Content of n-3 LC-PUFA in Breast Milk Four Months Postpartum is Associated with Infancy Blood Pressure in Boys and Infancy Blood Lipid Profile in Girls
Abstract
1. Introduction
2. Materials and Methods
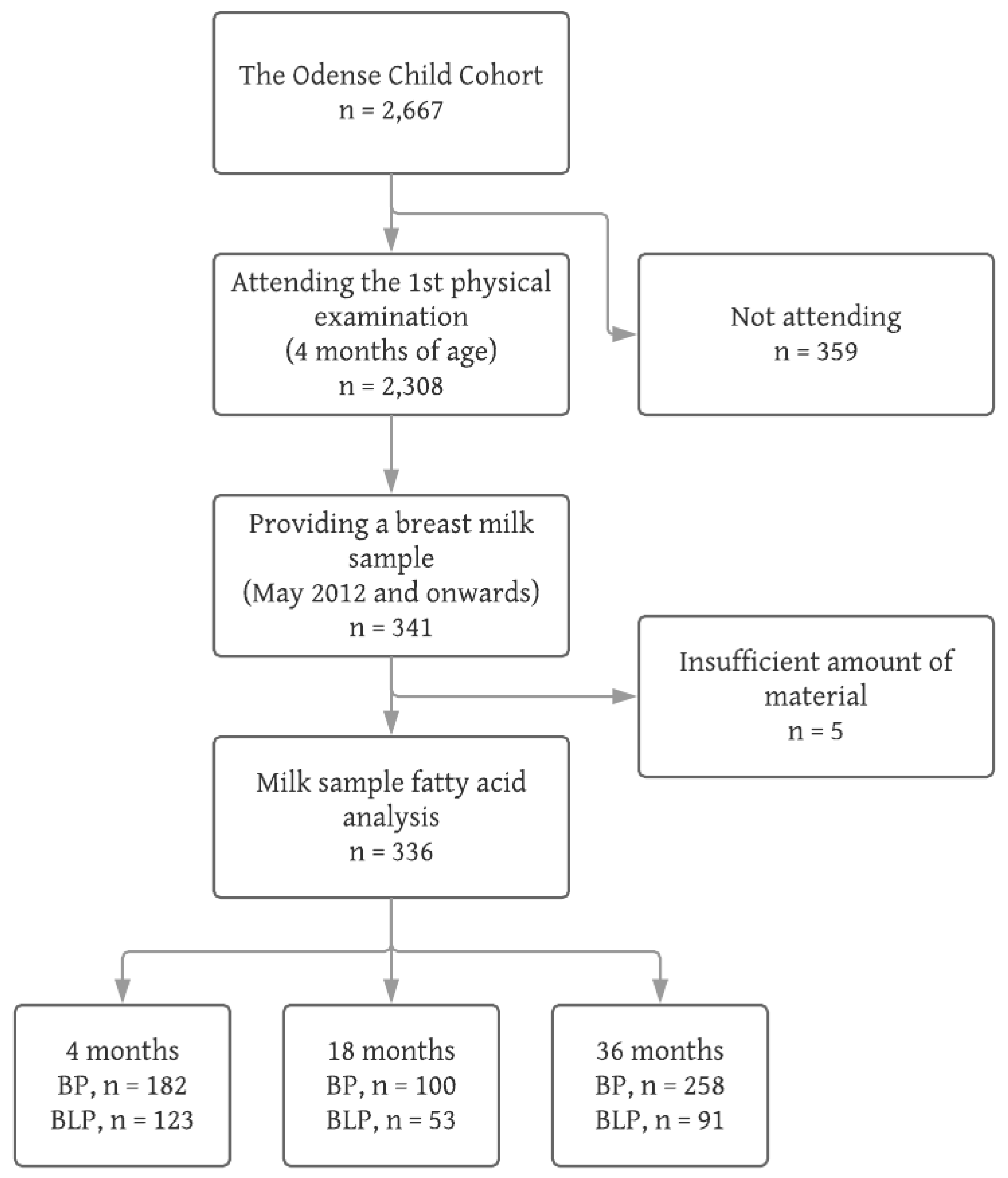
2.1. Participants
2.2. Breast Milk Fatty Acids
2.3. Blood Pressure Measurement
2.4. Blood Pressure Z-Scores and Percentiles
2.5. Blood Lipid Profile
2.6. Ethics
2.7. Statistics
- Those attending the physical examination at 4 months and delivering a breast milk sample (n = 336)
- Those attending the physical examination at 4 months, but not delivering a breast milk sample (n = 1967)
- Those not attending the physical examination at 4 months (n = 359)
3. Results
3.1. Participants
3.2. Breast Milk Fatty Acids
3.3. Blood Pressure and Blood Lipid Profile
3.4. Content of n-3 LC-PUFA in Breast Milk and Associations with Infancy Blood Pressure
3.5. Content of n-3 LC-PUFA in Breast Milk and Associations with Infancy Blood Lipid Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Wang, Y. Tracking of blood pressure from childhood to adulthood: A systematic review and meta-regression analysis. Circulation 2008, 117, 3171–3180. [Google Scholar] [CrossRef] [PubMed]
- Theodore, R.F.; Broadbent, J.; Nagin, D.; Ambler, A.; Hogan, S.; Ramrakha, S.; Cutfield, W.; Williams, M.J.; Harrington, H.; Moffitt, T.E.; et al. Childhood to early-midlife systolic blood pressure trajectories: Early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension 2015, 66, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.R.; Frontini, M.G.; Xu, J.; Berenson, G.S. Utility of childhood non-high-density lipoprotein cholesterol levels in predicting adult dyslipidemia and other cardiovascular risks: The Bogalusa Heart Study. Pediatrics 2006, 118, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Juhola, J.; Magnussen, C.G.; Viikari, J.S.A.; Kähönen, M.; Hutri-Kähönen, N.; Jula, A.; Lehtimäki, T.; Åkerblom, H.K.; Pietikäinen, M.; Laitinen, T.; et al. Tracking of serum lipid levels, blood pressure and body mass index from childhood to adulthood: The Cardiovascular Risk in Young Finns Study. J. Pediatr. 2011, 159, 584–590. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Available online: http://apps.who.int/iris/bitstream/10665/94384/1/9789241506236_eng.pdf?ua=1&ua=1 (accessed on 8 January 2018).
- Horta, B.L.; Victora, C.G.; World Health Organization. Long-Term Effects of Breastfeeding: A Systematic Review. 2013. Available online: http://apps.who.int/iris/bitstream/10665/79198/1/9789241505307_eng.pdf (accessed on 16 January 2018).
- Horta, B.L.; Loret de Mola, C.; Victora, C.G. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Whincup, P.H.; Cook, D.G. Breast-feeding and cardiovascular risk factors and outcomes in later life: Evidence from epidemiological studies. Proc. Nutr. Soc. 2011, 70, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Subcommittee on screening and management of high blood pressure in children. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140. [Google Scholar] [CrossRef] [PubMed]
- Falkner, B.; Gidding, S.S.; Ramirez-Garnica, G.; Wiltrout, S.A.; West, D.; Rappaport, E.B. The relationship of body mass index and blood pressure in primary care pediatric patients. J. Pediatr. 2006, 148, 195–200. [Google Scholar] [CrossRef]
- Perng, W.; Rifas-Shiman, S.L.; Kramer, M.S.; Haugaard, L.K.; Oken, E.; Gillman, M.W.; Belfort, M.B. Early weight gain, linear growth, and mid-childhood blood pressure: A prospective study in Project Viva. Hypertension 2016, 67, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.; Logan, K.M.; Santhakumaran, S.; Parkinson, J.R.; Hyde, M.J.; Modi, N. Effect of breastfeeding compared with formula feeding on infant body composition: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 656–669. [Google Scholar] [CrossRef]
- Rzehak, P.; Oddy, W.H.; Mearin, M.L.; Grote, V.; Mori, T.A.; Szajewska, H.; Shamir, R.; Koletzko, S.; Weber, M.; Beilin, L.J.; et al. WP10 working group of the Early Nutrition Project. Infant feeding and growth trajectory patterns in childhood and body composition in young adulthood. Am. J. Clin. Nutr. 2017, 106, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Lucas, A. Early origins of cardiovascular disease: Is there a unifying hypothesis? Lancet 2004, 363, 1642–1645. [Google Scholar] [CrossRef]
- Owen, C.G.; Whincup, P.H.; Odoki, K.; Gilg, J.A.; Cook, D.G. Infant feeding and blood cholesterol: A study in adolescents and a systematic review. Pediatrics 2002, 110, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2017, 3, CD000376. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.R.; Harsløf, L.B.; Schnurr, T.M.; Hansen, T.; Hellgren, L.I.; Michaelsen, K.F.; Lauritzen, L. A study of associations between early DHA status and fatty acid desaturase (FADS) SNP and developmental outcomes in children of obese mothers. Br. J. Nutr. 2017, 117, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Gibson, R.A.; Udell, T.; Ried, K.; International LCPUFA Investigators. Supplementation of infant formula with long-chain polyunsaturated fatty acids does not influence the growth of term infants. Am. J. Clin. Nutr. 2005, 81, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Pluymen, L.P.M.; Dalmeijer, G.W.; Smit, H.A.; Uiterwaal, C.S.P.M.; van der Ent, C.K.; van Rossem, L. Long-chain polyunsaturated fatty acids in infant formula and cardiovascular markers in childhood. Matern. Child Nutr. 2018, 14, e12523. [Google Scholar] [CrossRef]
- Lauritzen, L.; Jørgensen, M.H.; Hansen, H.S.; Michaelsen, K.F. Fluctuations in human milk long-chain PUFA levels in relation to dietary fish intake. Lipids 2002, 37, 237–244. [Google Scholar] [CrossRef]
- Jackson, K.H.; Harris, W.S. Should there be a target level of docosahexaenoic acid in breast milk? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 92–96. [Google Scholar] [CrossRef]
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: A meta-analysis of randomized controlled trials. Am. J. Hypertens. 2014, 27, 885–896. [Google Scholar] [CrossRef]
- Jensen, H.A.; Harsløf, L.B.S.; Nielsen, M.S.; Christensen, L.B.; Ritz, C.; Michaelsen, K.F.; Vogel, U.; Lauritzen, L. FADS single-nucleotide polymorphisms are associated with behavioural outcomes in children, and the effect varies between sexes and is dependent on PPAR genotype. Am. J. Clin. Nutr. 2014, 100, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Sørensen, L.B.; Harsløf, L.B.; Ritz, C.; Stark, K.D.; Astrup, A.; Dyssegaard, C.B.; Egelund, N.; Michaelsen, K.F.; Damsgaard, C.T. Mendelian randomization shows sex-specific associations between long-chain PUFA-related genotypes and cognitive performance in Danish schoolchildren. Am. J. Clin. Nutr. 2017, 106, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M. DHA supplementation during the perinatal period and neurodevelopment: Do some babies benefit more than others? Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Kyhl, H.B.; Jensen, T.K.; Barington, T.; Buhl, S.; Norberg, L.A.; Jørgensen, J.S.; Jensen, D.F.; Christesen, H.T.; Lamont, R.F.; Husby, S. The Odense Child Cohort: Aims, design, and cohort profile. Paediatr. Perinat. Epidemiol. 2015, 29, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Bruun, S.; Buhl, S.; Husby, S.; Jacobsen, L.N.; Michaelsen, K.F.; Sørensen, J.; Zachariassen, G. Breastfeeding, infant formula, and introduction to complementary foods—Comparing data obtained by questionnaires and health visitors’ reports to weekly short message service text messages. Breastfeed. Med. 2017, 12, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Metherel, A.H.; Buzikievich, L.M.; Charkhzarin, P.; Patterson, A.C.; Peel, A.C.; Howorth, A.M.; Kishi, D.M.; Stark, K.D. Omega-3 polyunsaturated fatty acid profiling using fingertip-prick whole blood does not require overnight fasting before blood collection. Nutr. Res. 2012, 32, 547–556. [Google Scholar] [CrossRef]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004, 114, 555–576. [Google Scholar] [CrossRef]
- Task Force on Blood Pressure Control in Children. The National Heart, Lung, and Blood Institute, Bethesda, Maryland. Report of the Second Task Force on Blood Pressure Control in Children—1987. Pediatrics 1987, 79, 3797155. [Google Scholar]
- Rosner, B.; (Harvard University, Boston, MA, USA). Personal communication, 2 November 2007.
- Childhood Blood Pressure Macro-Batch Mode. Available online: https://sites.google.com/a/channing.harvard.edu/bernardrosner/pediatric-blood-press/childhood-blood-pressure (accessed on 1 November 2017).
- Browne, J.; Awad, I.; Plant, R.; McAdoo, J.; Shorten, G. Topical amethocaine (Ametop) is superior to EMLA for intravenous cannulation. Eutectic mixture of local anesthetics. Can. J. Anaesth. 1999, 46, 1014–1018. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- EPONIG FOR, RRIN CHILDREN. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl. 5), S213–S256. [Google Scholar] [CrossRef] [PubMed]
- Wijga, A.; Houwelingen, A.C.; Smit, H.A.; Kerkhof, M.; Vos, A.P.; Neijens, H.J.; Brunekreef, B.; PIAMA Birth Cohort Study. Fatty acids in breast milk of allergic and non-allergic mothers: The PIAMA birth cohort study. Pediatr. Allergy Immunol. 2003, 14, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Rosenlund, H.; Fagerstedt, S.; Alm, J.; Mie, A. Breastmilk fatty acids in relation to sensitization—The ALADDIN birth cohort. Allergy 2016, 71, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, J.; Linderborg, K.; Niinikoshi, H.; Yang, B.; Lagström, H. Breast milk fatty acid composition differs between overweight and normal weight women: The STEPS Study. Eur. J. Nutr. 2013, 52, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Barrera, C.; Valenzuela, R.; Chamorro, R.; Bascuñán, K.; Sandoval, J.; Sabag, N.; Valenzuela, F.; Valencia, M.-P.; Puigrredon, C.; Valenzuela, A. The impact of maternal diet during pregnancy and lactation on the fatty acid composition of erythrocytes and breast milk of Chilean women. Nutrients 2018, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Halkjæer, L.B.; Mikkelsen, T.B.; Olsen, S.F.; Michaelsen, K.F.; Loland, L.; Bisgaard, H. Fatty acid composition of human milk in atopic Danish mothers. Am. J. Clin. Nutr. 2006, 84, 190–196. [Google Scholar] [CrossRef]
- Damsgaard, C.T.; Schack-Nielsen, L.; Michaelsen, K.F.; Fruekilde, M.B.; Hels, O.; Lauritzen, L. Fish oil affects blood pressure and the plasma lipid profile in healthy Danish infants. J. Nutr. 2006, 136, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Harsløf, L.B.; Damsgaard, C.T.; Hellgren, L.I.; Andersen, A.D.; Vogel, U.; Lauritzen, L. Effects on metabolic markers are modified by PPARG2 and COX2 polymorphisms in infants randomized to fish oil. Genes Nutr. 2014, 9, 396. [Google Scholar] [CrossRef]
- Ulbak, J.; Lauritzen, L.; Hansen, H.S.; Michaelsen, K.F. Diet and blood pressure in 2.5-y-old Danish children. Am. J. Clin. Nutr. 2004, 79, 1095–1102. [Google Scholar] [CrossRef]
- Asserhøj, M.; Nehammer, S.; Matthiessen, J.; Michaelsen, K.F.; Lauritzen, L. Maternal fish oil supplementation during lactation may adversely affect long-term blood pressure, energy intake, and physical activity of 7-year-old boys. J. Nutr. 2009, 139, 298–304. [Google Scholar] [CrossRef]
- Lauritzen, L.; Eriksen, S.E.; Hjorth, M.F.; Nielsen, M.S.; Olsen, S.F.; Stark, K.D.; Michaelsen, K.F.; Damsgaard, C.T. Maternal fish oil supplementation during lactation is associated with reduced height at 13 years of age and higher blood pressure in boys only. Br. J. Nutr. 2016, 116, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- See, V.H.L.; Mori, T.A.; Prescott, S.L.; Beilin, L.J.; Burrows, S.; Huang, R.C. Cardiometabolic risk factors at 5 years after omega-3 fatty acid supplementation in infancy. Pediatrics 2018, 142. [Google Scholar] [CrossRef] [PubMed]
- van Rossem, L.; Wijga, A.H.; de Jongste, J.C.; Koppelman, G.H.; Oldenwening, M.; Postma, D.S.; Abrahamse-Berkeveld, M.; van de Heijning, B.; Brunekreef, B.; Smit, H.A. Blood pressure in 12-year-old children is associated with fatty acid composition of human milk: The prevention and incidence of asthma and mite allergy birth cohort. Hypertension 2012, 60, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T. Recommended standards for assessing blood pressure in human research where blood pressure or hypertension is a major focus. Clin. Exp. Hypertens. 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Duncombe, S.L.; Voss, C.; Harris, K.C. Oscillometric and auscultatory blood pressure measurement methods in children: A systematic review and meta-analysis. J. Hypertens. 2017, 35, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Schmidt, R.J.; Hertz-Picciotto, I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int. J. Epidemiol. 2014, 43, 443–464. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Iwanaga, M.; Harada, E. Possible regulatory mechanism of DHA-induced anti-stress reaction in rats. Brain Res. 2003, 964, 136–143. [Google Scholar] [CrossRef]
- Fedorova, I.; Salem, N., Jr. Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 271–289. [Google Scholar] [CrossRef]
- Pérez, M.Á.; Terreros, G.; Dagnino-Subiabre, A. Long-term ω-3 fatty acid supplementation induces anti-stress effects and improves learning in rats. Behav. Brain Funct. 2013, 9, 25. [Google Scholar] [CrossRef]
- Cartier, L.J.; Collins, C.; Lagacé, M.; Douville, P. Comparison of fasting and non-fasting lipid profiles in a large cohort of patients presenting at a community hospital. Clin. Biochem. 2018, 52, 61–66. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Langsted, A.; Mora, S.; Kolovou, G.; Baum, H.; Bruckert, E.; Watts, G.F.; Sypniewska, G.; Wiklund, O.; Borén, J.; et al. Fasting is not routinely required for determination of a lipid profile: Clinical and laboratory implications including flagging at desirable concentration cutpoints—A joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Clin. Chem. 2016, 62, 930–946. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.H.; Mølgaard, C.; Hellgren, L.I.; Lauritzen, L. Effects of fish oil supplementation on markers of the metabolic syndrome. J. Pediatr. 2010, 157, 395–400. [Google Scholar] [CrossRef]
- Balk, E.M.; Lichtenstein, A.H. Omega-3 fatty acids and cardiovascular disease: Summary of the 2016 Agency of Healthcare Research and Quality Evidence Review. Nutrients 2017, 9, 865. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Sacks, F.; Rosner, B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 1993, 88, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Shi, M.Q.; Li, Z.H.; Yang, J.J.; Li, D. Fish, long-chain n-3 PUFA and incidence of elevated blood pressure: A meta-analysis of prospective cohort studies. Nutrients 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Davey Smith, G. Does having been breastfed in infancy influence lipid profile in later lafe? A review of the literature. Adv. Exp. Med. Biol. 2009, 646, 41–50. [Google Scholar] [CrossRef]
| Group | A | B | C |
|---|---|---|---|
| n | 336 | 1967 | 359 |
| Maternal Characteristics | |||
| Age at parturition, years | |||
| mean (SD) | 30.6 (4.1) | 30.4 (4.5) | 29.2 (5.0) |
| Pre-pregnancy BMI, kg/m2 | |||
| median (IQR) | 22.9 (21.1, 26.1) | 23.4 (21.3, 26.4) | 23.4 (20.8, 26.3) |
| Postdelivery parity, n (%) | |||
| primiparous | 172 (51.2) | 1106 (56.2) | 205 (57.1) |
| Parity 2 | 120 (35.7) | 658 (33.5) | 112 (31.2) |
| Parity 3 | 39 (11.6) | 158 (8.0) | 32 (8,9) |
| Parity ≥ 4 | 4 (1.2) | 34 (1.7) | 10 (2.8) |
| unknown | 1 (0.3) | 11 (0.6) | - |
| Educational level, n (%) | |||
| Low | 49 (14.6) | 391 (19.9) | 95 (26.5) |
| Intermediate | 154 (45.8) | 741 (37.7) | 106 (29.5) |
| High | 74 (22.0) | 329 (16.7) | 28 (7.8) |
| unknown | 59 (17.6) | 506 (25.7) | 130 (36.2) |
| Smoking status, n (%) | |||
| Smoker | 1 (0.3) | 70 (3.6) | 32 (8.9) |
| Nonsmoker 1 | 334 (99.4) | 1882 (95.7) | 323 (90.0) |
| unknown | 1 (0.3) | 15 (0.8) | 4 (1.1) |
| Birth type, n (%) | |||
| Vaginal | 276 (82.1) | 1511 (76.8) | 254 (70.8) |
| Caesarean section | 60 (17.9) | 456 (23.8) | 105 (29.2) |
| Children’s Characteristics | |||
| Sex, n (%) | |||
| Male | 175 (52.1) | 1025 (52.1) | 196 (54.6) |
| Female | 161 (47.9) | 942 (47.9) | 163 (45.4) |
| Gestational age, days | |||
| median (IQR) | 281 (274, 288) | 281 (273, 287) | 279 (271, 286) |
| Birth weight, g | |||
| mean (SD) | 3575 (487) | 3482 (567) | 3404 (639) |
| unknown | - | 3 | 1 |
| Birth weight Z-score, SD | |||
| mean (SD) | −0.01 (1.0) | −0.14 (1.0) | −0.19 (1.1) |
| unknown | - | 3 | 1 |
| Birth weight Z-score group, n (%) | |||
| BWZ < −2 SD | 6 (1.8) | 55 (2.8) | 16 (4.5) |
| −2 SD ≤ BWZ ≤ 2 SD | 319 (94.9) | 1,863 (94.7) | 332 (92.5) |
| BWZ > 2 SD | 11 (3.3) | 46 (2.3) | 10 (2.8) |
| unknown | - | 3 (0.2) | 1 (0.3) |
| FA wt% of Total FA | ||
|---|---|---|
| Girls | Boys | |
| n | 161 | 175 |
| Total SFAs | 42.49 (5.17) | 42.37 (4.14) |
| Total MUFAs | 40.49 (3.89) | 40.74 (3.35) |
| Total PUFAs | 13.68 (2.67) | 13.41 (2.38) |
| Total n-3 PUFA | 11.84 (2.34) | 11.66 (2.22) |
| Total LA 1 | 10.76 (2.26) | 10.58 (2.14) |
| Total AA | 0.37 (0.09) | 0.35 (0.08) |
| Total n-3 PUFA | 1.81 (0.61) | 1.73 (0.48) |
| Total ALA 2 | 1.09 (0.85, 1.46) | 1.10 (0.86, 1.39) |
| Total EPA | 0.08 (0.06, 0.12) | 0.08 (0.06, 0.12) |
| Total DHA | 0.25 (0.19, 0.34) | 0.24 (0.17, 0.35) |
| Total n-3 LC-PUFAs (EPA + DHA) | 0.34 (0.25, 0.47) | 0.33 (0.24, 0.46) |
| n-6/n-3 PUFA ratio | 6.80 (5.59, 8.03) | 6.81 (5.62, 8.15) |
| Girls | Boys | |||||
|---|---|---|---|---|---|---|
| 4 Months | 18 Months | 36 Months | 4 Months | 18 Months | 36 Months | |
| Attending, n | 161 | 98 | 143 | 175 | 115 | 152 |
| Age, months | 4.0 (3.4, 4.4) | 18.6 (18.4, 19.1) | 36.1 (35.9, 36.5) | 4.0 (3.4, 4.4) | 18.6 (18.2, 19.2) | 36.1 (35.9, 36.5) |
| Blood pressure, n | 90 1 | 46 2 | 133 3 | 92 | 56 | 133 4 |
| Age, months | 4.1 (3.6, 4.5) | 18.7 (18.3, 19.2) | 36.1 (35.9, 36.5) | 4.0 (3.6, 4.4) | 18.6 (18.2, 19.2) | 36.1 (35.9, 36.5) |
| Systolic BP, mmHg | 99.6 (11.3) | 103.5 (9.5) | 98.1 (7.3) | 103.8 (14.0) | 102.3 (9.1) | 98.4 (7.3) |
| Diastolic BP, mmHg | 61.1 (11.1) | 63.4 (6.9) | 62.3 (5.8) | 61.6 (9.6) | 63.6 (8.6) | 61.8 (5.6) |
| Systolic BP, percentile | 68.2 (23.6) | 87.9 (12.7) | 72.6 (18.5) | 72.2 (25.1) | 86.5 (16.3) | 74.9 (17.4) |
| Diastolic BP, percentile | 78.3 (23.2) | 93.4 (7.4) | 86.4 (10.8) | 83.3 (16.2) | 95.8 (4.9) | 91.7 (6.9) |
| Blood lipid profile, n | 63 5 | 21 6 | 44 | 60 | 32 | 47 |
| Age, months | 4.2 (3.8, 4.4) | 18.8 (18.5, 19.2) | 36.0 (35.7, 36.3) | 4.0 (3.7, 4.5) | 18.8 (18.3, 19.6) | 36.1 (35.9, 36.6) |
| Total cholesterol, mmol/L | 4.2 (3.8, 4.5) | 3.9 (3.5, 4.5) | 3.8 (3.5, 4.5) | 4.2 (3.7, 4.4) | 4.0 (3.6, 4.3) | 3.7 (3.3, 4.2) |
| HDL cholesterol, mmol/L | 1.1 (0.9, 1.2) | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.3) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) | 1.2 (1.1, 1.4) |
| LDL cholesterol, mmol/L | 2.0 (1.7, 2.4) | 2.3 (2.0, 2.8) | 2.3 (2.0, 2.8) | 2.0 (1.8, 2.4) | 2.2 (1.9, 2.6) | 2.2 (1.8, 2.5) |
| non-HDL cholesterol, mmol/L | 3.1 (2.7, 3.5) | 2.8 (2.4, 3.4) | 2.7 (2.3, 3.2) | 3.0 (2.6, 3.3) | 2.9 (2.3, 3.1) | 2.5 (2.1, 2.9) |
| Triglycerides, mmol/L | 2.1 (1.4, 3.0) | 1.0 (0.9, 1.2) | 0.9 (0.6, 1.3) | 2.1 (1.5, 2.7) | 1.3 (0.9, 1.4) | 0.8 (0.5, 1.0) |
| Group | Girls | Boys | ||||
|---|---|---|---|---|---|---|
| 4 Months | 18 Months | 36 Months | 4 Months | 18 Months | 36 Months | |
| Blood pressure, n | 76 1 | 39 2 | 108 3 | 79 | 49 | 113 4 |
| Systolic BP, mmHg | 2.4 (−10.3, 15.0) | −10.3 (−26.9, 6.3) | −0.5 (−7.5, 6.6) | −20.0 (−33.4, −6.7) B | 0.5 (−15.1, 16.1) | 2.5 (−4.0, 9.0) |
| Diastolic BP, mmHg | 3.6 (−8.2, 15.5) | −10.9 (−23.3, 1.4) | −0.2 (−5.3, 4.9) | −10.2 (−19.8, −0.5) A | −0.8 (−16.5, 14.9) | 4.7 (−0.3, 9.8) |
| Systolic BP, percentile | 7.3 (−18.4, 33.0) | −21.2 (−41.6, −0.9) A | 1.2 (−16.5, 18.9) | −35.7 (−60.2, −11.3) B | 14.3 (−9.7, 38.3) | 1.2 (−14.4, 16.7) |
| Diastolic BP, percentile | 15.5 (−10.3, 41.2) | −4.8 (−17.2, 7.7) | 0.6 (−8.6, 9.8) | −16.5 (−34.0, 1.0) | 4.1 (−4.4, 12.6) | 3.2 (−3.0, 9.4) |
| Blood lipid profile, n | 55 5 | 18 6 | 40 | 52 | 30 | 43 |
| Total cholesterol, mmol/L | 0.2 (−0.7, 1.0) | 0.2 (−3.1, 3.6) | −0.8 (−2.2, 0.5) | 0.2 (−0.8, 1.1) | 0.7 (−1.0, 2.3) | −0.1 (−1.2, 1.1) |
| HDL cholesterol, mmol/L | −0.7 (−1.1, −0.3) B | −0.2 (−1.1, 0.8) | −0.3 (−0.9, 0.3) | −0.1 (−0.6, 0.3) | −0.3 (−1.1, 0.5) | −0.2 (−0.7, 0.3) |
| LDL cholesterol, mmol/L | −0.1 (−1.0, 0.9) | 0.7 (−3.2, 4.5) | −0.8 (−2.0, 0.3) | 0.3 (−0.5, 1.1) | 0.7 (−0.5, 2.0) | −0.2 (−1.1, 0.7) |
| Triglycerides, mmol/L | 3.1 (1.0, 5.2)B | 0.6 (−2.5, 3.8) | 0.7 (−0.5, 1.9) | 0.2 (−1.8, 2.1) | 0.6 (−0.4, 1.6) | 0.6 (−0.1, 1.3) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruun, S.; van Rossem, L.; Lauritzen, L.; Husby, S.; Neergaard Jacobsen, L.; Michaelsen, K.F.; Boysen Sandberg, M.; Stark, K.D.; Sørensen, J.; Zachariassen, G. Content of n-3 LC-PUFA in Breast Milk Four Months Postpartum is Associated with Infancy Blood Pressure in Boys and Infancy Blood Lipid Profile in Girls. Nutrients 2019, 11, 235. https://doi.org/10.3390/nu11020235
Bruun S, van Rossem L, Lauritzen L, Husby S, Neergaard Jacobsen L, Michaelsen KF, Boysen Sandberg M, Stark KD, Sørensen J, Zachariassen G. Content of n-3 LC-PUFA in Breast Milk Four Months Postpartum is Associated with Infancy Blood Pressure in Boys and Infancy Blood Lipid Profile in Girls. Nutrients. 2019; 11(2):235. https://doi.org/10.3390/nu11020235
Chicago/Turabian StyleBruun, Signe, Lenie van Rossem, Lotte Lauritzen, Steffen Husby, Lotte Neergaard Jacobsen, Kim F. Michaelsen, Maria Boysen Sandberg, Ken D. Stark, Jan Sørensen, and Gitte Zachariassen. 2019. "Content of n-3 LC-PUFA in Breast Milk Four Months Postpartum is Associated with Infancy Blood Pressure in Boys and Infancy Blood Lipid Profile in Girls" Nutrients 11, no. 2: 235. https://doi.org/10.3390/nu11020235
APA StyleBruun, S., van Rossem, L., Lauritzen, L., Husby, S., Neergaard Jacobsen, L., Michaelsen, K. F., Boysen Sandberg, M., Stark, K. D., Sørensen, J., & Zachariassen, G. (2019). Content of n-3 LC-PUFA in Breast Milk Four Months Postpartum is Associated with Infancy Blood Pressure in Boys and Infancy Blood Lipid Profile in Girls. Nutrients, 11(2), 235. https://doi.org/10.3390/nu11020235





