.; Kidd, M.; Lochner, C.; Kotze, M.J.; van Rensburg, S.J. Fractional anisotropy of white matter, disability and blood iron parameters in multiple sclerosis. Metab. Brain Dis. 2018, 33, 545–557. [Google Scholar] [CrossRef] [PubMed]
- LeVine, S.M.; Chakrabarty, A. The role of iron in the pathogenesis of experimental allergic encephalomyelitis and multiple sclerosis. Ann. N. Y. Acad. Sci. 2004, 1012, 252–266. [Google Scholar] [CrossRef]
- Pakpoor, J.; Seminatore, B.; Graves, J.S.; Schreiner, T.; Waldman, A.T.; Lotze, T.E.; Belman, A.; Greenberg, B.M.; Weinstock-Guttman, B.; Aaen, G.; et al. Dietary factors and pediatric multiple sclerosis: A case-control study. Mult. Scler. J. 2018, 24, 1067–1076. [Google Scholar] [CrossRef]
- van Rensburg, S.J.; Kotze, M.J.; van Toorn, R. The conundrum of iron in multiple sclerosis—Time for an individualised approach. Metab. Brain Dis. 2012, 27, 239–253. [Google Scholar] [CrossRef]
- Trepanier, M.O.; Hildebrand, K.D.; Nyamoya, S.D.; Amor, S.; Bazinet, R.P.; Kipp, M. Phosphatidylcholine 36:1 concentration decreases along with demyelination in the cuprizone animal model and in post-mortem multiple sclerosis brain tissue. J. Neurochem. 2018, 145, 504–515. [Google Scholar] [CrossRef]
- De Riccardis, L.; Buccolieri, A.; Muci, M.; Pitotti, E.; De Robertis, F.; Trianni, G.; Manno, D.; Maffia, M. Copper and ceruloplasmin dyshomeostasis in serum and cerebrospinal fluid of multiple sclerosis subjects. Biochim. Et Biophys. Acta. Mol. Basis Dis. 2018, 1864 Pt A, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Nemazannikova, N.; Mikkelsen, K.; Stojanovska, L.; Blatch, G.L.; Apostolopoulos, V. Is there a Link between Vitamin B and Multiple Sclerosis? Med. Chem. 2018, 14, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Fan, Z.; Zhang, Y.; Yu, R.; Yang, H.; Zhou, C.; Luo, J.; Ke, Z.J. Thiamine deficiency promotes T cell infiltration in experimental autoimmune encephalomyelitis: The involvement of CCL2. J. Immunol. 2014, 193, 2157–2167. [Google Scholar] [CrossRef]
- Najafi, M.R.; Shaygannajad, V.; Mirpourian, M.; Gholamrezaei, A. Vitamin B(12) Deficiency and Multiple Sclerosis; Is there Any Association? Int. J. Prev. Med. 2012, 3, 286–289. [Google Scholar] [PubMed]
- Laye, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Vidaurre, O.G.; Haines, J.D.; Katz Sand, I.; Adula, K.P.; Huynh, J.L.; McGraw, C.A.; Zhang, F.; Varghese, M.; Sotirchos, E.; Bhargava, P.; et al. Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain 2014, 137 Pt 8, 2271–2286. [Google Scholar] [CrossRef]
- Moyano, A.L.; Li, G.; Boullerne, A.I.; Feinstein, D.L.; Hartman, E.; Skias, D.; Balavanov, R.; van Breemen, R.B.; Bongarzone, E.R.; Mansson, J.E.; et al. Sulfatides in extracellular vesicles isolated from plasma of multiple sclerosis patients. J. Neurosci. Res. 2016, 94, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, V.S.; Ponimaskin, E. Palmitoylation as a Functional Regulator of Neurotransmitter Receptors. Neural Plast. 2018, 2018, 5701348. [Google Scholar] [CrossRef] [PubMed]
- Messedi, M.; Naifar, M.; Grayaa, S.; Frikha, F.; Messoued, M.; Sethom, M.M.; Feki, M.; Kaabach, N.; Bahloul, Z.; Jamoussi, K.; et al. Plasma Saturated and Monounsaturated Fatty Acids in Behcet’s Disease. Open Rheumatol. J. 2018, 12, 139–151. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Gutierrez-Aguilar, R. The Role of the Novel Lipokine Palmitoleic Acid in Health and Disease. Adv. Nutr. 2017, 8, 173S–181S. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Watkins, S.M.; Hotamisligil, G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008, 134, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, H.; Xu, H.; Halim, V.; Zhang, W.; Wang, H.; Ong, K.T.; Woo, S.L.; Walzem, R.L.; Mashek, D.G.; et al. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS ONE 2012, 7, e39286. [Google Scholar] [CrossRef] [PubMed]
- Hadzovic-Dzuvo, A.; Lepara, O.; Valjevac, A.; Avdagic, N.; Hasic, S.; Kiseljakovic, E.; Ibragic, S.; Alajbegovic, A. Serum total antioxidant capacity in patients with multiple sclerosis. Bosn. J. Basic Med. Sci. 2011, 11, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Frei, B. Efficacy of dietary antioxidants to prevent oxidative damage and inhibit chronic disease. J. Nutr. 2004, 134, 3196S–3198S. [Google Scholar] [CrossRef] [PubMed]
| Variables | Values | Control (n = 83) | MS (n = 63) | p |
|---|---|---|---|---|
| Age at enrolment, year (M ± SD) | 40.6 ± 11.9 | 44.7 ± 14.0 | 0.056 | |
| Gender (n, %) | Female | 49, 59.0 | 42, 66.7 | 0.346 |
| Ethnicity (n, %) | Jew | 54, 65.1 | 41, 65.1 | 0.998 |
| BMI, kg/h2 (M ± SD) | 25.3 ± 4.7 | 25.0 ± 4.4 | 0.732 |
| Daily Consumption Data (/day) | Control | MS | p * | MS | p ** | |
|---|---|---|---|---|---|---|
| Mean | Mean | EDSS 0–3 | EDSS ≥ 3.5 | |||
| General Components | ||||||
| Food energy (Kcal) | 1973.91 a | 1675.62 | 0.013 | 1627.56 b | 1757.33 ab | 0.036 |
| Protein (g) | 87.07 a | 64.70 | 0.000 | 65.22 b | 63.81 b | 0.001 |
| Carbohydrates (g) | 224.79 | 196.73 | 0.108 | 195.09 | 199.38 | 0.273 |
| Moisture (g) | 3400.68 a | 2279.22 | 0.000 | 2370.08 b | 2124.76 b | 0.001 |
| Total Dietary Fibers (g) | 25.24 a | 19.53 | 0.008 | 18.42 b | 21.32 ab | 0.019 |
| Alcohol (g) | 1.13 | 0.35 | 0.210 | 0.57 | 0.00 | 0.380 |
| EER (kcal) | 2144.9 | 2009.3 | 0.341 | 2074.6 | 1903.6 | 0.456 |
| Total Sugars (g) | 66.16 | 60.73 | 0.455 | 55.49 | 69.21 | 0.327 |
| Minerals | ||||||
| Calcium (mg) | 830.17 a | 633.07 | 0.012 | 609.44 b | 673.26 b | 0.036 |
| Iron (mg) | 14.70 a | 10.69 | 0.008 | 10.08 b | 11.75 b | 0.023 |
| Magnesium (mg) | 519.80 a | 351.73 | 0.003 | 341.18 b | 369.68 b | 0.014 |
| Phosphorus (mg) | 1338.61 a | 999.57 | 0.000 | 1012.88 b | 976.95 b | 0.001 |
| Potassium (mg) | 3339.78 a | 2490.56 | 0.000 | 2434.61 b | 2590.67 b | 0.001 |
| Sodium (mg) | 3274.69 a | 2392.66 | 0.002 | 2321.24 b | 2520.47 ab | 0.006 |
| Zinc (mg) | 10.66 a | 7.51 | 0.000 | 7.68 b | 7.20 b | 0.000 |
| Copper (mg) | 2.11 | 1.44 | 0.051 | 1.29 | 1.67 | 0.112 |
| Vitamins | ||||||
| Vitamin A (µg) | 562.08 | 467.94 | 0.273 | 481.67 | 443.52 | 0.527 |
| Vitamin E (mg) | 7.97 | 7.37 | 0.530 | 7.39 | 7.32 | 0.821 |
| Vitamin C (mg) | 112.18 | 101.71 | 0.517 | 99.10 | 105.94 | 0.778 |
| Vitamin D (µg) | 46.29 | 30.52 | 0.230 | 30.77 | 30.19 | 0.512 |
| Thiamin (B1) (mg) | 1.27a | 0.93 | 0.001 | 0.89b | 0.99 ab | 0.005 |
| Riboflavin (B2) (mg) | 2.10 a | 1.52 | 0.003 | 1.46 b | 1.63 ab | 0.009 |
| Niacin (B3) (mg) | 24.36 | 19.56 | 0.045 | 18.10 | 21.92 | 0.072 |
| Vitamin B6 (mg) | 1.96 | 1.43 | 0.000 | 1.41 b | 1.47 b | 0.001 |
| Folate B9 (µg) | 359.75 a | 273.06 | 0.003 | 259.42 b | 296.26 ab | 0.010 |
| Vitamin B12 (µg) | 3.56 | 2.74 | 0.079 | 2.59 | 2.99 | 0.182 |
| Carotene (µg) | 3175.02 | 2936.18 | 0.682 | 3417.06 | 2075.67 | 0.283 |
| Fats | ||||||
| Total fat (g) | 78.88 | 67.39 | 0.091 | 62.98 | 74.90 | 0.117 |
| Cholesterol (mg) | 310.75 | 222.49 | 0.042 | 214.92 | 235.36 | 0.121 |
| Saturated fat (g) | 25.41 | 21.39 | 0.114 | 20.54 | 22.83 | 0.240 |
| Trans fatty acids (g) | 0.21 | 0.26 | 0.737 | 0.32 | 0.16 | 0.756 |
| Butyric C4_0 (g) | 0.51 | 0.43 | 0.335 | 0.44 | 0.42 | 0.624 |
| Caproic C6_0 (g) | 0.29 | 0.23 | 0.163 | 0.25 | 0.21 | 0.307 |
| Caprylic C8_0 (g) | 0.28 | 0.20 | 0.042 | 0.20 | 0.19 | 0.123 |
| Capric C10_0 (g) | 0.47 | 0.36 | 0.098 | 0.35 | 0.37 | 0.251 |
| Lauric C12_0 (g) | 0.82 | 0.52 | 0.167 | 0.47 | 0.60 | 0.354 |
| Myristic C14_0 (g) | 2.05 | 1.62 | 0.098 | 1.61 | 1.63 | 0.256 |
| Palmitic C16_0 (g) | 12.36 | 10.87 | 0.243 | 10.92 | 10.77 | 0.498 |
| Stearic C18_0 (g) | 5.56 | 4.66 | 0.174 | 4.46 | 5.01 | 0.340 |
| Oleic C18_1n9 (g) | 26.31 | 22.00 | 0.091 | 22.30 | 21.46 | 0.236 |
| Linolenic ALA C18_3n3 (g) | 1.69 ab | 1.61 | 0.680 | 1.31 b | 2.07 a | 0.026 |
| Arachidonic C20_4n6 (g) | 0.12 | 0.10 | 0.243 | 0.09 | 0.12 | 0.249 |
| Docosahexanoic DHA C22_6n3 (g) | 0.11 | 0.10 | 0.830 | 0.08 | 0.11 | 0.901 |
| Palmitoleic C16_1n7 (g) | 1.10 | 0.97 | 0.349 | 0.95 | 0.99 | 0.635 |
| Parinaric C18_4n3 (Conjugated) (g) | 0.01 | 0.00 | 0.179 | 0.01 | 0.00 | 0.368 |
| Gadoleic C20_1n11 (g) | 0.29 | 0.38 | 0.463 | 0.36 | 0.39 | 0.748 |
| Eicosapentaenoic EPA C20_5n3 (g) | 0.03 | 0.03 | 0.973 | 0.02 | 0.04 | 0.873 |
| Erucic C22_1n9 (g) | 0.04 | 0.03 | 0.530 | 0.03 | 0.04 | 0.755 |
| Docosapentaenoic DPA C22_5 (g) | 0.02 | 0.02 | 0.713 | 0.02 | 0.02 | 0.921 |
| Monosaturated (g) | 30.79 | 25.32 | 0.174 | 22.64 | 29.87 | 0.190 |
| Polysaturated (g) | 12.30 | 9.48 | 0.069 | 8.94 | 10.37 | 0.159 |
| Amino Acids | ||||||
| Isoleucine (g) | 1.80 | 1.30 | 0.029 | 1.22 | 1.42 | 0.077 |
| Leucine (g) | 3.14 | 2.21 | 0.019 | 2.10 | 2.39 | 0.056 |
| Valine (g) | 2.10 a | 1.50 | 0.017 | 1.42 b | 1.61 ab | 0.050 |
| Lysine (g) | 2.80 | 1.94 | 0.030 | 1.83 | 2.13 | 0.082 |
| Threonine (g) | 1.59 | 1.13 | 0.026 | 1.07 | 1.24 | 0.072 |
| Methionine (g) | 0.92 | 0.66 | 0.032 | 0.64 | 0.69 | 0.098 |
| Phenylalanine (g) | 1.82 a | 1.31 | 0.017 | 1.24 b | 1.42 b | 0.049 |
| Tryptophan (g) | 0.27 | 0.22 | 0.348 | 0.22 | 0.23 | 0.635 |
| Histidine (g) | 0.98 | 0.74 | 0.098 | 0.66 | 0.88 | 0.148 |
| Tyrosine (g) | 1.39 | 0.97 | 0.018 | 0.93 | 1.05 | 0.053 |
| Arginine (g) | 2.34 | 1.65 | 0.028 | 1.48 | 1.92 | 0.055 |
| Cystine (g) | 0.56 | 0.41 | 0.041 | 0.38 | 0.46 | 0.092 |
| Serine (g) | 1.86 a | 1.32 | 0.013 | 1.26 b | 1.42 ab | 0.041 |
| Variables | Control | MS | p * | EDSS 0–3 | EDSS ≥ 3.5 | p ** |
|---|---|---|---|---|---|---|
| Iron (µg/dL) | 78.7 ± 33.0 | 62.7 ± 35.3 | 0.043 | 69.9 ± 35.9 | 50.2 ± 31.8 | 0.054 |
| Ferritin (ng/mL) | 111.2 ± 93.8 | 78.9 ± 70.4 | 0.105 | 65.5 ± 62.0 | 101.3 ± 80.4 | 0.155 |
| Vitamin B12 (pg/mL) | 417.2 ± 144.9 | 447.9 ± 170.4 | 0.340 | 451.6 ± 164.6 | 440.8 ± 186.0 | 0.859 |
| Magnesium (mg %) | 2.0 ± 0.2 | 2.1 ± 0.1 | 0.377 | 2.2 ± 0.1 | 2.0 ± 0.1 | 0.066 |
| Folic acid (ng/mL) | 9.3 ± 4.0 | 10.0 ± 5.2 | 0.473 | 9.5 ± 4.9 | 10.9 ± 5.8 | 0.537 |
| Triglycerides (mg/dL) | 110.7 ± 64.3 | 109.0 ± 65.2 | 0.895 | 104.8 ± 61.2 | 117.3 ± 70.7 | 0.757 |
| Total Cholesterol (mg/dL) | 186.5 ± 46.6 | 178.2 ± 32.1 | 0.265 | 172.9 ± 26.5 | 186.6 ± 38.8 | 0.493 |
| HDL (mg/dL) | 50.5 ± 11.5 | 48.9 ± 13.1 | 0.515 | 46.7 ± 10.2 | 53.1 ± 17.0 | 0.197 |
| LDL (mg/dL) | 110.6 ± 37.7 | 109.5 ± 27.3 | 0.869 | 104.7 ± 25.6 | 118.8 ± 28.7 | 0.277 |
| Albumin (g %) | 4.1 ± 0.5 | 4.0 ± 0.5 | 0.303 | 4.1 ± 0.4 | 3.9 ± 0.5 | 0.106 |
| Variables | Control | MS | p * | EDSS 0–3 | EDSS ≥ 3.5 | p ** | ||
|---|---|---|---|---|---|---|---|---|
| Saturated | Myristic C14_0 | 0.25 ± 0.10 | 0.27 ± 0.15 | 0.399 | 0.25 ± 0.14 | 0.30 ± 0.16 | 0.283 | |
| Palmitic acid C16_0 | 21.2 ±1.0 b | 21.7 ± 1.1 | 0.024 | 21.5 ± 1.0 ab | 21.9 ± 1.2 a | 0.023 | ||
| Stearic C18_0 | 17.3 ± 1.4 a | 16.7 ± 1.9 | 0.036 | 16.9 ± 1.9 ab | 16.3 ± 2.1 b | 0.048 | ||
| Eicosanoic C20_0 | 0.20 ±0.06 a | 0.16 ± 0.05 | 0.000 | 0.17 ± 0.05 b | 0.15 ± 0.05 b | 0.000 | ||
| Docosanoic C22_0 | 0.41 ± 0.13 a | 0.31 ± 0.14 | 0.000 | 0.32 ± 0.13 b | 0.28 ± 0.15 b | 0.000 | ||
| Lignoceric C24_0 | 1.11 ± 0.40 a | 0.93 ± 0.36 | 0.010 | 0.98 ± 0.35 ab | 0.85 ± 0.37 b | 0.015 | ||
| Mono saturated | Palmitoleic C16_1n7 | 0.21 ± 0.16 b | 0.27 ± 0.22 | 0.066 | 0.23 ± 0.19 ab | 0.34 ± 0.25 a | 0.019 | |
| Oleic C18_1n9 | 14.6 ± 1.4 | 14.9 ± 1.5 | 0.200 | 14.8 ± 1.4 | 15.1 ± 1.6 | 0.291 | ||
| Eicosenoic C20_1n9 | 0.27 ± 0.06 | 0.25 ± 0.06 | 0.199 | 0.25 ± 0.07 | 0.25 ± 0.04 | 0.404 | ||
| Nervonic C24_1n9 | 1.12 ± 0.33 a | 0.97 ± 0.40 | 0.028 | 1.02 ± 0.41 ab | 0.89 ± 0.39 b | 0.033 | ||
| Polyunsaturated | Omega6 | Linoleic C18_2n6 | 12.8 ± 3.1 | 13.1 ± 3.1 | 0.646 | 12.8 ± 3.1 | 13.4 ± 3.2 | 0.718 |
| gamma Linolenic C18_3n6 | 0.10 ± 0.06 | 0.10 ± 0.06 | 0.868 | 0.09 ± 0.06 | 0.11 ± 0.06 | 0.575 | ||
| Eicosadienoic C20_2n6 | 0.31 ± 0.06 | 0.30 ± 0.06 | 0.347 | 0.30 ± 0.06 | 0.30 ± 0.05 | 0.644 | ||
| DGLA C20_3n6 | 1.75 ± 0.49 | 1.69 ± 0.30 | 0.406 | 1.67 ± 0.30 | 1.70 ± 0.30 | 0.688 | ||
| Arachidonic C20_4n6 | 16.6 ± 1.9 | 16.5 ± 2.1 | 0.856 | 16.5 ± 2.0 | 16.6 ± 2.2 | 0.963 | ||
| Docosatetraenoic C22_4n6 | 3.52 ± 1.03 | 3.65 ± 1.13 | 0.504 | 3.7 ± 1.1 | 3.6 ± 1.2 | 0.765 | ||
| Docosapentaenoic C22_5n6 | 0.97 ± 0.34 | 1.02 ± 0.33 | 0.379 | 1.01 ± 0.34 | 1.04 ± 0.32 | 0.649 | ||
| Omega3 | ALA C18_3n3 | 0.15 ± 0.09 | 0.16 ± 0.11 | 0.292 | 0.15 ± 0.12 | 0.18 ± 0.11 | 0.296 | |
| EPA C20_5n3 | 0.42 ± 0.23 | 0.50 ± 0.38 | 0.131 | 0.56 ± 0.44 | 0.42 ± 0.25 | 0.071 | ||
| Docosahexaenic C22_5n3 | 1.86 ± 0.44 | 1.96 ± 0.50 | 0.255 | 2.05 ± 0.53 | 1.80 ± 0.41 | 0.067 | ||
| DHA C22_6n3 | 4.31 ± 1.16 | 4.13 ± 1.43 | 0.436 | 4.3 ± 1.50 | 3.92 ± 1.37 | 0.457 | ||
| Omega3 Index | 4.76 ± 1.32 | 4.40 ± 1.59 | 0.214 | 4.47 ± 1.65 | 4.29 ± 1.54 | 0.419 | ||
| Trans | C16_1n7t trans | 0.07 ± 0.02 | 0.06 ± 0.03 | 0.068 | 0.06 ± 0.03 | 0.06 ± 0.03 | 0.186 | |
| C18_1t trans | 0.35 ± 0.12 a | 0.29 ± 0.14 | 0.007 | 0.28 ± 0.13 b | 0.29 ± 0.15 b | 0.026 | ||
| C18_2n6tt trans | 0.03 ± 0.07 | 0.03 ± 0.04 | 0.442 | 0.04 ± 0.05 | 0.03 ± 0.04 | 0.714 | ||
| C18_2n6ct trans | 0.02 ± 0.02 b | 0.03 ± 0.02 | 0.006 | 0.02 ± 0.02 b | 0.03 ± 0.03 a | 0.011 | ||
| C18_2n6tc trans | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.698 | 0.09 ± 0.03 | 0.10 ± 0.04 | 0.223 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armon-Omer, A.; Waldman, C.; Simaan, N.; Neuman, H.; Tamir, S.; Shahien, R. New Insights on the Nutrition Status and Antioxidant Capacity in Multiple Sclerosis Patients. Nutrients 2019, 11, 427. https://doi.org/10.3390/nu11020427
Armon-Omer A, Waldman C, Simaan N, Neuman H, Tamir S, Shahien R. New Insights on the Nutrition Status and Antioxidant Capacity in Multiple Sclerosis Patients. Nutrients. 2019; 11(2):427. https://doi.org/10.3390/nu11020427
Chicago/Turabian StyleArmon-Omer, Ayelet, Chen Waldman, Naaem Simaan, Hadar Neuman, Snait Tamir, and Radi Shahien. 2019. "New Insights on the Nutrition Status and Antioxidant Capacity in Multiple Sclerosis Patients" Nutrients 11, no. 2: 427. https://doi.org/10.3390/nu11020427
APA StyleArmon-Omer, A., Waldman, C., Simaan, N., Neuman, H., Tamir, S., & Shahien, R. (2019). New Insights on the Nutrition Status and Antioxidant Capacity in Multiple Sclerosis Patients. Nutrients, 11(2), 427. https://doi.org/10.3390/nu11020427





