Sourdough Fermented Breads are More Digestible than Those Started with Baker’s Yeast Alone: An In Vivo Challenge Dissecting Distinct Gastrointestinal Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Flour
2.2. Sourdough Preparation
2.3. Sourdoughs Characterization
2.4. Bread Making
2.5. Biochemical, Textural and Nnutritional Characterization of Breads
2.6. Volunteers Enrolment and Test Meal Administration
2.7. Sensory Analysis, Perception of Appetite and Satiety, and Gastrointestinal Symptoms
2.8. Gallbladder and Gastric Motility
2.9. Oro-Cecal Transit Time
2.10. Blood Analyses
2.11. Statistical Analysis
3. Results
3.1. Semi-Industrial Manufacture of Sourdough and Baker’s Yeast Breads
3.2. In Vitro Protein Digestibility
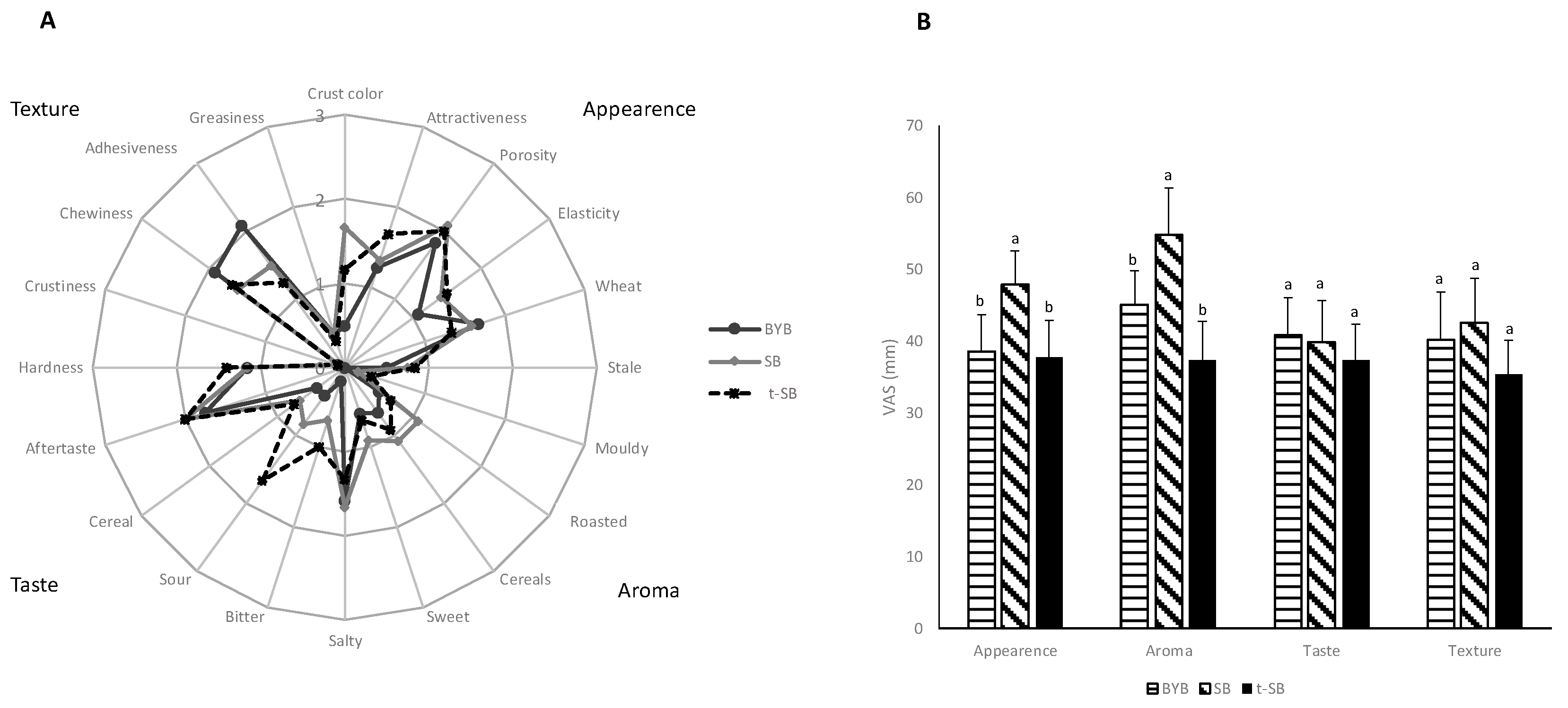
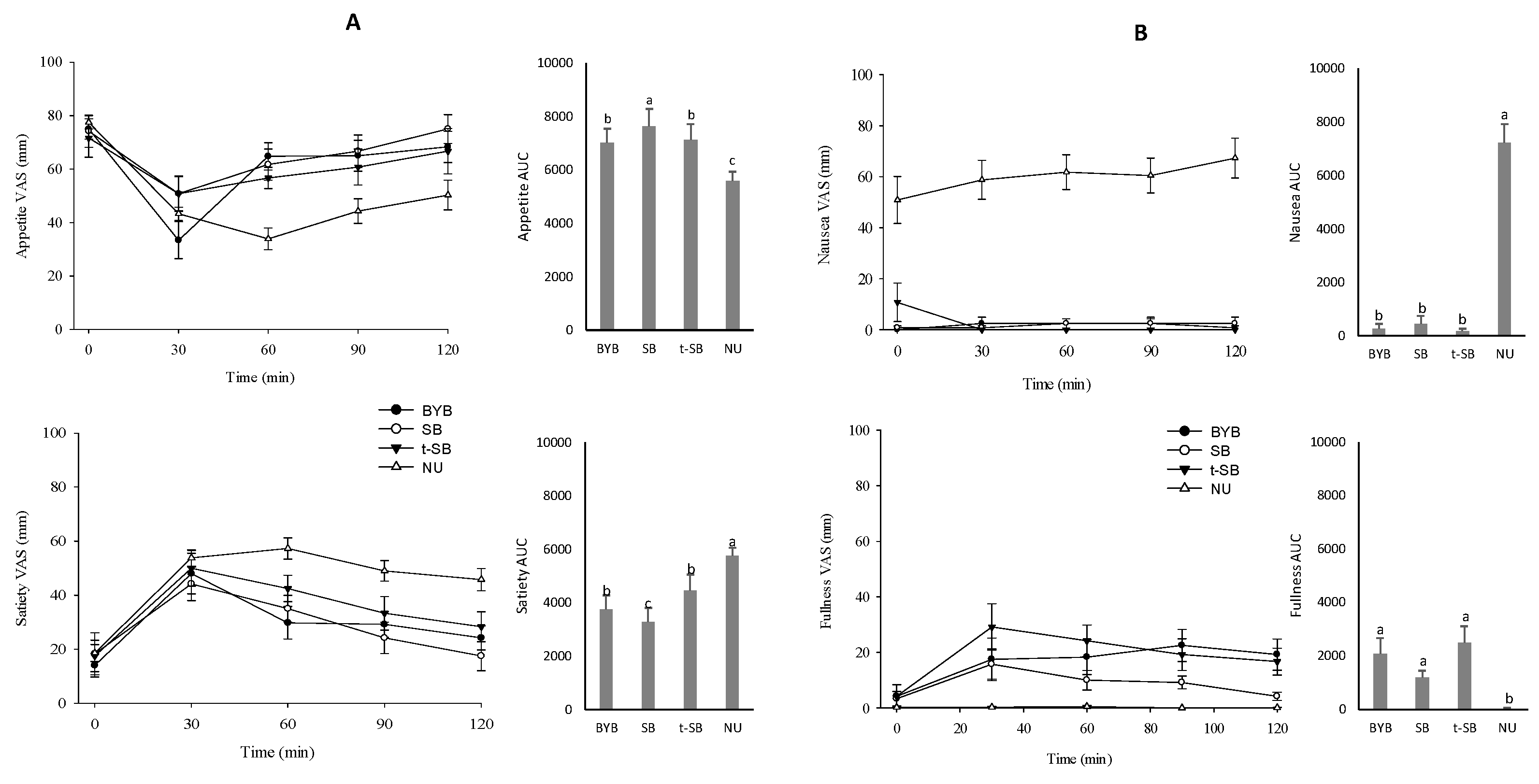
3.3. Breads Sensory Profile and Perception of Appetite, Satiety, and Gastrointestinal Symptoms
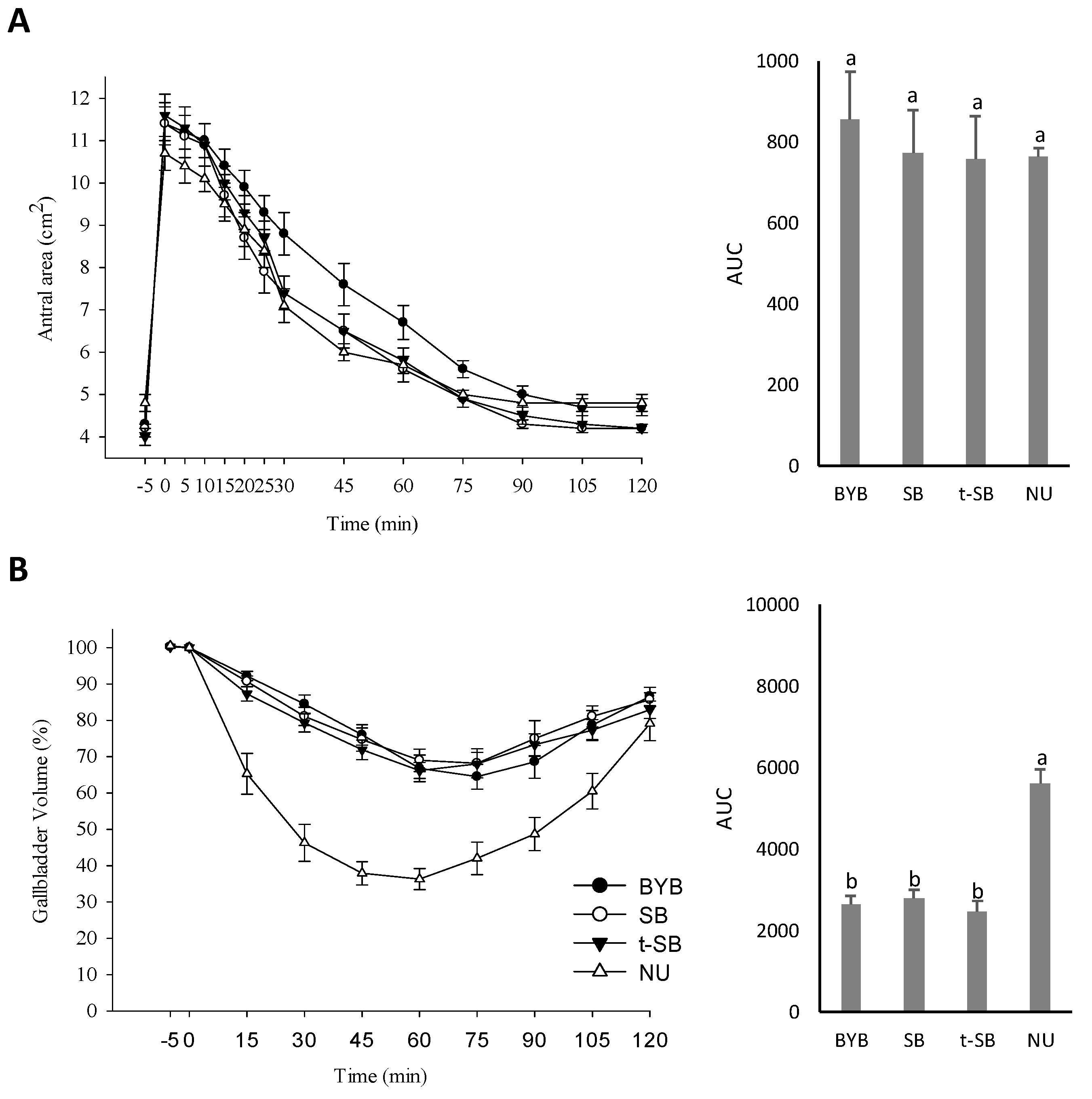
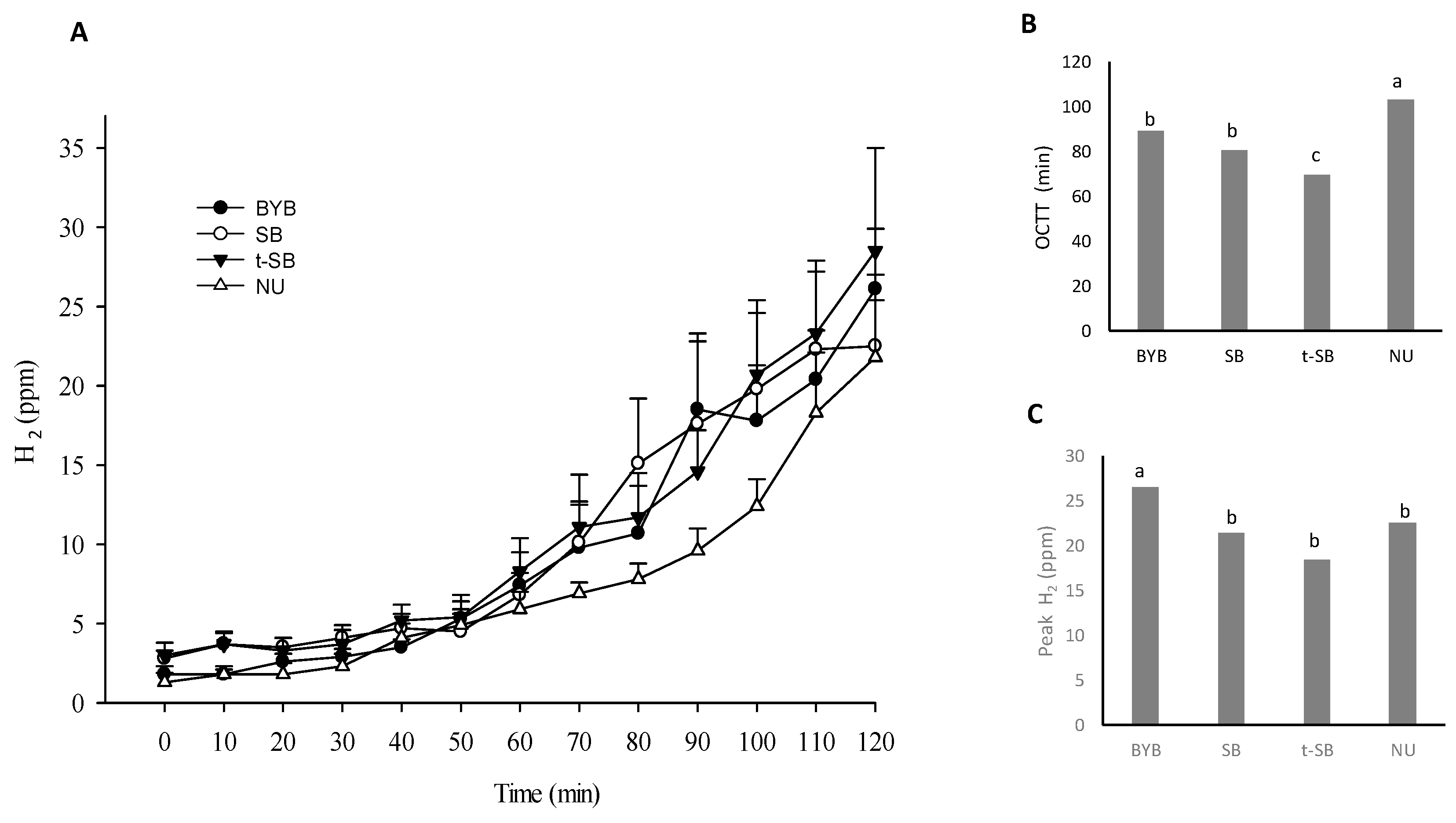
3.4. Gallbladder and Gastric Motility and Determination of the OCTT
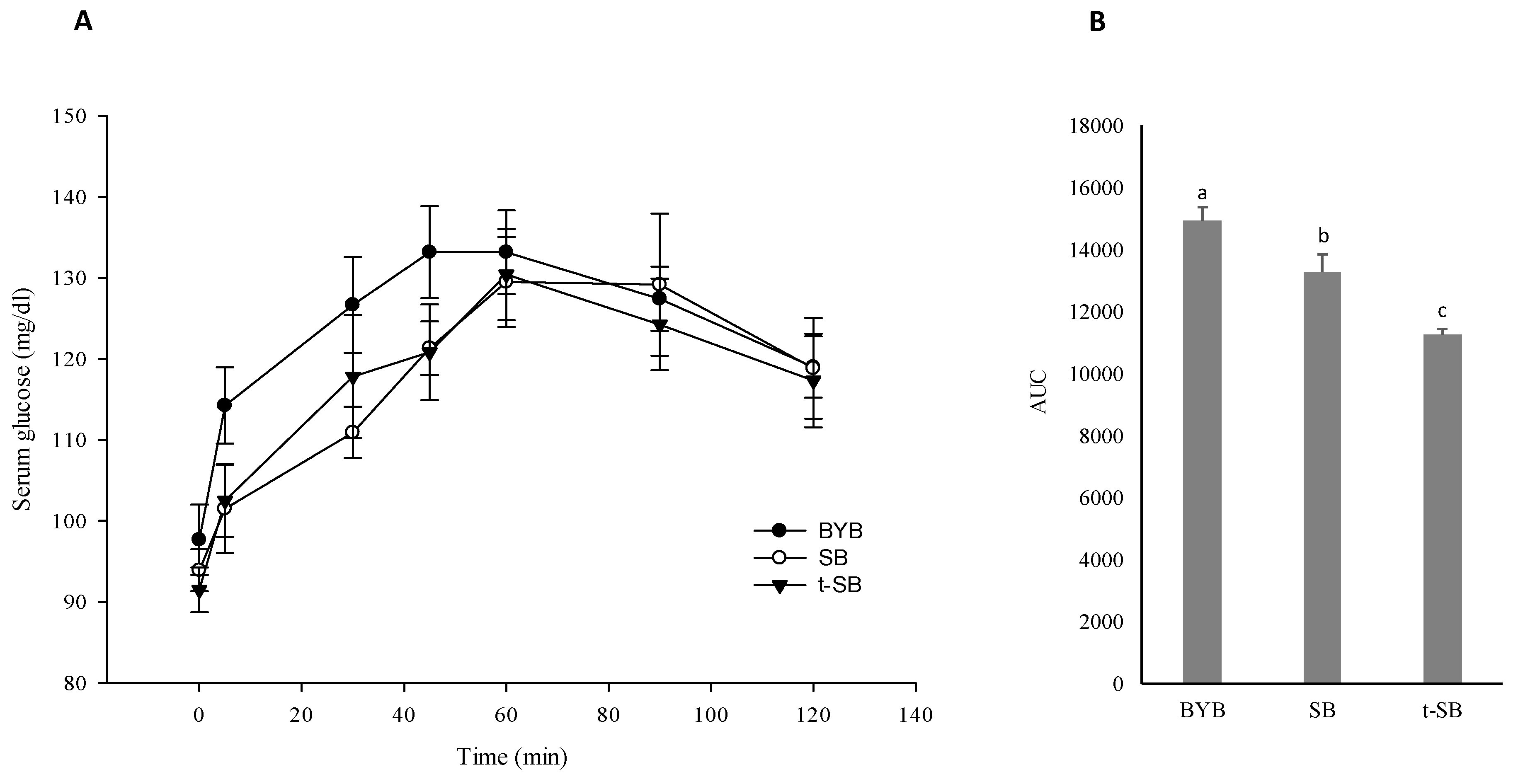
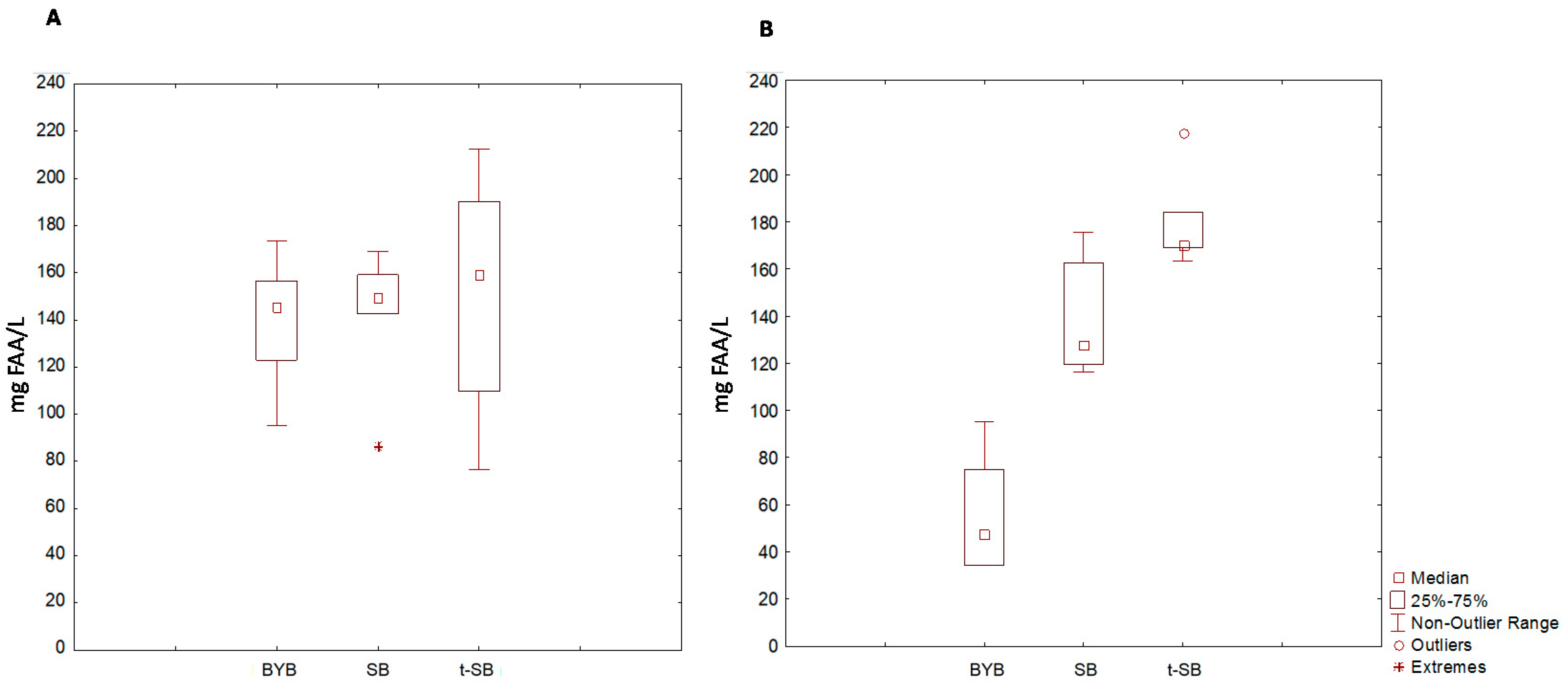
3.5. Postprandial Glycaemia and Free Amino Acids Absorption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- AIBI—Association Internationale de la Boulangerie Industrielle. Bread Market Report. Available online: http://www.aibi.eu/wp-content/uploads/draft-AIBI-Bread-Market-report-2013.pdf (accessed on 5 September 2019).
- Ercolini, D.; Pontonio, E.; De Filippis, F.; Minervini, F.; La Storia, A.; Gobbetti, M.; Di Cagno, R. Microbial ecology dynamics during rye and wheat sourdough preparation. Appl. Environ. Microbiol. 2013, 79, 7827–7836. [Google Scholar] [CrossRef] [PubMed]
- Hammes, W.P.; Gänzle, M.G. Sourdough Breads and Related Products. In Microbiology of Fermented Foods; Woods, B.J.B., Ed.; Blackie Academic/Professional: London, UK, 1998; pp. 199–216. [Google Scholar]
- De Vuyst, L.; Van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.-M.; Weckx, S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, M.A.; Vogel, R.F. Molecular taxonomy and genetics of sourdough lactic acid bacteria. Trends Food Sci. Technol. 2005, 16, 31–42. [Google Scholar] [CrossRef]
- Gobbetti, M. The sourdough microflora: Interactions of lactic acid bacteria and yeasts. Trends Food Sci. Technol. 1998, 9, 267–274. [Google Scholar] [CrossRef]
- De Vuyst, L.; Vrancken, G.; Ravyts, F.; Rimaux, T.; Weckx, S. Biodiversity, ecological determinants, and metabolic exploitation of sourdough microbiota. Food Microbiol. 2009, 26, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Montemurro, M.; Coda, R.; Rizzello, C.G. Recent advances in the use of sourdough biotechnology in pasta making. Foods 2019, 8, 129. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Hussain, R.; Kubow, S.; Sadiq, F.A.; Huang, W.; Imran, M. Sourdough bread: A contemporary cereal fermented product. J. Food Process. Preserv. 2019, 43, e13883. [Google Scholar] [CrossRef]
- Pontonio, E.; Di Cagno, R.; Mahony, J.; Lanera, A.; De Angelis, M.; van Sinderen, D.; Gobbetti, M. Sourdough authentication: Quantitative PCR to detect the lactic acid bacterial microbiota in breads. Sci. Rep. 2017, 7, 624. [Google Scholar] [CrossRef]
- Cappelle, S.; Guylaine, L.; Gänzle, M.; Gobbetti, M. History and Social Aspects of Sourdough. In Handbook on Sourdough Biotechnology; Gobbetti, M., Ganzle, G.M., Eds.; Springer: Berlin, Germany, 2013; pp. 105–154. [Google Scholar]
- Polese, B.; Nicolai, E.; Genovese, D.; Verlezza, V.; La Sala, C.N.; Aiello, M.; Inglese, M.; Incoronato, M.; Sarnelli, G.; De Rosa, T.; et al. Postprandial gastrointestinal function differs after acute administration of sourdough compared with brewer’s yeast bakery products in healthy adults. J. Nutr. 2018, 148, 202–208. [Google Scholar] [CrossRef]
- Camilleri, M. Functional dyspepsia and gastroparesis. Dig. Dis. 2016, 34, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, W.S.; Skidmore, P.M.; O’Brien, L.; Wilkinson, T.J.; Gearry, R.B. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: The evidence to date. Clin. Exp. Gastroenterol. 2016, 9, 131–142. [Google Scholar] [PubMed]
- De Angelis, M.; Rizzello, C.G.; Alfonsi, G.; Arnault, P.; Cappelle, S.; Di Cagno, R.; Gobbetti, M. Use of sourdough lactobacilli and oat fibre to decrease the glycaemic index of white wheat bread. Br. J. Nutr. 2007, 98, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Damiano, N.; Rizzello, C.G.; Cassone, A.; Di Cagno, R.; Gobbetti, M. Sourdough fermentation as a tool for the manufacture of low-glycemic index white wheat bread enriched in dietary fibre. Eur. Food Res. Technol. 2009, 229, 593–601. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Curiel, J.A.; Nionelli, L.; Vincentini, O.; Di Cagno, R.; Silano, M.; Gobbetti, M.; Coda, R. Use of fungal proteases and selected sourdough lactic acid bacteria for making wheat bread with an intermediate content of gluten. Food Microbiol. 2014, 37, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; De Angelis, M.; Gobbetti, M. Effect of sourdough fermentation on stabilisation, and chemical and nutritional characteristics of wheat germ. Food Chem. 2010, 119, 1079–1089. [Google Scholar] [CrossRef]
- Corsetti, A. Technology of Sourdough Fermentation and Sourdough Applications. In Handbook on Sourdough Biotechnology; Gobbetti, M., Gänzle, M., Eds.; Springer: Berlin, Germany, 2013; pp. 85–103. [Google Scholar]
- Minervini, F.; Lattanzi, A.; De Angelis, M.; Di Cagno, R.; Gobbetti, M. Influence of artisan bakery- or laboratory-propagated sourdoughs on the diversity of lactic acid bacterium and yeast microbiotas. Appl. Environ. Microbiol. 2012, 78, 5328–5340. [Google Scholar] [CrossRef]
- Minervini, F.; Pinto, D.; Di Cagno, R.; De Angelis, M.; Gobbetti, M. Scouting the application of sourdough to frozen dough bread technology. J. Cereal Sci. 2011, 54, 296–304. [Google Scholar] [CrossRef]
- Weiss, W.; Vogelmeier, C.; Gorg, A. Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers’asthma. Electrophoresis 1993, 14, 805–816. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Trani, A.; Gobbetti, M. Manufacture and characterization of functional emmer beverages fermented by selected lactic acid bacteria. Food Microbiol. 2011, 28, 526–536. [Google Scholar] [CrossRef]
- Portincasa, P.; Maggipinto, A.; Berardino, M.; Bonfrate, L.; Costin, S.; Todarello, O.; Palasciano, G.; Wang, D.Q.H.; Dimitrascu, D.L. Assessing gastrointestinal symptoms and perception, quality of life, motility, and autonomic neuropathy in clinical studies. J. Gastrointestin. Liver Dis. 2009, 18, 205–211. [Google Scholar] [PubMed]
- Bolondi, L.; Bortolotti, M.; Santi, V.; Calletti, T.; Gaiani, S.; Labo, G. Measurement of gastric emptying time by real-time ultrasonography. Gastroenterology 1985, 89, 752–759. [Google Scholar] [CrossRef]
- Maclean, W.; Harnly, J.; Chen, J.; Chevassus-Agnes, S.; Gilani, G.; Livesey, G.; Warwick, P. Food Energy—Methods of Analysis and Conversion Factors. In Food and Agriculture Organization of the United Nations Technical Workshop Report; FAO: Rome, Italy, 2003; Volume 77. [Google Scholar]
- Rizzello, C.G.; Coda, R.; Mazzacane, F.; Minervini, D.; Gobbetti, M. Micronized by-products from debranned durum wheat and sourdough fermentation enhanced the nutritional, textural and sensory features of bread. Food Res. Int. 2012, 46, 304–313. [Google Scholar] [CrossRef]
- Akeson, W.R.; Stahmann, M.A.A. pepsin pancreatin digest index of protein quality evaluation. J. Nutr. 1964, 83, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.; Latimer, G. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockville, MD, USA, 2006. [Google Scholar]
- Pinter-Szakács, M.; Molnan-Perl, I. Determination of tryptophan in unhydrolysed food and feed stuff by the acid ninhydrin method. J. Agric. Food Chem. 1990, 38, 720–726. [Google Scholar] [CrossRef]
- Millward, D.J. Amino acid scoring patterns for protein quality assessment. Br. J. Nutr. 2012, 108, 31–43. [Google Scholar] [CrossRef]
- Block, R.J.; Mitchel, H.H. The correlation of the amino acid composition of protein with their nutritive value. Nutr. Abstr. Rev. 1946, 16, 249–278. [Google Scholar]
- Oser, B.L. An Integrated Essential Amino Acid Index for Predicting the Biological Value of Proteins. In Protein and Amino Acid Acids in Nutrition; Albanese, A.A., Ed.; Academic Press: Cambridge, MA, USA, 1959; pp. 281–291. [Google Scholar]
- Ihekoronye, A.I.A. Rapid Enzymatic and Chromatographic Predictive Model for the In-Vivo Rat-Based Protein Efficiency Ratio. Ph.D. Thesis, University of Missouri, Columbia, MO, USA, 1981. [Google Scholar]
- Crisan, E.V.; Sands, A. Nutritional Value. In The Biology and Cultivation of Edible Mushrooms; Chang, S.T., Hayes, W.A., Eds.; Academic Press: Cambridge, MA, USA, 1978; pp. 137–165. [Google Scholar]
- Gasbarrini, A.; Corazza, G.R.; Gasbarrini, G.; Montalto, M.; Di, M.S.; Basilisco, G.; Parodi, A.; Usai-Satta, P.; Vernia, P.; Anania, C.; et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Aliment. Pharm. Ther. 2009, 29, 1–49. [Google Scholar]
- Muresan, C.; Surdea Blaga, T.; Muresan, L.; Dumitrascu, D.L. Abdominal ultrasound for the evaluation of gastric emptying revisited. J. Gastrointestin. Liver Dis. 2015, 24, 329–338. [Google Scholar]
- Civille, G.V.; Lapsley, K.; Huang, G.; Yada, S.; Seltsam, J. Development of an almond lexicon to assess the sensory properties of almond varieties. J. Sens. Stud. 2010, 25, 146–162. [Google Scholar] [CrossRef]
- Vitellio, P.; Celano, G.; Bonfrate, L.; Gobbetti, M.; Portincasa, P.; De Angelis, M. Effects of Bifidobacterium longum and Lactobacillus rhamnosus on Gut Microbiota in Patients with Lactose Intolerance and Persisting Functional Gastrointestinal Symptoms: A Randomised, Double-Blind, Cross-Over Study. Nutrients 2019, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Wang, D.Q.; Portincasa, P. Gallbladder and gastric motility in obese newborns, pre-adolescents and adults. J. Gastroenterol. Hepatol. 2012, 27, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Grattagliano, I.; Portincasa, P. Chronic alcoholics retain dyspeptic symptoms, pan-enteric dysmotility, and autonomic neuropathy before and after abstinence. J. Dig. Dis. 2016, 17, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Covelli, M.; Berardino, M.; Berardino, M.; Wang, D.Q.H.; Lapadula, G.; Palasciano, G.; Portincasa, P. Gastrointestinal symptoms and motility disorders in patients with systemic scleroderma. BMC Gastroenterol. 2008, 8, 7. [Google Scholar] [CrossRef]
- Portincasa, P.; Di Ciaula, A.; Baldassarre, G.; Palmieri, V.; Gentile, A.; Cimmino, A.; Palasciano, G. Gallbladder motor function in gallstone patients: Sonographic and in vitro studies on the role of gallstones, smooth muscle function and gallbladder wall inflammation. J. Hepatol. 1994, 21, 430–440. [Google Scholar] [CrossRef]
- Portincasa, P.; Di Ciaula, A.; Palmieri, V.; Van Berge-Henegouwen, G.P.; Palasciano, G. Effects of cholestyramine on gallbladder and gastric emptying in obese and lean subjects. Eur. J. Clin. Investig. 1995, 25, 746–753. [Google Scholar] [CrossRef]
- Portincasa, P.; Moschetta, A.; Berardino, M.; Di Ciaula, A.; Vacca, M.; Baldassarre, G.; Pietrapertosa, A.; Cammarota, R.; Tannoia, N.; Palasciano, G. Impaired gallbladder motility and delayed orocecal transit contribute to pigment gallstone and biliary sludge formation in beta-thalassemia major adults. World J. Gastroenterol. 2004, 10, 2383–2390. [Google Scholar] [CrossRef]
- Diella, G.; Di Ciaula, A.; Lorusso, M.P.; Summo, C.; Caggiano, G.; Caponio, F.; Montagna, M.T.; Portincasa, P. Distinct effects of two almond cultivars on agreeability and gastrointestinal motility in healthy subjects: More than mere nutraceuticals. J. Gastrointest. Liver Dis. 2018, 27, 31–39. [Google Scholar]
- Altomare, D.F.; Portincasa, P.; Rinaldi, M.; Di Ciaula, A.; Martinelli, E.; Amoruso, A.; Palasciano, G.; Memeo, V. Slow-transit constipation. Dis. Colon Rectum 1999, 42, 231–240. [Google Scholar] [CrossRef]
- Bonfrate, L.; Krawczyk, M.; Lembo, A.; Grattagliano, I.; Lammert, F.; Portincasa, P. Effects of dietary education, followed by a tailored fructose-restricted diet in adults with fructose malabsorption. Eur. J. Gastroenterol. Hepatol. 2015, 27, 785–796. [Google Scholar] [CrossRef]
- Portincasa, P.; Di Ciaula, A.; Vacca, M.; Montelli, R.; Wang, D.H.; Palasciano, G. Beneficial effects of oral tilactase on patients with hypolactasia. Eur. J. Clin. Investig. 2008, 38, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Farup, J.; Rahbek, S.K.; Storm, A.C.; Klitgaard, S.; Jørgensen, H.; Bibby, B.M.; Serena, A.; Vissing, K. Effect of degree of hydrolysis of whey protein on in vivo plasma amino acid appearance in humans. SpringerPlus 2016, 5, 382. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 2018, 302, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2018, 302, 103–113. [Google Scholar] [CrossRef] [PubMed]
- McCrory, M.A.; Saltzman, E.; Rolls, B.J.; Roberts, S.B. A twin study of the effects of energy density and palatability on energy intake of individual foods. Physiol. Behav. 2006, 87, 451–459. [Google Scholar] [CrossRef]
- Stubbs, R.J.; Hughes, D.A.; Johnstone, A.M.; Rowley, E.; Reid, C.; Elia, M.; Stratton, R.; Delargy, H.; King, N.; Blundell, J.E. The use of visual analogue scales to assess motivation to eat in human subjects: A review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br. J. Nutr. 2000, 84, 405–415. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Nordlund, E.; Mattila, I.; Katina, K.; Aura, A.M.; Kolehmainen, M.; Oresic, M.; Mykkanen, H.; Poutanen, K. Postprandial differences in the plasma metabolome of healthy Finnish subjects after intake of a sourdough fermented endosperm rye bread versus white wheat bread. Nutr. J. 2011, 10, 116. [Google Scholar] [CrossRef]
- Najjar, A.M.; Parsons, P.M.; Duncan, A.M.; Robinson, L.E.; Yada, R.Y.; Graham, T.E. The acute impact of ingestion of breads of varying composition on blood glucose, insulin and incretins following first and second meals. Br. J. Nutr. 2008, 101, 391–398. [Google Scholar] [CrossRef]
- Liljeberg, H.G.; Björck, I.M. Delayed gastric emptying rate as a potential mechanism for lowered glycemia after eating sourdough bread: Studies in humans and rats using test products with added organic acids or an organic salt. Am. J. Clin. Nutr. 1996, 64, 886–893. [Google Scholar] [CrossRef]
- Halawi, H.; Camilleri, M.; Acosta, A.; Vazquez-Roque, M.; Oduyebo, I.; Burton, D.; Busciglio, I.; Zinsmeister, A.R. Relationship of gastric emptying or accommodation with satiation, satiety, and postprandial symptoms in health. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, 442–447. [Google Scholar] [CrossRef]
- Groenen, M.; Fyfe, C.L.; Holtrop, G.; Horgan, G.W.; Meek, C.L.; Gribble, F.; Morgan, P.; Johnstone, A.M. Investigation of mechanisms of protein induced satiety: Meal structure and protein content effects on gastric emptying and gut hormone release. Proc. Nutr. Soc. 2017, 76, E30. [Google Scholar] [CrossRef]
- Sobrino Crespo, C.; Perianes Cachero, A.; Puebla Jiménez, L.; Barrios, V.; Arilla Ferreiro, E. Peptides and food intake. Front. Endocrinol. 2014, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Camboni, G.; Basilisco, G.; Bozzani, A.; Bianchi, P.A. Repeatability of lactulose hydrogen breath test in subjects with normal or prolonged orocecal transit. Dig. Dis. Sci. 1988, 33, 1525–1527. [Google Scholar] [CrossRef] [PubMed]
- Costabile, A.; Santarelli, S.; Claus, S.P.; Sanderson, J.; Hudspith, B.N.; Brostoff, J.; Ward, J.L.; Lovegrove, A.; Shewry, P.R.; Jones, H.E.; et al. Effect of breadmaking process on in vitro gut microbiota parameters in irritable bowel syndrome. PLoS ONE 2014, 9, e111225. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Leenhardt, F.; Lioger, D.; Scalbert, A.; Rémésy, C. Parameters controlling the glycaemic response to breads. Nutr. Res. Rev. 2006, 19, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Liljeberg, H.; Lönner, C.; Björck, I. Sourdough fermentation or addition of organic acids or corresponding salts to bread improves nutritional properties of starch in healthy humans. J. Nutr. 1995, 125, 1503–1511. [Google Scholar]
- Östman, E. Fermentation as a Means of Optimizing the Glycaemic Index and Food Mechanisms and Metabolic Merits with Emphasis on Lactic Acid in Cereal Products. Ph.D. Thesis, Lund University, Lund, Sweden, 2003. [Google Scholar]
- Jung, E.H.; Ran Kim, S.; Hwang, I.K.; Youl, H.T. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J. Agric. Food Chem. 2007, 55, 9800–9804. [Google Scholar] [CrossRef]
| n | 36 |
|---|---|
| Males:Females | 18:18 |
| Age years (range) | 25 ± 1.1 (20–31) |
| BMI, kg/m2 (range) | 22.4 ± 0.9 (18.6–29.4) |
| Fullness (severity) * | 0.8 ± 0.2 |
| Fullness (frequency) ** | 0.7 ± 0.2 |
| Fullness (duration) *** | 0.7 ± 0.2 |
| Epigastric pain (severity) | 0.9 ± 0.4 |
| Epigastric pain (frequency) | 0.6 ± 0.3 |
| Epigastric pain (duration) | 0.6 ± 0.3 |
| Nausea/vomiting (severity) | 0.2 ± 0.2 |
| Nausea/vomiting (frequency) | 0.2 ± 0.1 |
| Nausea/vomiting (duration) | 0.6 ± 0.3 |
| Heartburn (severity) | 0.5 ± 0.2 |
| Heartburn (frequency) | 0.5 ± 0.2 |
| Heartburn (duration) | 0.4 ± 0.2 |
| S | S4 | S24 | |
|---|---|---|---|
| pH | 3.89 ± 0.02 b | 4.26 ± 0.01 a | 3.55 ± 0.01 c |
| TTA Total titratable acidity (mL NaOH 0.1M/10g) | 6.60 ± 0.11 b | 3.20 ± 0.09 c | 9.00 ± 0.15 a |
| LAB (Log cfu/g) | 9.53 ± 0.13 b | 9.18 ± 0.10 b | 9.75 ± 0.08 a |
| Yeasts (Log cfu/g) | 7.33 ± 0.05 b | 7.07 ± 0.07 c | 7.50 ± 0.05 a |
| Lactic acid (mmol/kg) | 43.3 ± 0.3 b | 28.2 ± 0.2 c | 93.3 ± 0.3 a |
| Acetic acid (mmol/kg) | 10.2 ± 0.2 b | 6.2 ± 0.2 c | 20.3 ± 0.2 a |
| Fermentation Quotient (FQ) | 4.3 ± 0.2 | 4.5 ± 0.2 | 4.6 ± 0.2 |
| Total Free Amino Acids (g/kg) | 0.722 ± 0.018 c | 1.380 ± 0.024 b | 7.174 ± 0.032 a |
| BYB | SB | t-SB | |
|---|---|---|---|
| Dough (before baking) | |||
| pH | 5.6 ± 0.1 a | 4.9 ± 0.1 b | 4.4 ± 0.1 c |
| TTA (mL NaOH 0.1m/10g) | 3.0 ± 0.1 c | 5.0 ± 0.2 b | 6.9 ± 0.2 a |
| Lactic acid (mmol/kg) | 1.7 ± 0.1 c | 7.2 ± 0.1 b | 26.4 ± 0.3 a |
| Acetic acid (mmol/kg) | nd | 1.4 ± 0.1 b | 5.8 ± 0.1 a |
| FQ | nd | 4.2 ± 0.1 b | 4.5 ± 0.2 a |
| Total FAA (g/kg) | 0.70 ± 0.02 c | 1.33 ± 0.02 b | 1.71 ± 0.02 a |
| Bread | |||
| Volume increase (%) | 231 ± 5 b | 245 ± 7 a | 223 ± 4 c |
| Specific volume (cm2/g) | 3.3 ± 0.1 b | 3.6 ± 0.1 a | 2.9 ± 0.1 c |
| Textural parameters | |||
| Hardness (g) | 3210 ± 10 b | 3150 ± 21 c | 3472 ± 11 a |
| Resilience | 0.85 ± 0.02 a | 0.81 ± 0.05 a | 0.72 ± 0.02 b |
| Fracturability (g) | 3075 ± 5 a | 2956 ± 10 b | 2282 ± 10 c |
| Image analysis | |||
| Black pixel area (%) | 44.0 ± 1.8 b | 52.6 ± 2.2 a | 44.4 ± 1.5 b |
| Color analysis | |||
| L | 60.2 ± 1.6 a | 60.4 ± 0.7 a | 54.7 ± 0.8 b |
| A | 10.4 ± 0.8 b | 9.3 ± 0.6 b | 11.7 ± 0.2 a |
| B | 35.7 ± 1.0 a | 34.1 ± 0.2 a | 33.6 ± 0.5 b |
| ΔE | 48.1 ± 1.7 b | 46.6 ± 0.6 b | 51.3 ± 0.6 a |
| Nutritional indexes | |||
| In vitro protein digestibility (IVPD, %) | 63.7 ± 1.2 c | 71.6 ± 1.1 b | 79.8 ± 1.4 a |
| Limiting Amino Acids | Lysine Methionine Tryptophan | Lysine Methionine Tryptophan | Lysine Methionine Tryptophan |
| Protein score (%) | 18.2 ± 0.2 c | 24.2 ± 0.2 b | 59.2 ± 0.5 a |
| Essential amino acids Index (EAAI) | 43.2 ± 0.4 c | 56.3 ± 0.5 b | 72.3 ± 0.5 a |
| Biological value (BV) | 35.4 ± 0.3 c | 39.7 ± 0.3 b | 54.1 ± 0.4 a |
| Protein Efficiency Ratio (PER) | 21.1 ± 0.2 c | 23.5 ± 0.2 b | 53.5 ± 0.3 a |
| Nutritional Index (NI) | 2.8 ± 0.1 c | 3.4 ± 0.1 b | 5.5 ± 0.1 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzello, C.G.; Portincasa, P.; Montemurro, M.; Di Palo, D.M.; Lorusso, M.P.; De Angelis, M.; Bonfrate, L.; Genot, B.; Gobbetti, M. Sourdough Fermented Breads are More Digestible than Those Started with Baker’s Yeast Alone: An In Vivo Challenge Dissecting Distinct Gastrointestinal Responses. Nutrients 2019, 11, 2954. https://doi.org/10.3390/nu11122954
Rizzello CG, Portincasa P, Montemurro M, Di Palo DM, Lorusso MP, De Angelis M, Bonfrate L, Genot B, Gobbetti M. Sourdough Fermented Breads are More Digestible than Those Started with Baker’s Yeast Alone: An In Vivo Challenge Dissecting Distinct Gastrointestinal Responses. Nutrients. 2019; 11(12):2954. https://doi.org/10.3390/nu11122954
Chicago/Turabian StyleRizzello, Carlo Giuseppe, Piero Portincasa, Marco Montemurro, Domenica Maria Di Palo, Michele Pio Lorusso, Maria De Angelis, Leonilde Bonfrate, Bernard Genot, and Marco Gobbetti. 2019. "Sourdough Fermented Breads are More Digestible than Those Started with Baker’s Yeast Alone: An In Vivo Challenge Dissecting Distinct Gastrointestinal Responses" Nutrients 11, no. 12: 2954. https://doi.org/10.3390/nu11122954
APA StyleRizzello, C. G., Portincasa, P., Montemurro, M., Di Palo, D. M., Lorusso, M. P., De Angelis, M., Bonfrate, L., Genot, B., & Gobbetti, M. (2019). Sourdough Fermented Breads are More Digestible than Those Started with Baker’s Yeast Alone: An In Vivo Challenge Dissecting Distinct Gastrointestinal Responses. Nutrients, 11(12), 2954. https://doi.org/10.3390/nu11122954






