A Comparison of Dietary and Caloric Restriction Models on Body Composition, Physical Performance, and Metabolic Health in Young Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Interventions
2.2. Measurement of Body Composition
2.3. Measurement of Physical Fitness
2.4. Glucose Tolerance Test Protocol
2.5. Glucose Homeostasis
2.6. Biochemical Analyses
2.7. Data Analyses
3. Results
3.1. Overview
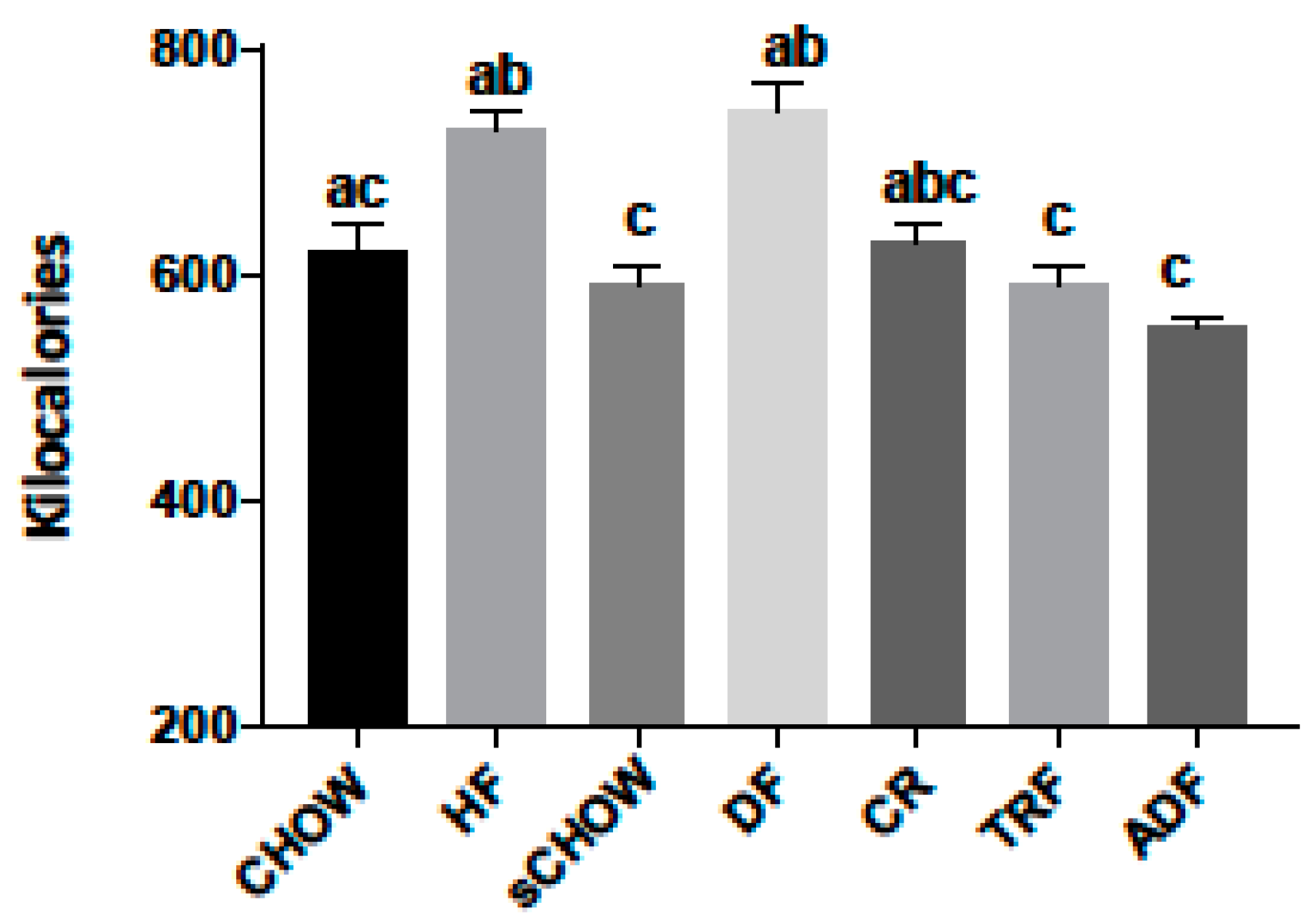
3.2. Caloric Consumption Data
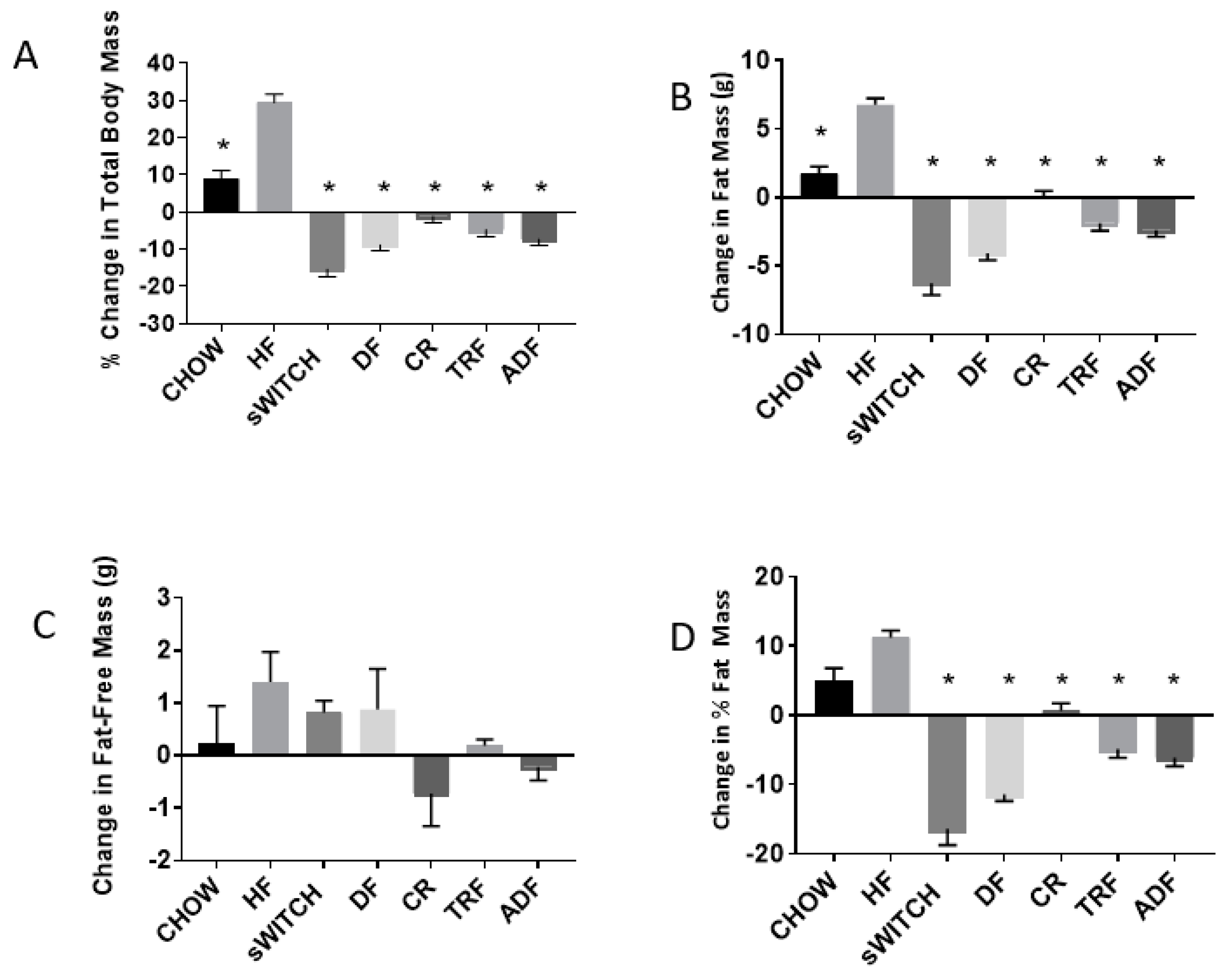
3.3. Anthropometric Data
3.4. Run-Time-To-Exhaustion Data
3.5. Blood Lipids
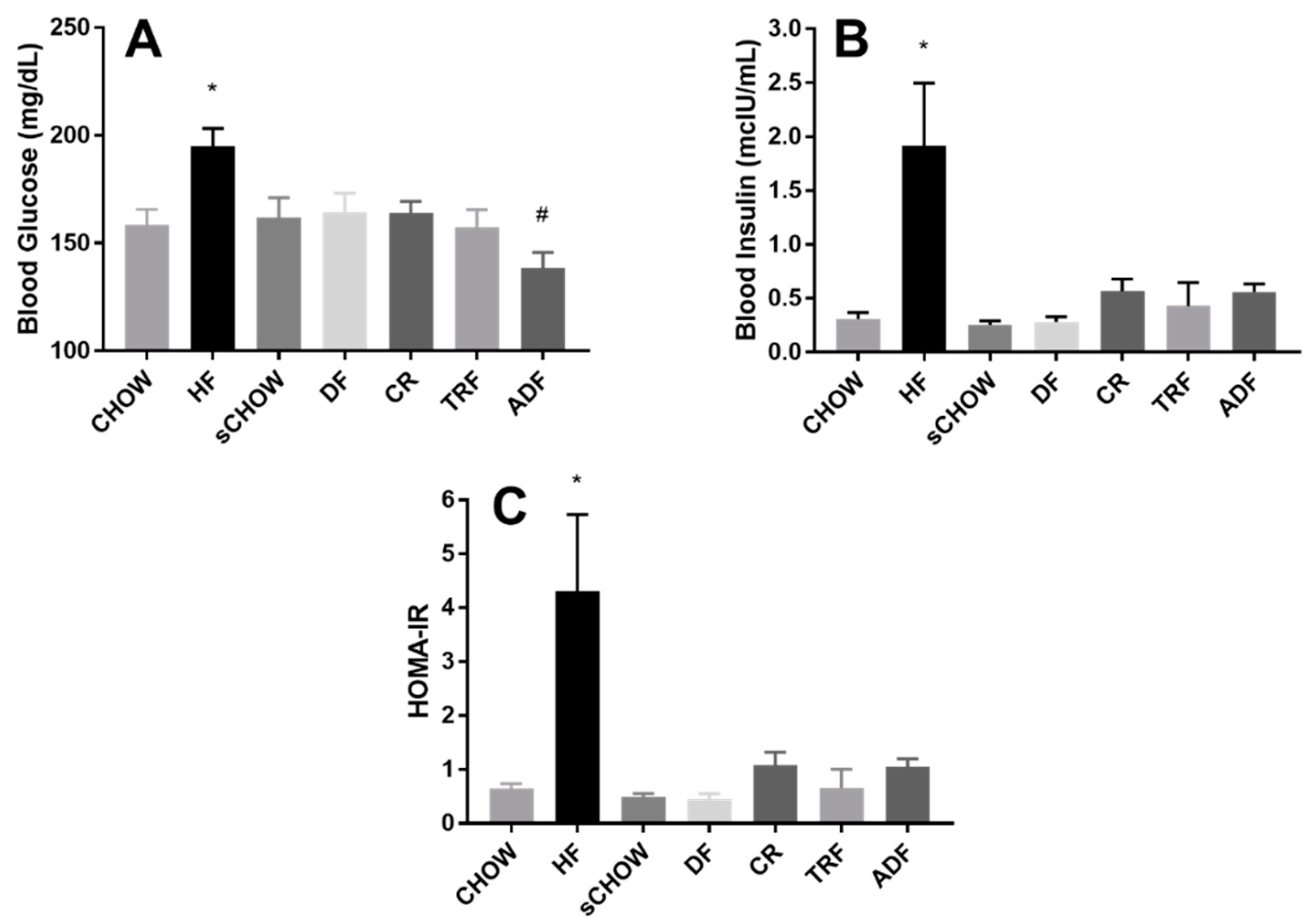
3.6. Fasting Blood Glucose
3.7. Fasting Blood Insulin
3.8. HOMA-IR
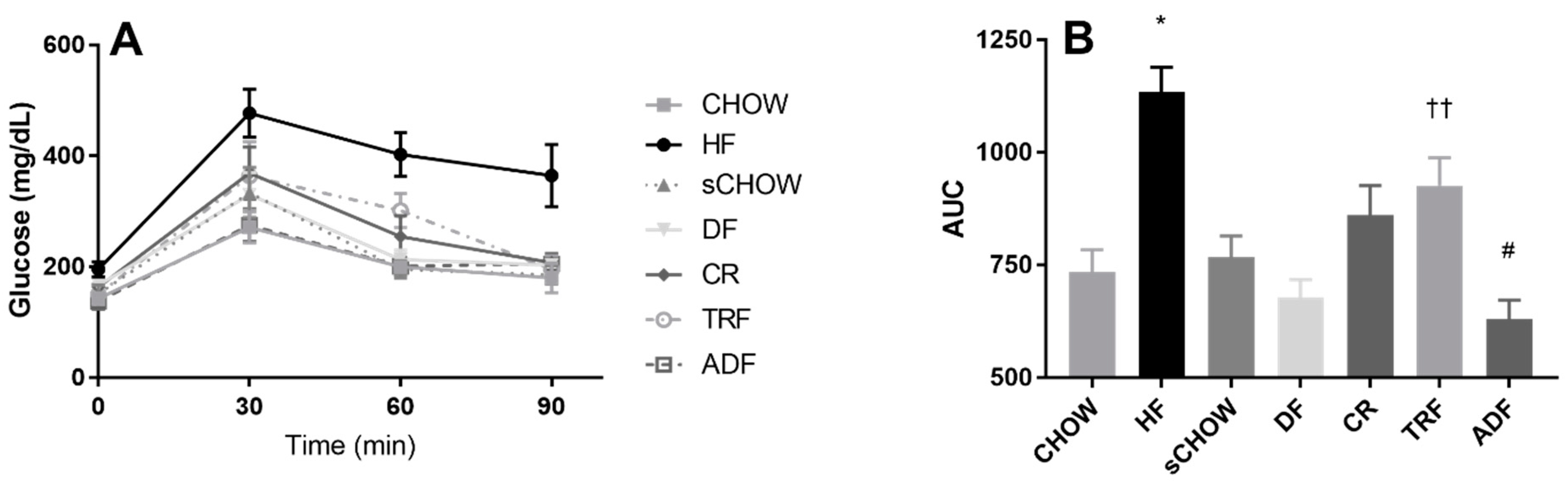
3.9. Glucose Area under the Curve
4. Discussion
4.1. Anthropometric Findings
4.2. Run-Time-To-Exhaustion Findings
4.3. Metabolic Findings
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ogden, C.L.; Carroll, M.D.; Fryar, C.D.; Flegal, K.M. Prevalence of Obesity among Adults and Youth: United States, 2011–2014; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Washington, DC, USA, 2015.
- Moyer, V.A. Screening for and management of obesity in adults: Us preventive services task force recommendation statement. Ann. Intern. Med. 2012, 157, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, A.; Rinne, M.; Vasankari, T.; Santtila, M.; Häkkinen, K.; Kyröläinen, H. Association of physical fitness with health-related quality of life in finnish young men. Health Qual. Life Outcomes 2010, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Stote, K.S.; Baer, D.J.; Spears, K.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Strycula, P.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007, 85, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Sherman, H.; Frumin, I.; Gutman, R.; Chapnik, N.; Lorentz, A.; Meylan, J.; le Coutre, J.; Froy, O. Long-term restricted feeding alters circadian expression and reduces the level of inflammatory and disease markers. J. Cell. Mol. Med. 2011, 15, 2745–2759. [Google Scholar] [CrossRef]
- Sherman, H.; Genzer, Y.; Cohen, R.; Chapnik, N.; Madar, Z.; Froy, O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012, 26, 3493–3502. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Gill, S.; Le, H.D.; Melkani, G.C.; Panda, S. Time-restricted feeding attenuates age-related cardiac decline in drosophila. Science 2015, 347, 1265–1269. [Google Scholar] [CrossRef]
- Fakhrzadeh, H.; Larijani, B.; Sanjari, M.; Baradar-Jalili, R.; Amini, M.R. Effect of ramadan fasting on clinical and biochemical parameters in healthy adults. Ann. Saudi Med. 2003, 23, 223–226. [Google Scholar] [CrossRef]
- Mirzaei, B.; Rahmani-Nia, F.; Moghadam, M.G.; Ziyaolhagh, S.J.; Rezaei, A. The effect of ramadan fasting on biochemical and performance parameters in collegiate wrestlers. Iran. J. Basic Med. Sci. 2012, 15, 1215–1220. [Google Scholar] [PubMed]
- Ziaee, V.; Razaei, M.; Ahmadinejad, Z.; Shaikh, H.; Yousefi, R.; Yarmohammadi, L.; Bozorgi, F.; Behjati, M.J. The changes of metabolic profile and weight during ramadan fasting. Singap. Med. J. 2006, 47, 409–414. [Google Scholar]
- Farooq, N.; Priyamvada, S.; Arivarasu, N.A.; Salim, S.; Khan, F.; Yusufi, A.N. Influence of ramadan-type fasting on enzymes of carbohydrate metabolism and brush border membrane in small intestine and liver of rat used as a model. Br. J. Nutr. 2006, 96, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Hoddy, K.K.; Gibbons, C.; Kroeger, C.M.; Trepanowski, J.F.; Barnosky, A.; Bhutani, S.; Gabel, K.; Finlayson, G.; Varady, K.A. Changes in hunger and fullness in relation to gut peptides before and after 8 weeks of alternate day fasting. Clin. Nutr. 2016, 35, 1380–1385. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-term modified alternate-day fasting: A novel dietary strategy for weight loss and cardioprotection in obese adults. Am. J. Clin. Nutr. 2009, 90, 1138–1143. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity 2013, 21, 1370–1379. [Google Scholar] [CrossRef]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Troy Donahoo, W. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Smith, S.R.; Martin, C.K.; Anton, S.D.; Ravussin, E. Alternate-day fasting in nonobese subjects: Effects on body weight, body composition, and energy metabolism. Am. J. Clin. Nutr. 2005, 81, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Hoddy, K.K.; Kroeger, C.M.; Trepanowski, J.F.; Barnosky, A.; Bhutani, S.; Varady, K.A. Meal timing during alternate day fasting: Impact on body weight and cardiovascular disease risk in obese adults. Obesity (Silver Spring) 2014, 22, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Roohk, D.J.; Loe, Y.C.; McEvoy-Hein, B.K.; Hellerstein, M.K. Effects of modified alternate-day fasting regimens on adipocyte size, triglyceride metabolism, and plasma adiponectin levels in mice. J. Lipid Res. 2007, 48, 2212–2219. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Kabir, M.M.; Canale, R.E.; Trepanowski, J.F.; Marshall, K.E.; Farney, T.M.; Hammond, K.G. Effect of a 21 day daniel fast on metabolic and cardiovascular disease risk factors in men and women. Lipids Health Dis. 2010, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Summer, W.; Cutler, R.G.; Martin, B.; Hyun, D.H.; Dixit, V.D.; Pearson, M.; Nassar, M.; Telljohann, R.; Maudsley, S.; et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007, 42, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Joslin, P.M.N.; Bell, R.K.; Swoap, S.J. Obese mice on a high-fat alternate-day fasting regimen lose weight and improve glucose tolerance. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1036–1045. [Google Scholar] [CrossRef]
- Alleman, R.J.; Harvey, I.C.; Farney, T.M.; Bloomer, R.J. Both a traditional and modified daniel fast improve the cardio-metabolic profile in men and women. Lipids Health Dis. 2013, 12, 114. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Kabir, M.M.; Trepanowski, J.F.; Canale, R.E.; Farney, T.M. A 21 day daniel fast improves selected biomarkers of antioxidant status and oxidative stress in men and women. Nutr. Metab. 2011, 8, 17. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kabir, M.M.; Alleman, R.J.; Bloomer, R.J. A 21-day daniel fast with or without krill oil supplementation improves anthropometric parameters and the cardiometabolic profile in men and women. Nutr. Metab. 2012, 9, 82. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Schriefer, J.H.M.; Gunnels, T.A.; Lee, S.R.; Sable, H.J.; van der Merwe, M.; Buddington, R.K.; Buddington, K.K. Nutrient intake and physical exercise significantly impact physical performance, body composition, blood lipids, oxidative stress, and inflammation in male rats. Nutrients 2018, 10, 1109. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J. Influence of a 6-month modified or traditional daniel fast on measures of health in men and women. J. Nutr. Biol. 2015, 1, 22–32. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Gunnels, T.A.; Schriefer, J.M. Comparison of a restricted and unrestricted vegan diet plan with a restricted omnivorous diet plan on health-specific measures. Healthcare (Basel) 2015, 3, 544–555. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Toline, A.H. Participant compliance to a six-month traditional and modified daniel fast. J. Fasting Health 2014, 2, 90–95. [Google Scholar]
- Von Schulze, A.; Gunnels, T.A.; Schriefer, J.H.M.; Lee, S.R.; MacDonnchadh, J.J.; Buddington, R.K.; Buddington, K.K.; van der Merwe, M.; Sable, H.J.; Bloomer, R.J.; et al. Insulin resistance precedes the impairment of basal skeletal muscle heat shock protein expression in sedentary and aerobically trained rats fed a western diet. 2018. in review. [Google Scholar]
- Delahaye, L.B.; Bloomer, R.J.; Butawan, M.B.; Wyman, J.M.; Hill, J.L.; Lee, H.W.; Liu, A.C.; McAllan, L.; Han, J.C.; van der Merwe, M. Time-restricted feeding of a high fat diet in c57bl/6 male mice reduces adiposity, but does not protect against increased systemic inflammation. Appl. Physiol. Nutr. Metab. (Physiologie Appliquee, Nutrition et Metabolism) 2018, 43, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Cacho, J.; Sevillano, J.; de Castro, J.; Herrera, E.; Ramos, M.P. Validation of simple indexes to assess insulin sensitivity during pregnancy in wistar and sprague-dawley rats. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1269–E1276. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Guo, J.; Jou, W.; Gavrilova, O.; Hall, K.D. Persistent diet-induced obesity in male c57bl/6 mice resulting from temporary obesigenic diets. PLoS ONE 2009, 4, e5370. [Google Scholar] [CrossRef]
- Yang, Y.; Smith, D.L., Jr.; Keating, K.D.; Allison, D.B.; Nagy, T.R. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in c57bl/6j mice. Obesity 2014, 22, 2147–2155. [Google Scholar] [CrossRef]
- Gargiulo, S.; Gramanzini, M.; Megna, R.; Greco, A.; Albanese, S.; Manfredi, C.; Brunetti, A. Evaluation of growth patterns and body composition in c57bl/6j mice using dual energy x-ray absorptiometry. BioMed Res. Int. 2014, 2014, 253067. [Google Scholar] [CrossRef]
- Buckley, J.D.; Howe, P.R. Long-chain omega-3 polyunsaturated fatty acids may be beneficial for reducing obesity—A review. Nutrients 2010, 2, 1212–1230. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Miles-Brown, J.; Pellizzon, M.; Ulman, E.; Ricci, M.; Zhang, L.; Patterson, A.D.; Vijay-Kumar, M.; Gewirtz, A.T. Lack of soluble fiber drives diet-induced adiposity in mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 309, G528–G541. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Delgado, R.; Angeles-Castellanos, M.; Saderi, N.; Buijs, R.M.; Escobar, C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology 2010, 151, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Ahmet, I.; Wan, R.; Mattson, M.P.; Lakatta, E.G.; Talan, M. Cardioprotection by intermittent fasting in rats. Circulation 2005, 112, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Camandola, S.; Mattson, M.P. Intermittent fasting and dietary supplementation with 2-deoxy-d-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J. 2003, 17, 1133–1134. [Google Scholar] [CrossRef] [PubMed]
- Dorighello, G.G.; Rovani, J.C.; Luhman, C.J.; Paim, B.A.; Raposo, H.F.; Vercesi, A.E.; Oliveira, H.C. Food restriction by intermittent fasting induces diabetes and obesity and aggravates spontaneous atherosclerosis development in hypercholesterolaemic mice. Br. J. Nutr. 2014, 111, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Denies, M.S.; Johnson, J.; Maliphol, A.B.; Bruno, M.; Kim, A.; Rizvi, A.; Rustici, K.; Medler, S. Diet-induced obesity alters skeletal muscle fiber types of male but not female mice. Physiol. Rep. 2014, 2, e00204. [Google Scholar] [CrossRef] [PubMed]
- Alhamdan, B.A.; Garcia-Alvarez, A.; Alzahrnai, A.H.; Karanxha, J.; Stretchberry, D.R.; Contrera, K.J.; Utria, A.F.; Cheskin, L.J. Alternate-day versus daily energy restriction diets: Which is more effective for weight loss? A systematic review and meta-analysis. Obes. Sci. Pract. 2016, 2, 293–302. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: A randomized clinical trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- Donohoe, C.L.; Doyle, S.L.; Reynolds, J.V. Visceral adiposity, insulin resistance and cancer risk. Diabetol. Metab. Syndr. 2011, 3, 12. [Google Scholar] [CrossRef]
- Hardy, O.T.; Czech, M.P.; Corvera, S. What causes the insulin resistance underlying obesity? Current Opin. Endocrinol. Diabetes Obes. 2012, 19, 81–87. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Lamendola, C.; Liu, A.; Abbasi, F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J. Clin. Endocrinol. Metab. 2011, 96, E1756–E1760. [Google Scholar] [CrossRef] [PubMed]
- de Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and insulin resistance. Mol. Med. (Cambridge, Mass.) 2008, 14, 741–751. [Google Scholar]
| DF (Daniel Fast) | HF (High-Fat) | CHOW | |
|---|---|---|---|
| Macronutrient | kcal% | kcal% | kcal% |
| Protein | 15 | 20 | 24 |
| Carbohydrate | 59 | 35 | 58 |
| Fat | 25 | 45 | 18 |
| kcal/gm | 3.9 | 4.7 | 3.1 |
| DF | HF | |||
|---|---|---|---|---|
| Nutrient | gm | kcal | gm | kcal |
| Casein | 0 | 0 | 200 | 800 |
| Soy Protein | 170 | 680 | 0 | 0 |
| DL-Methionine | 3 | 12 | 0 | 0 |
| Corn Starch | 0 | 0 | 72.8 | 291 |
| Corn Starch-Hi Maize 260 | 533.5 | 2134 | 0 | 0 |
| (70% Amylose and 30% Amylopectin) | ||||
| Maltodextrin | 150 | 600 | 100 | 400 |
| Sucrose | 0 | 0 | 172.8 | 691 |
| L-Cystine | 0 | 0 | 3 | 12 |
| Cellulose, BW200 | 100 | 0 | 50 | 0 |
| Inulin | 50 | 50 | 0 | 0 |
| Soybean Oil | 25 | 225 | ||
| Lard | 0 | 0 | 177.5 | 1598 |
| Flaxseed Oil | 71 | 639 | 0 | 0 |
| Safflower Oil, High Oleic | 59 | 531 | 0 | 0 |
| Ethoxyquin | 0.04 | 0 | 0 | 0 |
| Dicalcium Phosphate | 13 | 0 | ||
| Mineral Mix S10001 | 35 | 0 | 10 | 40 |
| Calcium Carbonate | 4 | 0 | 5.5 | 0 |
| Mineral Mix S10026 | 10 | 0 | ||
| Vitamin Mix V10001 | 10 | 40 | 0 | 0 |
| Choline Bitartrate | 2 | 0 | 2 | 0 |
| Ascorbic Acid Phosphate, 33% active | 0.41 | 0 | 0 | 0 |
| Potassium Citrate, 1 H2O | 0 | 0 | 16.5 | 0 |
| Cholesterol | 0 | 0 | 0 | 0 |
| FD&C Red Dye #40 | 0.05 | 0 | 0.05 | 0 |
| Micronutrients | Amino Acids | ||||
|---|---|---|---|---|---|
| Calcium | % | 1 | Aspartic Acid | % | 1.4 |
| Phosphorous | % | 0.7 | Glutamic Acid | % | 3.4 |
| Non-Phytate Phosphorous | % | 0.4 | Alanine | % | 1.1 |
| Sodium | % | 0.2 | Glycine | % | 0.8 |
| Potassium | % | 0.6 | Threonine | % | 0.7 |
| Chloride | % | 0.4 | Proline | % | 1.6 |
| Magnesium | % | 0.3 | Serine | % | 1.1 |
| Zinc | mg/kg | 70 | Leucine | % | 1.8 |
| Manganese | mg/kg | 100 | Isoleucine | % | 0.8 |
| Copper | mg/kg | 15 | Valine | % | 0.9 |
| Iodine | mg/kg | 6 | Phenylalanine | % | 1 |
| Iron | mg/kg | 200 | Tyrosine | % | 0.6 |
| Selenium | mg/kg | 0.23 | Methionine | % | 0.4 |
| Vitamins | Cysteine | % | 0.3 | ||
| Vitamin A | IU/g | 15 | Lysine | % | 0.9 |
| Vitamin D3 | IU/g | 1.5 | Histidine | % | 0.4 |
| Vitamin E | IU/kg | 110 | Arginine | % | 1 |
| Vitamin K3 (menadione) | mg/kg | 50 | Tryptophan | % | 0.2 |
| Vitamin B1 (thiamin) | mg/kg | 17 | Fatty Acids | ||
| Vitamin B2 (riboflavin) | mg/kg | 15 | C16:0 Palmitic | % | 0.7 |
| Niacin (nicotinic acid) | mg/kg | 70 | C18:0 Stearic | % | 0.2 |
| Vitamin B6 (pyridoxine) | mg/kg | 18 | C18:1ω9 Oleic | % | 1.2 |
| Pantothenic Acid | mg/kg | 33 | C18:2ω6 Linoleic | % | 3.1 |
| Vitamin B12 (cyanocobalamin) | mg/kg | 0.08 | C18:3ω3 Linolenic | % | 0.3 |
| Biotin | mg/kg | 0.4 | Total Saturated | % | 0.9 |
| Folate | mg/kg | 4 | Total Monounsaturated | % | 1.3 |
| Choline | mg/kg | 1200 | Total Polyunsaturated | % | 3.4 |
| Body Mass (g) | FM (g) | FFM (g) | % FM | Run Time to Exhaustion (min) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention Group | PRE | POST | PRE | POST | PRE | POST | PRE | POST | POST |
| CHOW | 25.49 ± 0.66 | 27.79 ± 1.03 § | 2.48 ± 0.38 | 4.21 ± 0.71 § | 21.05 ± 0.48 | 21.30 ± 0.39 | 9.6 ± 1.4 | 14.6 ± 2.3 | 39.86 ± 7.87 |
| HF | 30.54 ± 1.01 | 39.60 ± 1.71* | 7.96 ± 0.72 | 14.72 ± 0.92 * | 20.64 ± 0.37 | 22.03 ± 0.85 | 25.8 ± 1.6 | 37.0 ± 0.9 | 16.38 ± 3.85 |
| sCHOW | 33.90 ± 0.79 | 28.65 ± 0.83 ¥ | 10.71 ± 0.68 | 4.43 ± 0.89 ¥ | 21.20 ± 0.34 | 22.02 ± 0.34 | 31.4 ± 1.4 | 15.0 ± 2.5 | 42.13 ± 9.01 |
| DF | 31.83 ± 1.02 | 28.91 ± 0.5 | 7.82 ± 0.84 | 3.72 ± 0.44 † | 21.97 ± 0.57 | 22.85 ± 0.76 | 24.5 ± 1.9 | 12.8 ± 1.4 | 39.00 ± 9.4 |
| CR | 34.12 ± 1.05 | 33.69 ± 1.01 | 10.55 ± 0.82 | 10.56 ± 0.62 | 21.50 ± 0.30 | 20.78 ± 0.86 | 30.6 ± 1.6 | 31.3 ± 1.4 | 30.63 ± 5.38 |
| TRF | 30.03 ± 1.06 | 28.47 ± 0.63 | 7.80 ± 0.89 | 5.91 ± 0.51 | 20.31 ± 0.31 | 20.50 ± 0.26 | 25.4 ± 2.2 | 20.5 ± 1.5 | 40.00 ± 10.68 |
| ADF | 30.96 ± 0.56 | 28.64 ± 0.40 | 7.75 ± 0.63 | 5.36 ± 0.35 | 21.13 ± 0.23 | 20.91 ± 0.38 | 24.8 ± 1.6 | 18.7 ± 1.1 | 27.78 ± 3.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, N.J.G.; Caldwell, J.L.; van der Merwe, M.; Sharma, S.; Butawan, M.; Puppa, M.; Bloomer, R.J. A Comparison of Dietary and Caloric Restriction Models on Body Composition, Physical Performance, and Metabolic Health in Young Mice. Nutrients 2019, 11, 350. https://doi.org/10.3390/nu11020350
Smith NJG, Caldwell JL, van der Merwe M, Sharma S, Butawan M, Puppa M, Bloomer RJ. A Comparison of Dietary and Caloric Restriction Models on Body Composition, Physical Performance, and Metabolic Health in Young Mice. Nutrients. 2019; 11(2):350. https://doi.org/10.3390/nu11020350
Chicago/Turabian StyleSmith, Nicholas J. G., Jade L. Caldwell, Marie van der Merwe, Sunita Sharma, Matthew Butawan, Melissa Puppa, and Richard J. Bloomer. 2019. "A Comparison of Dietary and Caloric Restriction Models on Body Composition, Physical Performance, and Metabolic Health in Young Mice" Nutrients 11, no. 2: 350. https://doi.org/10.3390/nu11020350





