Eucommia ulmoides Leaf Extract Ameliorates Steatosis Induced by High-fat Diet in Rats by Increasing Lysosomal Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Purification of EUL
2.2. Animals and Experimental Set-Up
2.3. Biochemical Analyses
2.4. Immunoblotting
2.5. Lysosomal Fractionation
2.6. Cathepsin B Activity
2.7. OxyBlot Assay
2.8. Cellular Reactive Oxygen Species (ROS) Detection
2.9. Analysis of Lipid Peroxidation
2.10. Histological and Immunohistochemical Analyses
2.11. Oil Red O Staining
2.12. Statistical Analysis
3. Results
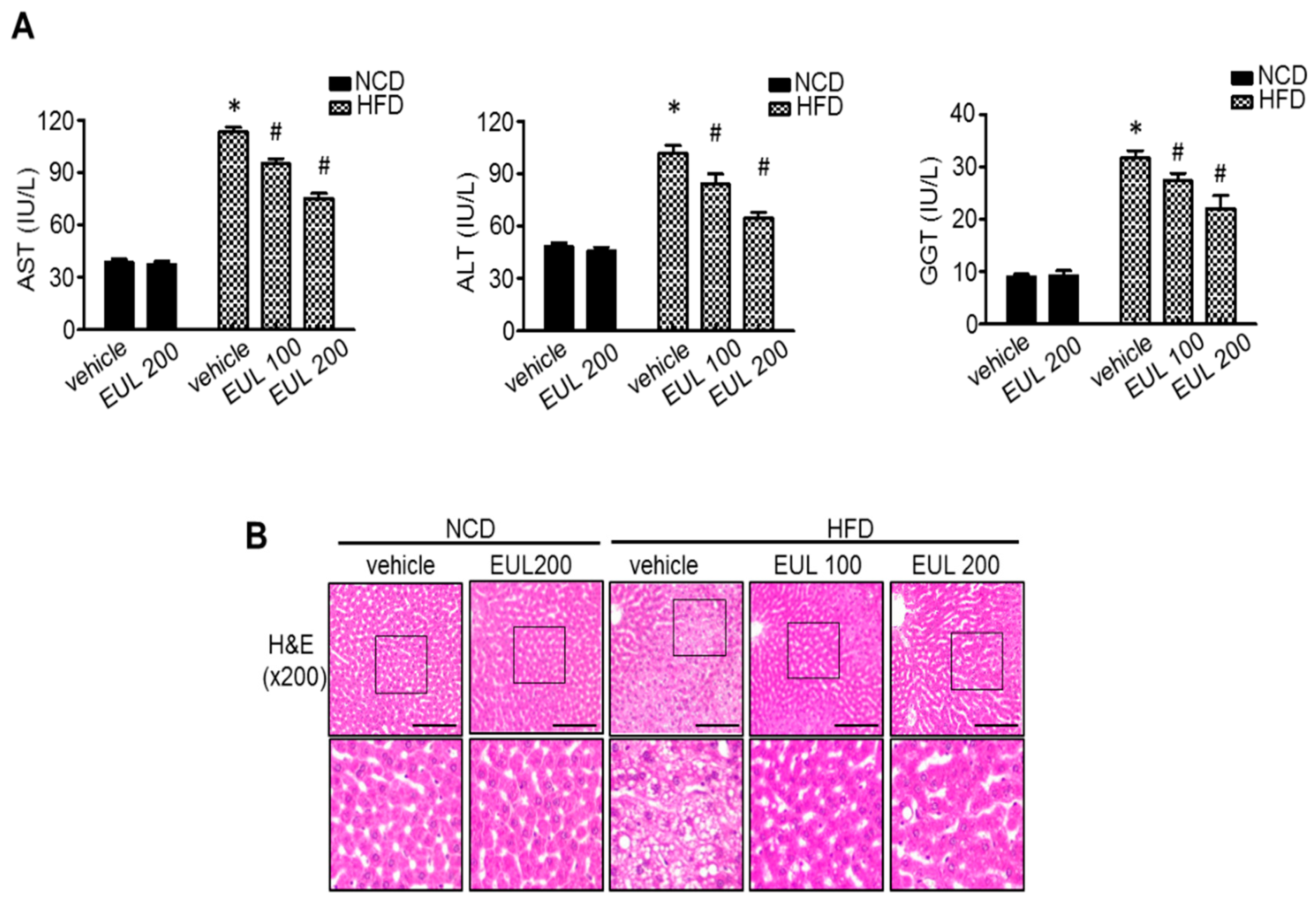
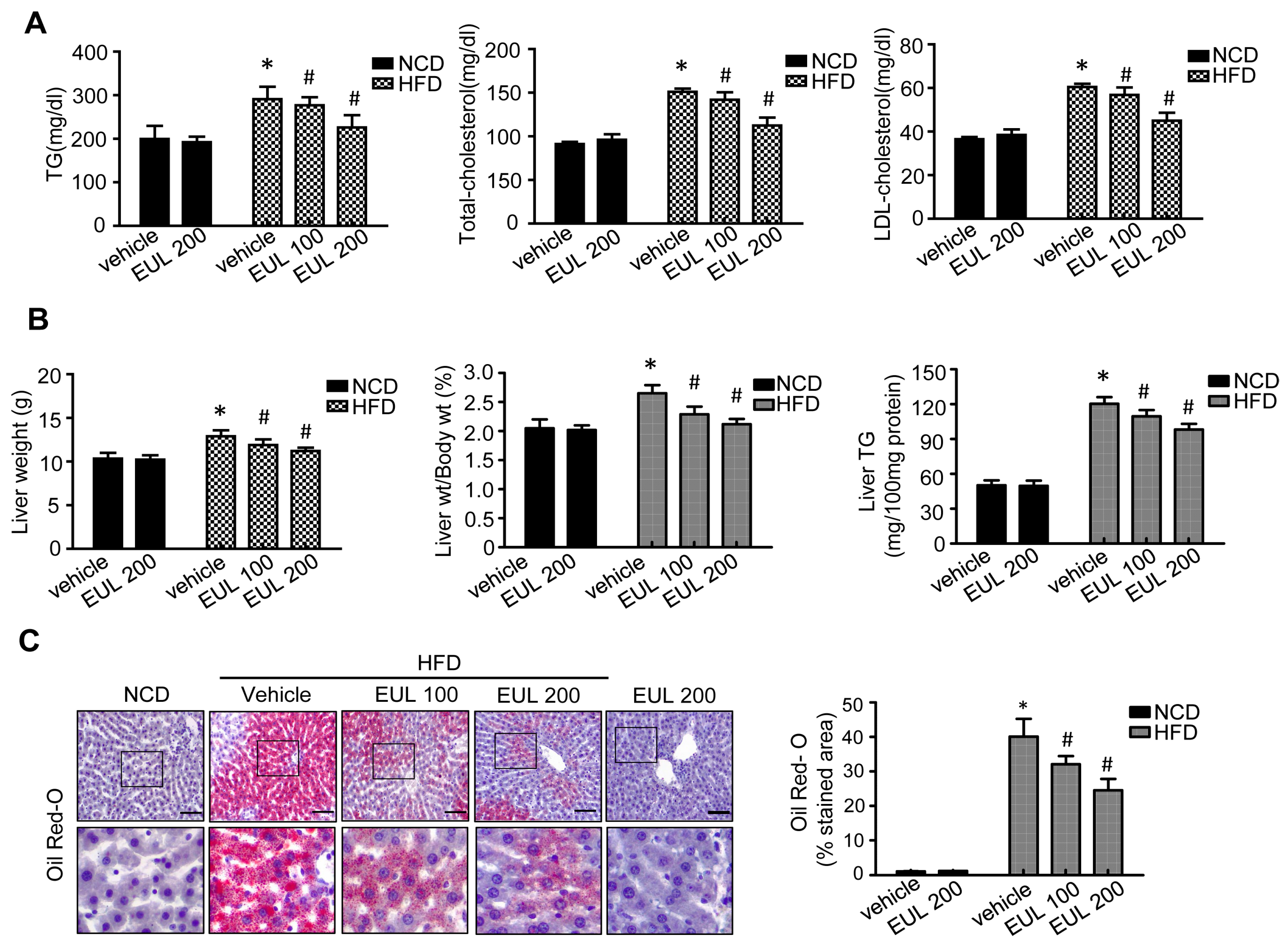
3.1. EUL Prevents HFD-Induced Hepatic Steatosis
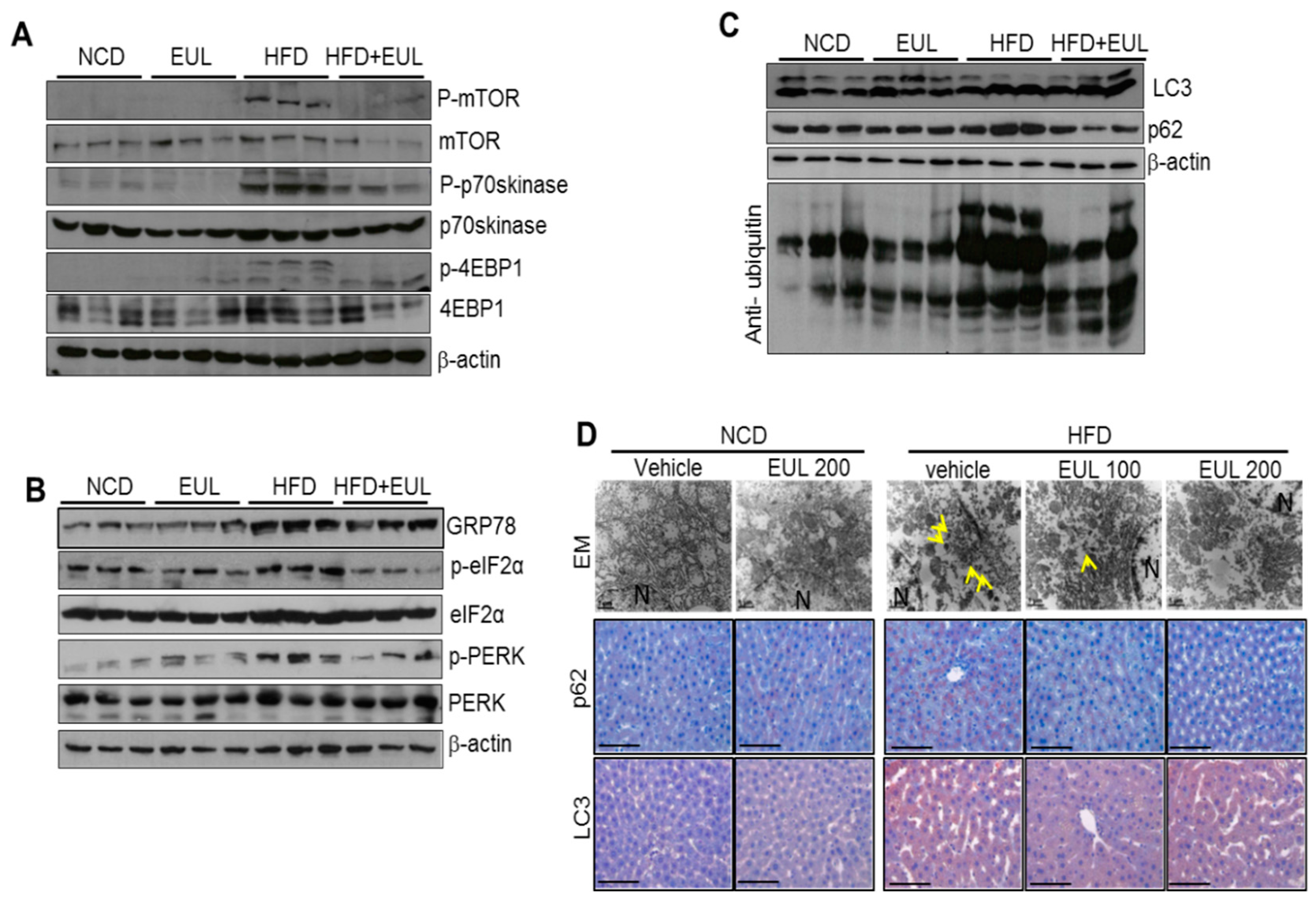
3.2. EUL Inhibits the mTORC1-ER Stress Response in HFD-fed Rats
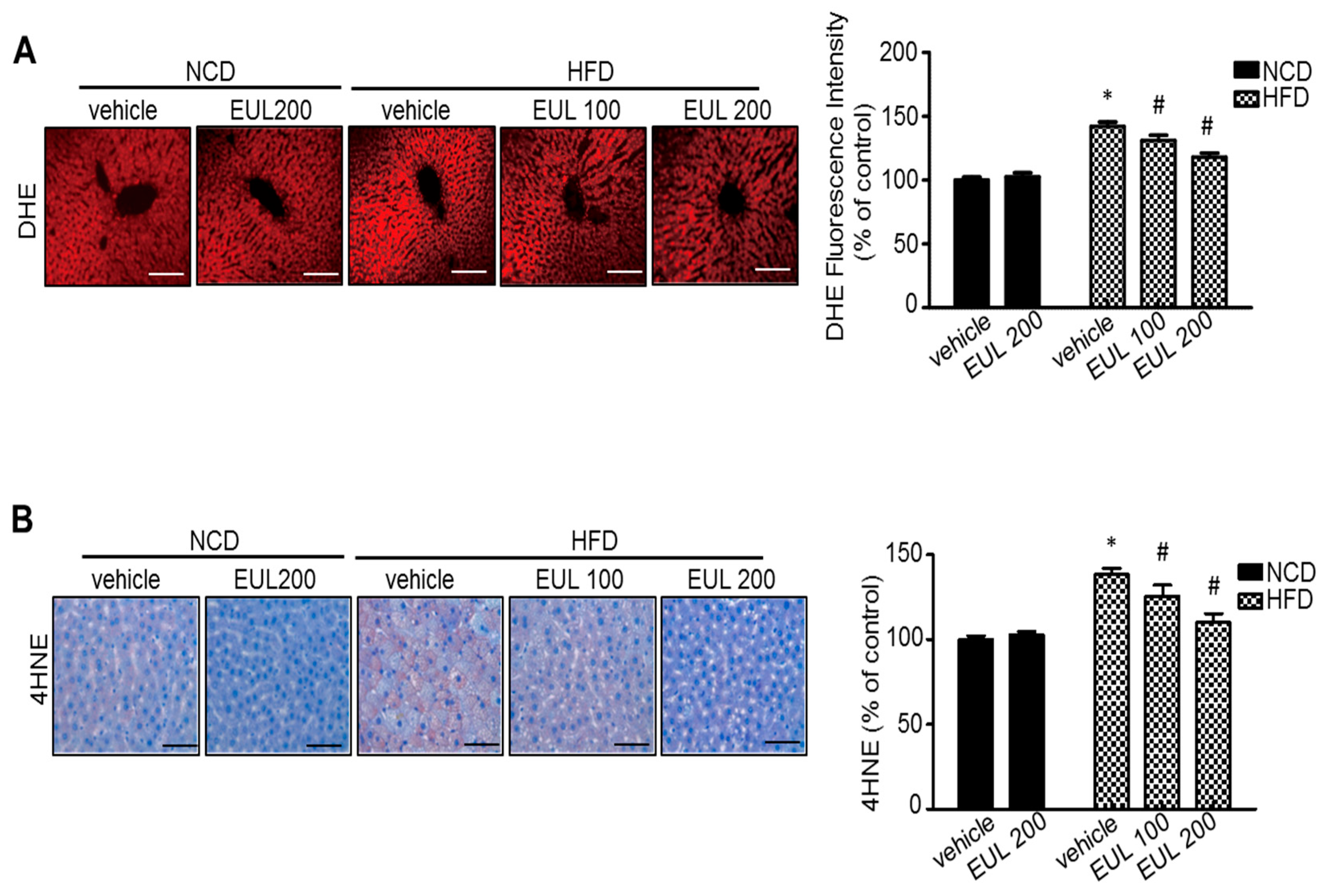
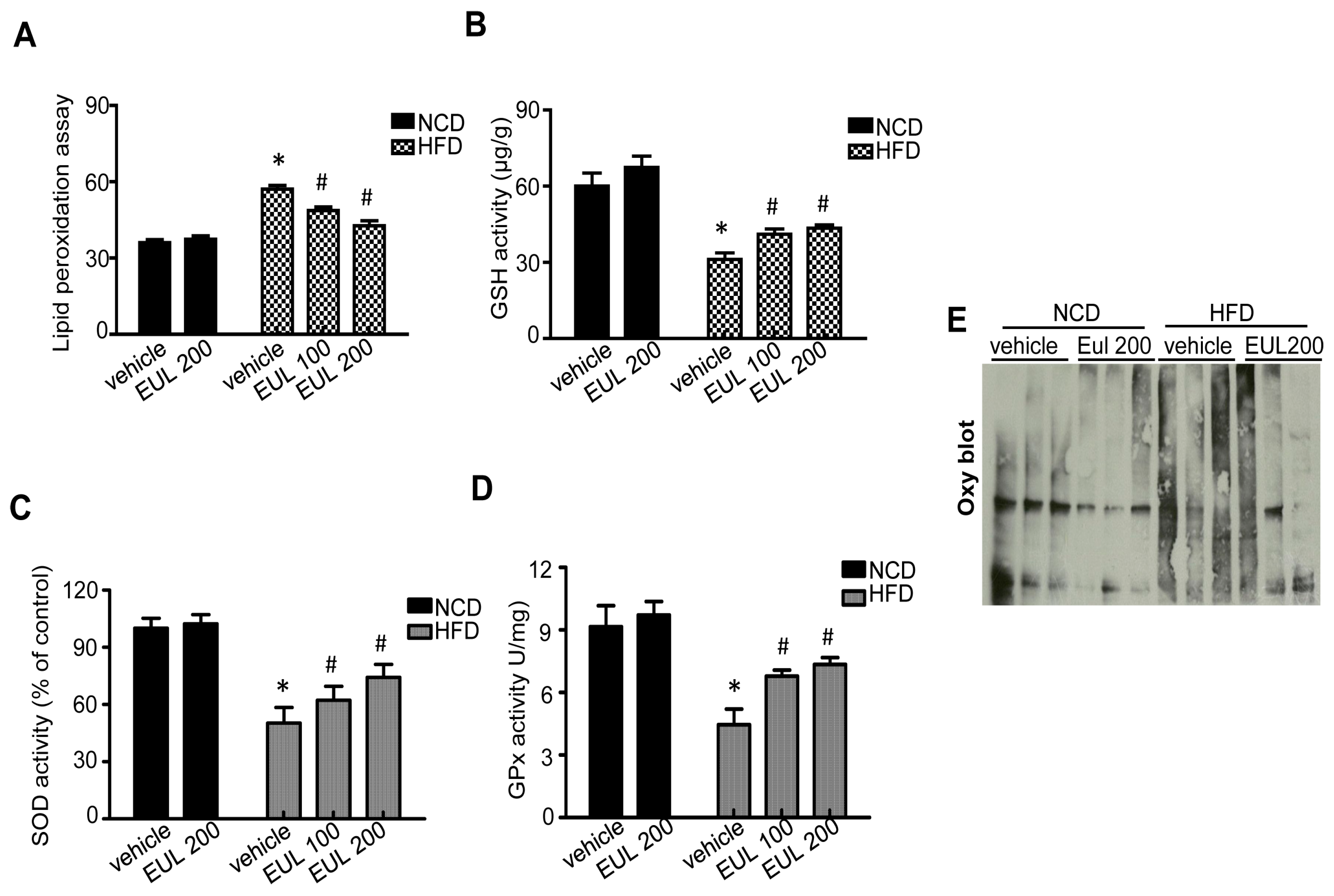
3.3. EUL Suppresses Hepatic Oxidative Stress
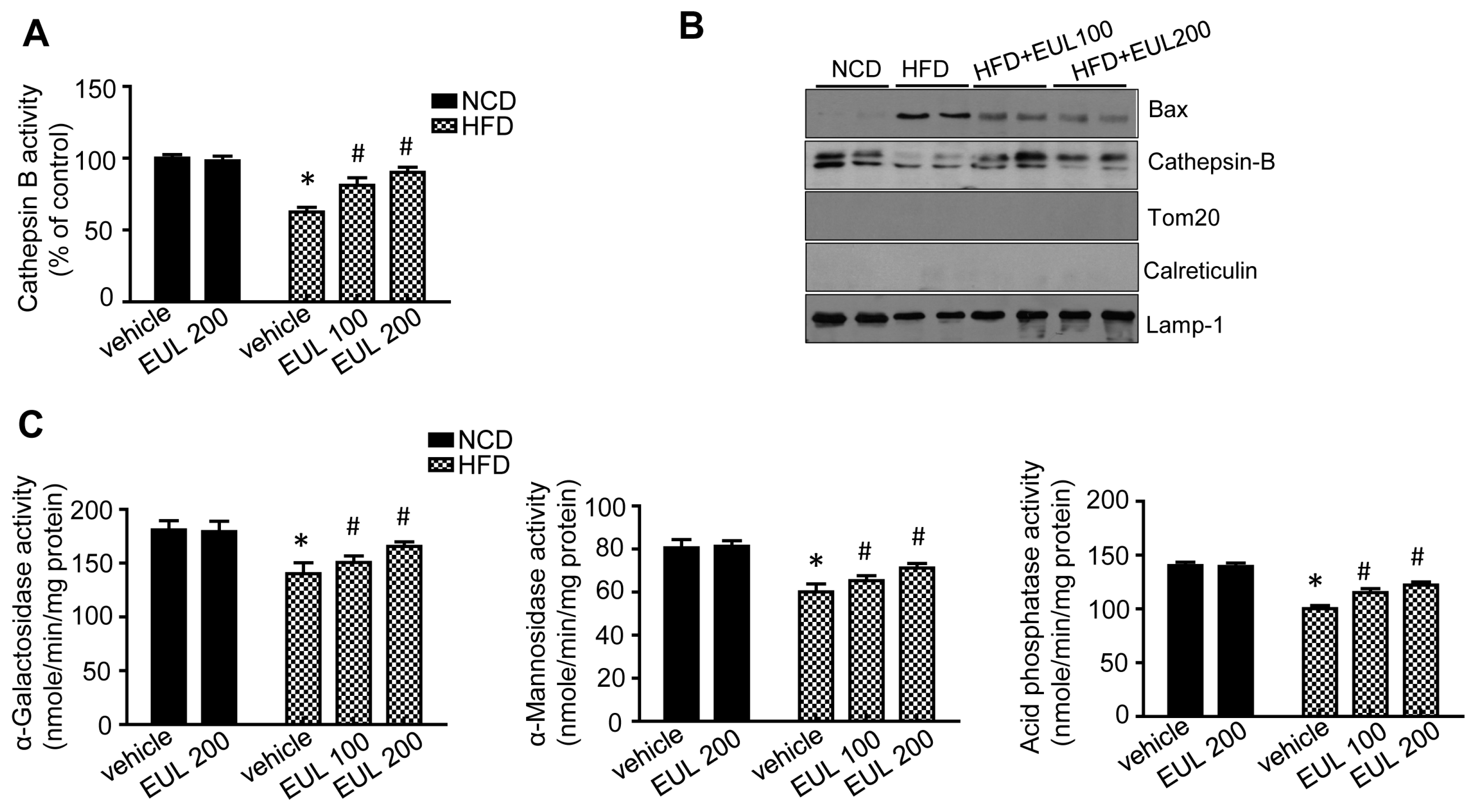
3.4. EUL Enhances Lysosomal Enzyme Activation
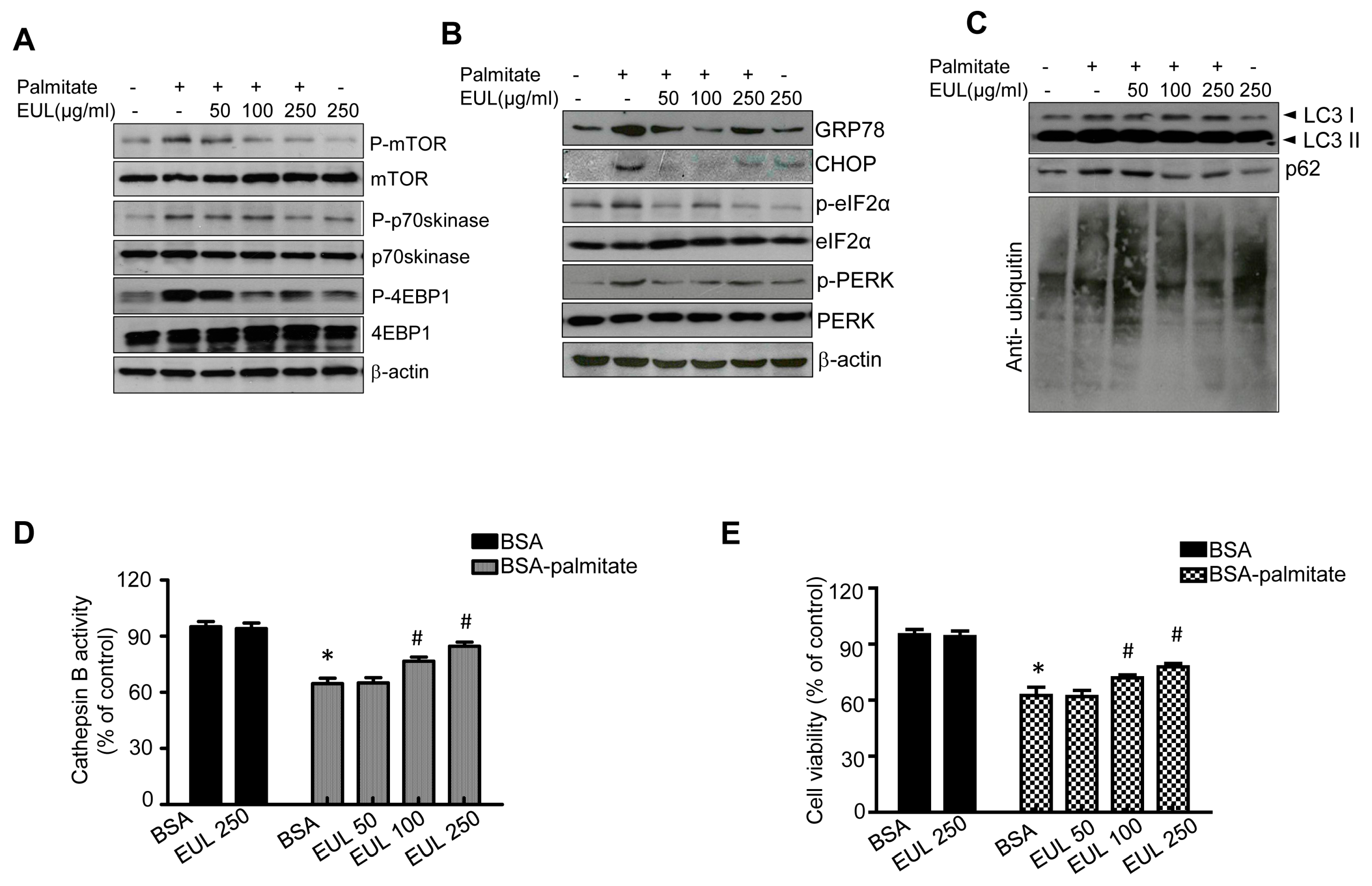
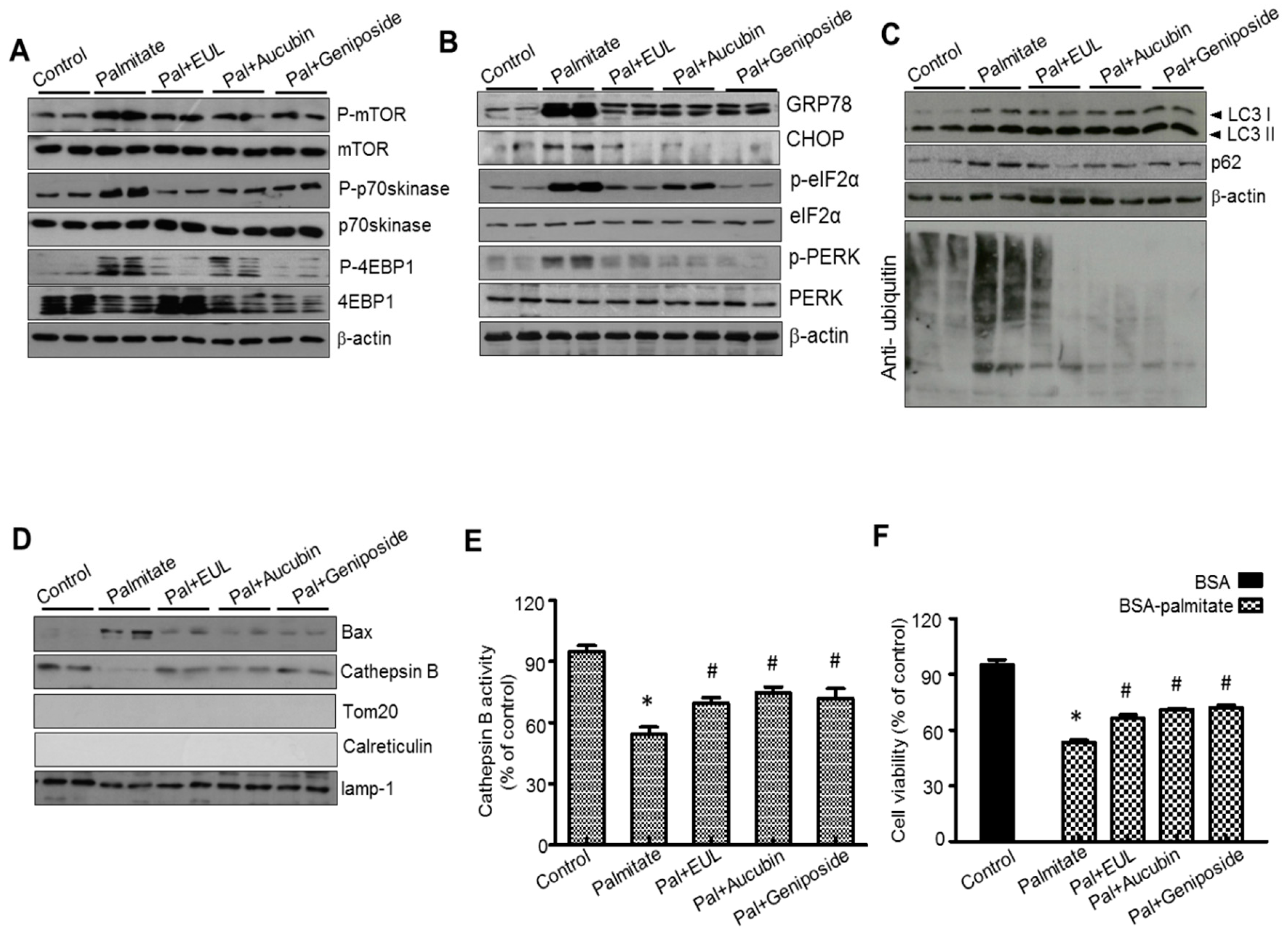
3.5. EUL Prevents Palmitate-Induced Lipid Accumulation by Inhibiting the mTORC1-ER Stress Pathway in HepG2 Cells
3.6. The Major Component of EUL, Aucubin, and Another Well-Known Major Component of EUO Cortex, Geniposide Regulate Lysosomal Function in HepG2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.; Du, H.; Shao, S.; Bo, T.; Yu, C.; Chen, W.; Zhao, L.; Li, Q.; Wang, L.; Liu, X.; et al. Cyclophilin D deficiency attenuates mitochondrial perturbation and ameliorates hepatic steatosis. Hepatology 2018. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.T.; Cui, C.; Qing, L.; Wang, L.S.; He, T.Y.; Yan, F.; Liu, F.Q.; Shen, Y.H.; Hou, X.G.; Chen, L. Activation of the STING-IRF3 pathway promotes hepatocyte inflammation, apoptosis and induces metabolic disorders in nonalcoholic fatty liver disease. Metabolism 2018, 81, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, Z.; Dai, H.; Miao, R.; Shu, J.; Gu, H.; Liu, X.; Huang, Z.; Yang, G.; Chen, A.F.; et al. Hepatic leukocyte immunoglobulin-like receptor B4 (LILRB4) attenuates nonalcoholic fatty liver disease via SHP1-TRAF6 pathway. Hepatology 2018, 67, 1303–1319. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Hikita, H.; Tatsumi, T.; Sakamori, R.; Nozaki, Y.; Sakane, S.; Shiode, Y.; Nakabori, T.; Saito, Y.; Hiramatsu, N.; et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology 2016, 64, 1994–2014. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cheng, J.; Chen, L.; Li, C.; Chen, G.; Gui, L.; Shen, B.; Zhang, Q. Endoplasmic reticulum stress involved in high-fat diet and palmitic acid-induced vascular damages and fenofibrate intervention. Biochem. Biophys. Res. Commun. 2015, 458, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kwon, J.; Kim, M.S.; Park, H.; Ji, Y.; Holzapfel, W.; Hyun, C.K. Protective effects of Bacillus probiotics against high-fat diet-induced metabolic disorders in mice. PloS ONE 2018, 13, e0210120. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, S.W.; Lee, G.H.; Choi, M.K.; Chung, H.W.; Lee, Y.C.; Kim, H.R.; Kwon, H.J.; Chae, H.J. Curcumin and Curcuma longa L. extract ameliorate lipid accumulation through the regulation of the endoplasmic reticulum redox and ER stress. Sci. Rep. 2017, 7, 6513. [Google Scholar] [CrossRef]
- Lee, G.H.; Lee, H.Y.; Choi, M.K.; Choi, A.H.; Shin, T.S.; Chae, H.J. Eucommia ulmoides leaf (EUL) extract enhances NO production in ox-LDL-treated human endothelial cells. Biomed. Pharmacother. 2018, 97, 1164–1172. [Google Scholar] [CrossRef]
- Choi, J.W.; Ohn, J.H.; Jung, H.S.; Park, Y.J.; Jang, H.C.; Chung, S.S.; Park, K.S. Carnitine induces autophagy and restores high-fat diet-induced mitochondrial dysfunction. Metabolism 2018, 78, 43–51. [Google Scholar] [CrossRef]
- Birse, R.T.; Choi, J.; Reardon, K.; Rodriguez, J.; Graham, S.; Diop, S.; Ocorr, K.; Bodmer, R.; Oldham, S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010, 12, 533–544. [Google Scholar] [CrossRef]
- Oldham, S. Obesity and nutrient sensing TOR pathway in flies and vertebrates: Functional conservation of genetic mechanisms. Trends Endocrinol. Metab. 2011, 22, 45–52. [Google Scholar] [CrossRef]
- Jung, C.H.; Ro, S.H.; Cao, J.; Otto, N.M.; Kim, D.H. mTOR regulation of autophagy. FEBS Letters 2010, 584, 1287–1295. [Google Scholar] [CrossRef]
- Singh, R.; Wang, Y.; Schattenberg, J.M.; Xiang, Y.; Czaja, M.J. Chronic oxidative stress sensitizes hepatocytes to death from 4-hydroxynonenal by JNK/c-Jun overactivation. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G907–G917. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Lee, G.H.; Lee, M.R.; Lee, H.Y.; Kim, S.H.; Kim, H.K.; Kim, H.R.; Chae, H.J. Eucommia ulmoides cortex, geniposide and aucubin regulate lipotoxicity through the inhibition of lysosomal BAX. PloS ONE 2014, 9, e88017. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; An, J.; Li, W.; Zhang, Z.; Chen, W.; Wang, C.; Wang, Z. UPLC-MS/MS determination and gender-related pharmacokinetic study of five active ingredients in rat plasma after oral administration of Eucommia cortex extract. J. Ethnopharmacol. 2015, 169, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Hirata, T.; Hosoo, S.; Nakajima, K.; Wada, A.; Yurugi, Y.; Soya, H.; Matsui, T.; Yamaguchi, A.; Ogata, M.; et al. Asperuloside stimulates metabolic function in rats across several organs under high-fat diet conditions, acting like the major ingredient of Eucommia leaves with anti-obesity activity. J. Nutr. Sci. 2012, 1, e10. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in growth, metabolism, and disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rodriguez, A.; Mayoral, R.; Agra, N.; Valdecantos, M.P.; Pardo, V.; Miquilena-Colina, M.E.; Vargas-Castrillon, J.; Lo Iacono, O.; Corazzari, M.; Fimia, G.M.; et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014, 5, e1179. [Google Scholar] [CrossRef]
- Balakumar, M.; Raji, L.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Mohan, V.; Balasubramanyam, M. High-fructose diet is as detrimental as high-fat diet in the induction of insulin resistance and diabetes mediated by hepatic/pancreatic endoplasmic reticulum (ER) stress. Mol. Cell. Biochem. 2016, 423, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kim, Y.; Lee, E.S.; Huh, J.H.; Chung, C.H. Caffeic acid ameliorates hepatic steatosis and reduces ER stress in high fat diet-induced obese mice by regulating autophagy. Nutrition 2018, 55–56, 63–70. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, G.H.; Lee, M.R.; Kim, H.K.; Kim, N.Y.; Kim, S.H.; Lee, Y.C.; Kim, H.R.; Chae, H.J. Eucommia ulmoides Oliver extract, aucubin, and geniposide enhance lysosomal activity to regulate ER stress and hepatic lipid accumulation. PloS ONE 2013, 8, e81349. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.M.; Ryu, J.C.; Park, Y.C.; Yun, H.S.; Yang, K.H. Protective activities of aucubin against carbon tetrachloride-induced liver damage in mice. Drug Chem. Toxicol. 1983, 6, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Ma, S.X.; Hong, S.I.; Kim, S.Y.; Lee, S.Y.; Jang, C.G. Eucommia ulmoides Oliv. bark. attenuates 6-hydroxydopamine-induced neuronal cell death through inhibition of oxidative stress in SH-SY5Y cells. J. Ethnopharmacol. 2014, 152, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Yen, G.C. Antioxidant actions of du-zhong (Eucommia ulmoides Oliv.) toward oxidative damage in biomolecules. Life Sci. 2000, 66, 1387–1400. [Google Scholar] [CrossRef]
- Choi, M.S.; Jung, U.J.; Kim, H.J.; Do, G.M.; Jeon, S.M.; Kim, M.J.; Lee, M.K. Du-zhong (Eucommia ulmoides Oliver) leaf extract mediates hypolipidemic action in hamsters fed a high-fat diet. Am. J. Chin. Med. 2008, 36, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Or, T.C.; Yang, C.L.; Lau, A.S. Anti-inflammatory activity of iridoid and catechol derivatives from Eucommia ulmoides Oliver. ACS Chem. Neurosci. 2014, 5, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Deng, X.L.; Huang, W.H.; Li, L.; Li, H.; Jing, X.; Tian, Y.Y.; Lv, P.Y.; Yang, T.L.; Zhou, H.H.; et al. Lignans from the bark of Eucommia ulmoides inhibited Ang II-stimulated extracellular matrix biosynthesis in mesangial cells. Chin Med 2014, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, J.; Li, M.; Hao, D.; Yang, Y.; Zhang, C.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.-H.; Lee, H.-Y.; Park, S.-A.; Shin, T.-S.; Chae, H.-J. Eucommia ulmoides Leaf Extract Ameliorates Steatosis Induced by High-fat Diet in Rats by Increasing Lysosomal Function. Nutrients 2019, 11, 426. https://doi.org/10.3390/nu11020426
Lee G-H, Lee H-Y, Park S-A, Shin T-S, Chae H-J. Eucommia ulmoides Leaf Extract Ameliorates Steatosis Induced by High-fat Diet in Rats by Increasing Lysosomal Function. Nutrients. 2019; 11(2):426. https://doi.org/10.3390/nu11020426
Chicago/Turabian StyleLee, Geum-Hwa, Hwa-Young Lee, Sun-Ah Park, Tai-Sun Shin, and Han-Jung Chae. 2019. "Eucommia ulmoides Leaf Extract Ameliorates Steatosis Induced by High-fat Diet in Rats by Increasing Lysosomal Function" Nutrients 11, no. 2: 426. https://doi.org/10.3390/nu11020426
APA StyleLee, G.-H., Lee, H.-Y., Park, S.-A., Shin, T.-S., & Chae, H.-J. (2019). Eucommia ulmoides Leaf Extract Ameliorates Steatosis Induced by High-fat Diet in Rats by Increasing Lysosomal Function. Nutrients, 11(2), 426. https://doi.org/10.3390/nu11020426