Giardia intestinalis and Fructose Malabsorption: A Frequent Association
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Parasitological Examination
2.3. Analytical Determinations
2.4. Statistical Analysis
2.5. Ethical Statement
3. Results
3.1. Presence of Intestinal Parasites
3.2. Comparison of the Effectiveness of the Methods Used for G. intestinalis Diagnosis
3.3. Analytical Changes in the Study Population
3.4. Potential Risk Factors for Intestinal Parasites
3.5. Analysis of the Association of Intestinal Parasites and Carbohydrate Malabsorption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Ahmadi, A. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Solano, L.; Acuña, I.; Barón, M.A.; Morón de Salim, A.; Sánchez, A. Influencia de las parasitosis intestinales y otros antecedentes infecciosos sobre el estatus nutricional antropométrico de niños en situación de pobreza. Parasitología Latinoamericana 2008, 63, 12–19. [Google Scholar] [CrossRef]
- Fouad, S.A.; Esmat, S.; Basyoni, M.M.; Farhan, M.S.; Kobaisi, M.H. Molecular identification of Giardia intestinalis in patients with dyspepsia. Digestion 2014, 90, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Moya-Camarena, S.Y.; Sotelo, N.; Valencia, M.E. Effects of asymptomatic Giardia intestinalis infection on carbohydrate absorption in well-nourished Mexican children. Am. J. Trop. Med. Hyg. 2002, 66, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, B.; Matera, G.; Laratta, C.; Schipani, G.; Guarnieri, G.; Spiniello, E.; Imeneo, M.; Amorosi, A.; Focà, A.; Luzza, F. Giardia lamblia infection in patients with irritable bowel syndrome and dyspepsia: A prospective study. World J. Gastroenterol. 2006, 12, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Stark, D.; Van Hal, S.; Marriott, D.; Ellis, J.; Harkness, J. Irritable bowel syndrome: A review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int. J. Parasitol. 2007, 37, 11–20. [Google Scholar] [CrossRef]
- Litleskare, S.; Rortveit, G.; Eide, G.E.; Hanevik, K.; Langeland, N.; Wensaas, K.A. Prevalence of irritable bowel syndrome and chronic fatigue 10 years after Giardia infection. Clin. Gastroenterol. Hepatol. 2018, 16, 1064–1072. [Google Scholar] [CrossRef]
- Einarsson, E.; Troell, K.; Hoeppner, M.P.; Grabherr, M.; Ribacke, U.; Svärd, S.G. Coordinated changes in gene expression thorough encystation of Giardia intestinalis. PLoS Negl. Trop. Dis. 2016, 10, e0004571. [Google Scholar] [CrossRef]
- Ryan, U.; Cacciò, S.M. Zoonotic potential of Giardia. Int. J. Parasitol. 2013, 43, 943–956. [Google Scholar] [CrossRef]
- Ryan, U.; Hijjawi, N.; Feng, Y.; Xiao, L. Giardia: An under-reported foodborne parasite. Int. J. Parasitol. 2018, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Horton, B.; Bridle, H.; Alexander, C.L.; Katzer, F. Giardia duodenalis in the UK: Current knowledge of risk factors and public health implications. Int. J. Parasitol. 2018, 35, 1181–1190. [Google Scholar]
- Torgerson, P.R.; de Silva, N.R.; Fevre, E.M.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Sripa, B.; Gargouri, N.; Willingham, A.L.; Stein, C. The global burden of foodborne parasitic diseases: An update. Trends Parasitol. 2014, 30, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Odoi, A.; Martin, S.W.; Michel, P.; Holt, J.; Middleton, D.; Wilson, J. Determinants of the geographical distribution of endemic giardiosis in Ontario, Canada: A spatial modelling approach. Epidemiol. Infect. 2004, 132, 967–976. [Google Scholar] [CrossRef]
- Minetti, C.; Lamden, K.; Durband, C.; Cheesbrough, J.; Platt, K.; Charlett, A.; Wastling, J.M. Case-control study of risk factors for sporadic giardiasis and parasite assemblages in North West England. J. Clin. Microbiol. 2015, 53, 3133–3140. [Google Scholar] [CrossRef]
- Vega-Franco, L.; Meza, C.; Romero, J.L.; Alanis, S.E.; Meijerink, J. Breath hydrogen test in children with giardiasis. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 365–368. [Google Scholar] [CrossRef]
- Pettoello Mantovani, M.; Guandalini, S.; Ecuba, P.; Corvino, C.; di Martino, L. Lactose malabsorption in children with symptomatic Giardia lamblia infection: Feasibility of yogurt supplementation. J. Pediatr. Gastroenterol. Nutr. 1989, 9, 295–300. [Google Scholar] [CrossRef]
- Cordingley, F.T.; Crawford, G.P.M. Giardia infection causes vitamin b12 deficiency. Aust. N. Z. J. Med. 1986, 16, 78–79. [Google Scholar] [CrossRef]
- Cotton, J.A.; Beaty, J.K.; Buret, A.G. Host parasite interactions and pathophysiology in Giardia infections. Int. J. Parasitol. 2011, 41, 925–933. [Google Scholar] [CrossRef]
- Troeger, H.; Epple, H.J.; Schneider, T.; Wahnschaffe, U.; Ullrich, R.; Burchard, G.D.; Jelinek, T.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Effect of chronic Giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut 2007, 56, 328–335. [Google Scholar] [CrossRef]
- Halliez, M.C.; Buret, A.G. Extra-intestinal and long-term consequences of Giardia duodenalis infections. World J. Gastroenterol. 2013, 47, 8974–8985. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, L.A.; Sartor, R.B. Advances in understanding Giardia: Determinants and mechanisms of chronic sequelae. F1000prime Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Atlanta. Giardiasis. 2017. Available online: https://www.cdc.gov/parasites/giardia/ (accessed on 9 October 2019).
- Koehler, A.V.; Jex, A.R.; Haydon, S.R.; Stevens, M.A.; Gasser, R.B. Giardia/giardiasis—A perspective on diagnostic and analytical tools. Biotechnol. Adv. 2014, 32, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, J.; Jafri, W.; Abid, S.; Jafri, N.; Hamid, S.; Shah, H.A.; Shaikh, H. Giardiasis in patients with dyspeptic symptoms. World J. Gastroenterol. 2005, 11, 6667. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Borjas, D.M.; Díaz-Rivadeneyra, J.; Doña-Leyva, A.; Zambrano-Villa, S.A.; Mascaró, C.; Osuna, A.; Ortiz-Ortiz, L. Secretory immune response to membrane antigens during Giardia lamblia infection in humans. Infect. Immun. 1998, 66, 756–759. [Google Scholar] [PubMed]
- Hassan, S.M.T.; Maachee, M.; Cordova, O.M.; de la Guardia, R.D.; Martins, M.; Osuna, A. Human secretory immune response to fatty acid-binding protein fraction from Giardia lamblia. Infect. Immun. 2002, 70, 2226–2229. [Google Scholar] [CrossRef]
- Hassan, S.M.T.; Maache, M.; de la Guardia, R.D.; Cordova, O.M.; García, J.G.; Galiana, M.; Osuna, A. Binding properties and immunolocalization of a fatty acid-binding protein in Giardia lamblia. J. Parasitol. 2005, 91, 284–293. [Google Scholar] [CrossRef]
- El-Gebaly, N.S.; Halawa, E.F.; Moussa, H.M.; Rabia, I.; Abu-Zekry, M. Saliva and sera IgA and IgG in Egyptian Giardia-infected children. Parasitol. Res. 2012, 111, 571–575. [Google Scholar] [CrossRef]
- Alliende, F. Intolerancia a la lactosa y otros disacáridos. Gastr Latinoam 2007, 18, 152–156. [Google Scholar]
- Zugasti, A. Intolerancia alimentaria. Endocrinología y Nutrición 2009, 56, 241–250. [Google Scholar] [CrossRef]
- Riveros, M.J.; Parada, A.; Pettinelli, P. Consumo de fructosa y sus implicaciones para la salud: Malabsorción de fructosa e hígado graso no alcohólico. Nutrición Hospitalaria 2014, 29, 491–499. [Google Scholar] [PubMed]
- Brown-Esters, O.; Mc Namara, P.; Savaiano, D. Dietary and biological factors influencing lactose intolerance. Int. Dairy J. 2012, 22, 98–103. [Google Scholar] [CrossRef]
- Yang, J.; Deng, Y.; Chu, H.; Cong, Y.; Zhao, J.; Pohl, D.; Misselwitz, B.; Fried, M.; Dai, N.; Fox, M. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2013, 11, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Huerta, J.U.; De la Cruz-Patiño, E.; Ramirez-Gutiérrez de Velasco, A.; Zamundo, C.; Remes-Troche, J.M. Intolerancia a la fructosa en pacientes con síndrome de intestino irritable; un estudio de casos y controles. Revista de Gastroenterología de México 2010, 4, 405–411. [Google Scholar]
- Wilder-Smith, C.H.; Li, X.; Ho, S.S.; Leong, S.M.; Wong, R.K.; Koay, E.S.; Ferraris, R.P. Fructose trasnporters GLUT-5 and GLUT-2 expression in adult patients with fructose intolerance. U. Eur. Gastroenterol. J. 2014, 2, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Lindshield, B.L.; Adhikari, K. Online and campus college students like using an open educational resource instead of a traditional textbook. J. Online Learn. Teach. 2013, 9, 26–38. [Google Scholar]
- Gibson, P.R.; Newnham, E.; Barrett, J.S.; Shepherd, S.J.; Muir, J.G. Review article: Fructose malabsorption and the bigger picture. Aliment. Pharmacol. Ther. 2007, 25, 349–363. [Google Scholar] [CrossRef]
- Ghosal, U. How to interpret Hydrogen Breath Test. J. Neurogastroenterol. Motil. 2011, 17, 312–317. [Google Scholar] [CrossRef]
- Hinojosa-Guadix, J.H.; Gamarro, M.P.; Sánchez, I.M. Malabsorción e intolerancia a la fructosa: Fructosa-sorbitol en patología funcional. Revista Andaluza de Patología Digestive 2017, 40, 119–124. [Google Scholar]
- Paul-Dauphin, A.; Guillemin, F.; Virion, J.M.; Briançon, S. Bias and precision in visual analogue scales: A randomized controlled trial. Am. J. Epidemiol. 1999, 150, 1117–1127. [Google Scholar] [CrossRef]
- Fletcher, S.M.; Stark, D.; Harkness, J.; Ellis, J. Enteric Protozoa in the Developed World: A Public Health Perspective. Clin. Microbiol. Rev. 2012, 25, 420–449. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, O.L.; Hagel, I.; González, Y.; Roque, M.E.; Vásquez, N.; López, E.; Di Prisco, M.C. Secretory IgA antibody responses in Venezuelan children infected with Giardia duodenalis. J. Trop. Pediatr. 2004, 50, 68–72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abulhasan, M.; Elshazly, T.A.; Eida, M.; Albadry, A. Giardia intestinalis in patients with nonulcer dyspepsia. Arab J. Gastroenterol. 2013, 14, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Cedillo-Rivera, R.; Leal, Y.A.; Yépez-Mulia, L.; Gómez-Delgado, A.; Ortega-Pierres, G.; Tapia-Conyer, R.; Muñoz, O. Seroepidemiology of giardiasis in Mexico. Am. J. Trop. Med. Hyg. 2009, 80, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.S. Giardiasis. Clin. Microbiol. Rev. 1992, 5, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, J.; Jafri, W.; Jafri, N.; Khan, R.; Islam, M.; Beg, M.A.; Zaman, V. Irritable bowel syndrome: In search of an etiology: Role of Blastocystis hominis. Am. J. Trop. Med. Hyg. 2004, 70, 383–385. [Google Scholar] [CrossRef]
- Yakoob, J.; Jafri, W.; Beg, M.A.; Abbas, Z.; Naz, S.; Islam, M.; Khan, R. Irritable bowel syndrome: Is it associated with genotypes of Blastocystis hominis. Parasitol. Res. 2010, 106, 1033–1038. [Google Scholar] [CrossRef]
- Losada-Ocaña, C.; Cuenca-Gómez, J.A.; Cabezas-Fernández, M.T.; Vázquez-Villegas, J.; Soriano-Pérez, M.J.; Cabeza-Barrera, I.; Salas-Coronas, J. Clinical and epidemiological characteristics of intestinal parasite infection by Blastocystis hominis. Rev. Clin. Esp. (Engl. Ed.) 2018, 218, 115–120. [Google Scholar] [CrossRef]
- Cifre, S.; Gozalbo, M.; Ortiz, V.; Soriano, J.M.; Merino, J.F.; Trelis, M. Blastocystis subtypes and their association with irritable bowel syndrome. Med. Hypotheses 2018, 116, 4–9. [Google Scholar] [CrossRef]
- Scanlan, P.D.; Stensvold, C.R. Blastocystis: Getting to grips with our guileful guest. Trends Parasitol. 2013, 29, 523–529. [Google Scholar] [CrossRef]
- National Centre of Epidemiology, Health Institute Carlos III (Spain). Results of Epidemiological Surveillance of Communicable Diseases. Annual Report. 2016. Available online: http://gesdoc.isciii.es/gesdoccontroller?action=download&id=25/01/2019-d8ee271b6f (accessed on 6 May 2018).
- Rivera, M.; de la Parte, M.A.; Hurtado, P.; Magaldi, L.; Collazo, M. Giardiasis intestinal. Mini-revisión. Investigación Clínica 2002, 43, 119–128. [Google Scholar] [PubMed]
- Alparo, I. Giardiasis y desnutrición. Revista de la Sociedad Boliviana de Pediatría 2005, 44, 166–173. [Google Scholar]
- Khandait, D.W.; Vasudeo, N.D.; Zodpey, S.P.; Kumbhalkar, D.T. Brief report. Risk factors for subclinical vitamin A deficiency in children under the age of 6 years. J. Trop. Pediatr. 2000, 46, 239–241. [Google Scholar] [PubMed]
- Ponce-Macotela, M.; González-Maciel, A.; Reynoso-Robles, R.; Martínez-Gordillo, M.N. Goblet cells: Are they an unspecific barrier against Giardia intestinalis or a gate. Parasitol. Res. 2008, 102, 509–513. [Google Scholar] [CrossRef]
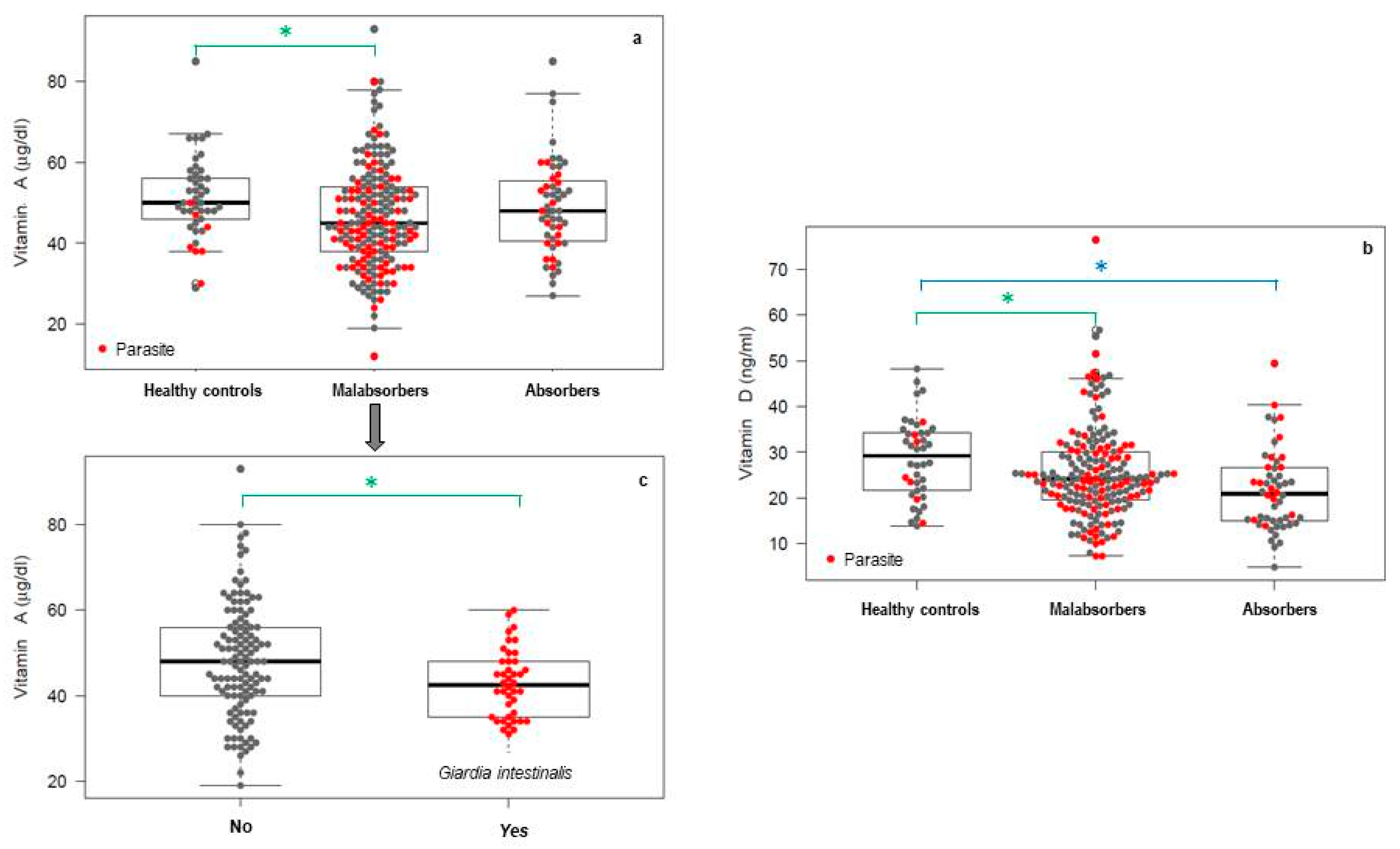
- Quihui, C.; Astiazarán-García, V.; Morales, F.; Lopez, M.; Ortiz, V. Impact of Giardia intestinalis on vitamin A status in schoolchildren from northwest Mexico. Int. J. Vitam. Nutr. Res. 2008, 78, 51–56. [Google Scholar] [CrossRef]
- Gil, Á.; Sánchez de Medina Contreras, F. Tratado de Nutrición, 2nd ed.; Médica Panamericana: Madrid, Spain, 2010; p. 3980. [Google Scholar]
- Ascensión, M. Inmunonutrición: En la Salud y la Enfermedad; Médica Panamericana: Madrid, Spain, 2011; p. 640. [Google Scholar]
- Mahalanabis, D.; Simpson, T.W.; Chakraborty, M.L.; Ganguli, C.; Bhattacharjee, A.K.; Mukherjee, K.L. Malabsorption of water miscible vitamin A in children with giardiasis and ascariasis. Am. J. Clin. Nutr. 1979, 32, 313–318. [Google Scholar] [CrossRef]
- Latham, M.C. Nutrición Humana en el Mundo en Desarrollo; FAO: Roma, Italy, 2002. [Google Scholar]
- Gilaberte, Y.; Aguilera, J.; Carrascosa, J.M.; Figueroa, F.L.; de Gabriel, J.R.; Nagore, E. La vitamina D: Evidencias y controversias. Actas Dermo-Sifiliográficas 2011, 102, 572–588. [Google Scholar] [CrossRef]
- Valles, L.E.T.; Salas, A.; Lozada, C.; Cárdenas, E.; Martín, J.; Agobian, G. Detección de enteroparásitos en lechugas que se comercializan en el estado Lara, Venezuela. Luz y Vida Revista Médico-Científica 2013, 4, 7–11. [Google Scholar]
- Amorós, I.; Alonso, J.L.; Cuesta, G. Cryptosporidium oocysts and Giardia cysts on salad products irrigated with contaminated water. J. Food Prot. 2010, 73, 1138–1140. [Google Scholar] [CrossRef]
| Malabsorbers (213) | Absorbers (56) | |
|---|---|---|
| Gender | ||
| Female, n (%) | 172 (80.8%) | 37 (66.1%) |
| Male, n (%) | 41 (19.2%) | 19 (33.9%) |
| Age | ||
| Years (mean) | 40.5 | 47.4 |
| minimum‒maximum | 29‒55 | 30‒64 |
| Type of malabsorption | ||
| Fructose, n (%) | 171 (80.3%) | |
| Lactose, n (%) | 127 (59.6%) | |
| Combined, n (%) | 84 (39.4%) | |
| Gastrointestinal symptoms | ||
| Diarrhea, n (%) | 204 (95.8%) | 50 (89.3%) |
| Constipation, n (%) | 112 (52.6%) | 22 (39.3%) |
| Abdominal distension (VAS > 5), n (%) | 197 (92.5%) | 49 (87.5%) |
| Flatulence (VAS > 5), n (%) | 203 (95.3%) | 51 (91.1%) |
| Comorbidities | ||
| Irritable Bowel Syndrome, n (%) | 22 (10.3%) | 7 (39.2%) |
| Inflammatory Bowel Disease, n (%) | 5 (2.4%) | |
| Celiac Disease, n (%) | 6 (2.8%) | |
| Intestinal Parasites | Patients (269) | Grouped Patients (269) | Controls (82) | |||||
|---|---|---|---|---|---|---|---|---|
| Malabsorbers (213) | Absorbers (56) | |||||||
| n | % | n | % | n | % | n | % | |
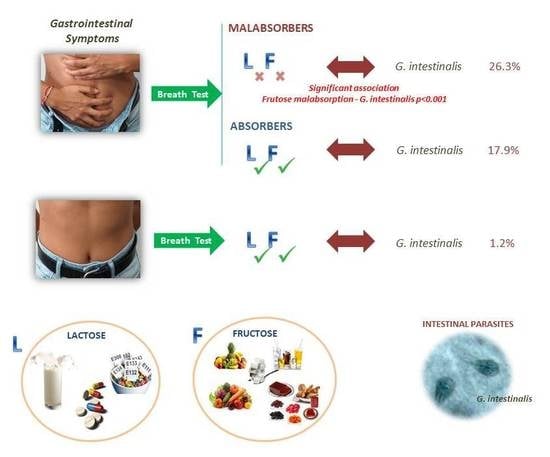
| Giardia intestinalis # * | 56 | 26.3 * | 10 | 17.9 * | 66 | 24.5 | 1 | 1.2 |
| Blastocystis sp. | 29 | 13.6 | 5 | 8.9 | 34 | 12.6 | 11 | 13.4 |
| Endolimax nana | 1 | 0.5 | 2 | 3.6 | 3 | 1.1 | 0 | 0.0 |
| Entamoeba coli | 0 | 0.0 | 3 | 5.4 | 3 | 1.1 | 0 | 0.0 |
| Entamoeba hartmanni | 1 | 0.5 | 3 | 5.4 | 4 | 1.1 | 0 | 0.0 |
| Cryptosporidium parvum | 2 | 0.9 | 0 | 0.0 | 2 | 0.7 | 0 | 0.0 |
| Iodamoeba buetschlii | 0 | 0.0 | 1 | 1.8 | 1 | 0.4 | 0 | 0.0 |
| Total | 89 | 41.8 | 24 | 42.9 | 113 | 42.0 | 12 | 14.6 |
| Feces | Saliva | ||
|---|---|---|---|
| Immunochromatography | Light Microscopy | Indirect ELISA | |
| Positive (69) | 10 (2.8%) | 17 (4.8%) | 62 (17.7%) |
| Negative (282) | 341 (97.2%) | 334 (95.2%) | 289 (82.3%) |
| Variables | N | With IP | OR | 95% CI | p Value | |
|---|---|---|---|---|---|---|
| n | % | |||||
| Nationality of risk | 17 | 9 | 52.9 | 1.32 | 0.41, 4.29 | 0.640 |
| Profession of risk | 56 | 26 | 46.4 | 2.16 | 1.14, 4.21 | 0.022 * |
| Contact with animals | 89 | 37 | 41.6 | 1.67 | 0.95, 2.92 | 0.072 |
| Regular consumption of ecological food | 180 | 95 | 52.8 | 6.81 | 4.03, 11.9 | 0.001 * |
| Having travelled to endemic countries in the last five years | 75 | 37 | 49.3 | 1.90 | 1.03, 3.57 | 0.040 * |
| Type of Malabsorption | G. intestinalis | Blastocystis sp. | ||||||
|---|---|---|---|---|---|---|---|---|
| Association | OR | 95% CI | p Value | Association | OR | 95% CI | p Value | |
| Fructose | 89.5% | 3.21 | 1.8‒5.7 | ˂0.001 * | 51.1% | 0.85 | 0.4‒1.6 | 0.076 |
| Lactose | 50.0% | 1.23 | 0.7‒2.3 | 0.089 | 42.2% | 1.24 | 0.6‒2.4 | 0.099 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trelis, M.; Taroncher-Ferrer, S.; Gozalbo, M.; Ortiz, V.; Soriano, J.M.; Osuna, A.; Merino-Torres, J.F. Giardia intestinalis and Fructose Malabsorption: A Frequent Association. Nutrients 2019, 11, 2973. https://doi.org/10.3390/nu11122973
Trelis M, Taroncher-Ferrer S, Gozalbo M, Ortiz V, Soriano JM, Osuna A, Merino-Torres JF. Giardia intestinalis and Fructose Malabsorption: A Frequent Association. Nutrients. 2019; 11(12):2973. https://doi.org/10.3390/nu11122973
Chicago/Turabian StyleTrelis, María, Silvia Taroncher-Ferrer, Mónica Gozalbo, Vicente Ortiz, José M. Soriano, Antonio Osuna, and Juan F. Merino-Torres. 2019. "Giardia intestinalis and Fructose Malabsorption: A Frequent Association" Nutrients 11, no. 12: 2973. https://doi.org/10.3390/nu11122973
APA StyleTrelis, M., Taroncher-Ferrer, S., Gozalbo, M., Ortiz, V., Soriano, J. M., Osuna, A., & Merino-Torres, J. F. (2019). Giardia intestinalis and Fructose Malabsorption: A Frequent Association. Nutrients, 11(12), 2973. https://doi.org/10.3390/nu11122973