The Role of Vitamin D and Omega-3 PUFAs in Islet Transplantation
Abstract
:1. Introduction
2. Vitamin D
3. Omega-3 PUFAs
4. Syngeneic Islet Transplantation
5. Allogeneic Islet Transplantation
6. Role of Vitamin D in Clinical Solid Organ Transplantation
7. Discussion
- prevent IBMIR and promote islet engraftment in the immediate post-transplant period
- prevent acute allograft rejection
- prevent or delay recurrence of autoimmunity by restoring immune tolerance
- promote long-term islet graft survival and function
- modulate the reduction in the administered dose of immunosuppressive drugs and associated adverse events (e.g., opportunistic infections, bone loss and beta-cell toxicity)
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hering, B.J.; Clarke, W.R.; Bridges, N.D.; Eggerman, T.L.; Alejandro, R.; Bellin, M.D.; Chaloner, K.; Czarniecki, C.W.; Goldstein, J.S.; Hunsicker, L.G.; et al. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care 2016, 39, 1230–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickels, M.R.; Stock, P.G.; de Koning, E.J.P.; Piemonti, L.; Pratschke, J.; Alejandro, R.; Bellin, M.D.; Berney, T.; Choudhary, H.; Johnson, P.R.; et al. Defining outcomes for β-cell replacement therapy in the treatment of diabetes: a consensus report on the Igls criteria from the IPITA/EPITA opinion leaders workshop. Transpl. Int. 2018, 31, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lablanche, S.; Vantyghem, M.C.; Kessler, L.; Wojtusciszyn, A.; Borot, S.; Thivolet, C.; Girerd, S.; Bosco, D.; Bosson, J.L.; Colin, C.; et al. Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 527–537. [Google Scholar] [CrossRef]
- Poggioli, R.; Faradji, R.N.; Ponte, G.; Betancourt, A.; Messinger, S.; Baidal, D.A.; Froud, T.; Ricordi, C.; Alejandro, R. Quality of life after islet transplantation. Am. J. Transplant. 2006, 6, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Rickels, M.R.; Robertson, R.P. Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions. Endocr. Rev. 2019, 40, 631–668. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef]
- Collaborative Islet Transplant Registry Tenth Annual Report. 2017. Available online: https://citregistry.Org/system/files/10th_ar.Pdf. (accessed on 26 October 2019).
- Ryan, E.A.; Paty, B.W.; Senior, P.A.; Bigam, D.; Alfadhli, E.; Kneteman, N.M.; Lakey, J.R.; Shapiro, A.M. Five-year follow-up after clinical islet transplantation. Diabetes 2005, 54, 2060–2069. [Google Scholar] [CrossRef] [Green Version]
- Merani, S.; Toso, C.; Emamaullee, J.; Shapiro, A.M. Optimal implantation site for pancreatic islet transplantation. Br. J. Surg. 2008, 95, 1449–1461. [Google Scholar] [CrossRef]
- Cantarelli, E.; Piemonti, L. Alternative transplantation sites for pancreatic islet grafts. Curr. Diab. Rep. 2011, 11, 364–374. [Google Scholar] [CrossRef]
- Bennet, W.; Groth, C.G.; Larsson, R.; Nilsson, B.; Korsgren, O. Isolated human islets trigger an instant blood mediated inflammatory reaction: Implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups. J. Med. Sci. 2000, 105, 125–133. [Google Scholar] [CrossRef]
- Kanak, M.A.; Saravanan, P.B.; Levy, M.F. Inflammatory response and its impact on outcome of islet transplantation. CellR4 2019, 7, e2739. [Google Scholar]
- Yin, D.; Ding, J.W.; Shen, J.; Ma, L.; Hara, M.; Chong, A.S. Liver ischemia contributes to early islet failure following intraportal transplantation: Benefits of liver ischemic-preconditioning. Am. J. Transplant. 2006, 6, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Bottino, R.; Fernandez, L.A.; Ricordi, C.; Lehmann, R.; Tsan, M.F.; Oliver, R.; Inverardi, L. Transplantation of allogeneic islets of langerhans in the rat liver: Effects of macrophage depletion on graft survival and microenvironment activation. Diabetes 1998, 47, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Barshes, N.R.; Wyllie, S.; Goss, J.A. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: Implications for intrahepatic grafts. J. Leukoc. Biol. 2005, 77, 587–597. [Google Scholar] [CrossRef]
- Sakata, N.; Hayes, P.; Tan, A.; Chan, N.K.; Mace, J.; Peverini, R.; Sowers, L.; Pearce, W.J.; Chinnock, R.; Obenaus, A.; et al. Mri assessment of ischemic liver after intraportal islet transplantation. Transplantation 2009, 87, 825–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, A.M.; Gallant, H.L.; Hao, E.G.; Lakey, J.R.; McCready, T.; Rajotte, R.V.; Yatscoff, R.W.; Kneteman, N.M. The portal immunosuppressive storm: Relevance to islet transplantation? Ther. Drug Monit. 2005, 27, 35–37. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Calanchini, M.; Mammi, C.; Fabbri, A. Vitamin d: Not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eat. Weight Disord. 2017, 22, 27–41. [Google Scholar] [CrossRef]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin d in autoimmunity: Molecular mechanisms and therapeutic potential. Front. Immunol. 2016, 7, 697. [Google Scholar] [CrossRef] [Green Version]
- Webb, A.R.; Pilbeam, C.; Hanafin, N.; Holick, M.F. An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin d in an elderly nursing home population in boston. Am. J. Clin. Nutr. 1990, 51, 1075–1081. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin d deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Schmid, A.; Walther, B. Natural vitamin d content in animal products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Keast, D.R.; Fulgoni, V.L.; Nicklas, T.A.; O’Neil, C.E. Food sources of energy and nutrients among children in the united states: National health and nutrition examination survey 2003–2006. Nutrients 2013, 5, 283–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neil, C.E.; Keast, D.R.; Fulgoni, V.L.; Nicklas, T.A. Food sources of energy and nutrients among adults in the US: Nhanes 2003–2006. Nutrients 2012, 4, 2097–2120. [Google Scholar]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin d: Metabolism, molecular mechanism of action and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- White, J.H. Vitamin d metabolism and signaling in the immune system. Rev. Endocr. Metab. Disord. 2012, 13, 21–29. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin d and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Overbergh, L.; Decallonne, B.; Valckx, D.; Verstuyf, A.; Depovere, J.; Laureys, J.; Rutgeerts, O.; Saint-Arnaud, R.; Bouillon, R.; Mathieu, C. Identification and immune regulation of 25-hydroxyvitamin d-1-alpha-hydroxylase in murine macrophages. Clin. Exp. Immunol. 2000, 120, 139–146. [Google Scholar] [CrossRef]
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin-d3-1alpha-hydroxylase in human monocytes. J. Bone Miner. Res. 2006, 21, 37–47. [Google Scholar] [CrossRef]
- Overbergh, L.; Stoffels, K.; Waer, M.; Verstuyf, A.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin d-1alpha-hydroxylase in human monocytic thp1 cells: Mechanisms of interferon-gamma-mediated induction. J. Clin. Endocrinol. Metab. 2006, 91, 3566–3574. [Google Scholar] [CrossRef] [Green Version]
- Kapetanovic, R.; Fairbairn, L.; Beraldi, D.; Sester, D.P.; Archibald, A.L.; Tuggle, C.K.; Hume, D.A. Pig bone marrow-derived macrophages resemble human macrophages in their response to bacterial lipopolysaccharide. J. Immunol. 2012, 188, 3382–3394. [Google Scholar] [CrossRef]
- Amado Diago, C.A.; García-Unzueta, M.T.; Fariñas, M.e.C.; Amado, J.A. Calcitriol-modulated human antibiotics: New pathophysiological aspects of vitamin d. Endocrinol. Nutr. 2016, 63, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin d inhibits monocyte/macrophage proinflammatory cytokine production by targeting mapk phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korf, H.; Wenes, M.; Stijlemans, B.; Takiishi, T.; Robert, S.; Miani, M.; Eizirik, D.L.; Gysemans, C.; Mathieu, C. 1,25-dihydroxyvitamin d3 curtails the inflammatory and t cell stimulatory capacity of macrophages through an il-10-dependent mechanism. Immunobiology 2012, 217, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, M.; Guo, Y.; Song, Z.; Liu, B. 1,25-dihydroxyvitamin d3 promotes high glucose-induced m1 macrophage switching to m2 via the vdr-pparγ signaling pathway. Biomed. Res. Int. 2015, 2015, 157834. [Google Scholar] [PubMed] [Green Version]
- Piemonti, L.; Monti, P.; Sironi, M.; Fraticelli, P.; Leone, B.E.; Dal Cin, E.; Allavena, P.; Di Carlo, V. Vitamin d3 affects differentiation, maturation and function of human monocyte-derived dendritic cells. J. Immunol. 2000, 164, 4443–4451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penna, G.; Adorini, L. 1 alpha,25-dihydroxyvitamin d3 inhibits differentiation, maturation, activation and survival of dendritic cells leading to impaired alloreactive t cell activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef] [Green Version]
- Gauzzi, M.C.; Purificato, C.; Donato, K.; Jin, Y.; Wang, L.; Daniel, K.C.; Maghazachi, A.A.; Belardelli, F.; Adorini, L.; Gessani, S. Suppressive effect of 1alpha,25-dihydroxyvitamin d3 on type i ifn-mediated monocyte differentiation into dendritic cells: Impairment of functional activities and chemotaxis. J. Immunol. 2005, 174, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, G.B.; van Etten, E.; Verstuyf, A.; Waer, M.; Overbergh, L.; Gysemans, C.; Mathieu, C. 1,25-dihydroxyvitamin d3 alters murine dendritic cell behaviour in vitro and in vivo. Diabetes Metab. Res. Rev. 2011, 27, 933–941. [Google Scholar] [CrossRef]
- Saul, L.; Mair, I.; Ivens, A.; Brown, P.; Samuel, K.; Campbell, J.D.M.; Soong, D.Y.; Kamenjarin, N.; Mellanby, R.J. 1,25-dihydroxyvitamin d3 restrains CD4+ T cell priming ability of CD11c+ dendritic cells by upregulating expression of CD31. Front. Immunol. 2019, 10, 600. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-dihydroxyvitamin d3 and il-2 combine to inhibit t cell production of inflammatory cytokines and promote development of regulatory t cells expressing ctla-4 and foxp3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef] [Green Version]
- Overbergh, L.; Decallonne, B.; Waer, M.; Rutgeerts, O.; Valckx, D.; Casteels, K.M.; Laureys, J.; Bouillon, R.; Mathieu, C. 1alpha,25-dihydroxyvitamin d3 induces an autoantigen-specific t-helper 1/t-helper 2 immune shift in nod mice immunized with gad65 (p524-543). Diabetes 2000, 49, 1301–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.; O’Garra, A. 1alpha,25-dihydroxyvitamin d3 has a direct effect on naive cd4(+) t cells to enhance the development of th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouillon, R.; Lieben, L.; Mathieu, C.; Verstuyf, A.; Carmeliet, G. Vitamin d action: Lessons from vdr and cyp27b1 null mice. Pediatric Endocrinol. Rev. 2013, 10, 354–366. [Google Scholar]
- Mathieu, C.; Waer, M.; Laureys, J.; Rutgeerts, O.; Bouillon, R. Prevention of autoimmune diabetes in nod mice by 1,25 dihydroxyvitamin d3. Diabetologia 1994, 37, 552–558. [Google Scholar] [CrossRef]
- Mathieu, C.; Waer, M.; Casteels, K.; Laureys, J.; Bouillon, R. Prevention of type i diabetes in nod mice by nonhypercalcemic doses of a new structural analog of 1,25-dihydroxyvitamin d3, kh1060. Endocrinology 1995, 136, 866–872. [Google Scholar] [CrossRef]
- Mathieu, C.; Laureys, J.; Sobis, H.; Vandeputte, M.; Waer, M.; Bouillon, R. 1,25-dihydroxyvitamin d3 prevents insulitis in nod mice. Diabetes 1992, 41, 1491–1495. [Google Scholar] [CrossRef]
- Casteels, K.M.; Mathieu, C.; Waer, M.; Valckx, D.; Overbergh, L.; Laureys, J.M.; Bouillon, R. Prevention of type i diabetes in nonobese diabetic mice by late intervention with nonhypercalcemic analogs of 1,25-dihydroxyvitamin d3 in combination with a short induction course of cyclosporin a. Endocrinology 1998, 139, 95–102. [Google Scholar] [CrossRef]
- Gregori, S.; Giarratana, N.; Smiroldo, S.; Uskokovic, M.; Adorini, L. A 1alpha,25-dihydroxyvitamin d(3) analog enhances regulatory t-cells and arrests autoimmune diabetes in nod mice. Diabetes 2002, 51, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Infante, M.; Ricordi, C.; Sanchez, J.; Clare-Salzler, M.J.; Padilla, N.; Fuenmayor, V.; Chavez, C.; Alvarez, A.; Baidal, D.; Alejandro, R.; et al. Influence of vitamin d on islet autoimmunity and beta-cell function in type 1 diabetes. Nutrients 2019, 11, 2185. [Google Scholar] [CrossRef] [Green Version]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Fredman, G.; Bäckhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endres, S.; Ghorbani, R.; Kelley, V.E.; Georgilis, K.; Lonnemann, G.; van der Meer, J.W.; Cannon, J.G.; Rogers, T.S.; Klempner, M.S.; Weber, P.C. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Engl. J. Med. 1989, 320, 265–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razavi, M.; Jamilian, M.; Samimi, M.; Afshar Ebrahimi, F.; Taghizadeh, M.; Bekhradi, R.; Seyed Hosseini, E.; Haddad Kashani, H.; Karamali, M.; Asemi, Z. The effects of vitamin d and omega-3 fatty acids co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutr. Metab. 2017, 14, 80. [Google Scholar] [CrossRef] [Green Version]
- Jamilian, M.; Samimi, M.; Mirhosseini, N.; Afshar Ebrahimi, F.; Aghadavod, E.; Talaee, R.; Jafarnejad, S.; Hashemi Dizaji, S.; Asemi, Z. The influences of vitamin d and omega-3 co-supplementation on clinical, metabolic and genetic parameters in women with polycystic ovary syndrome. J. Affect. Disord. 2018, 238, 32–38. [Google Scholar] [CrossRef]
- Kagohashi, Y.; Otani, H. Diet with a low n-6/n-3 essential fatty acid ratio when started immediately after the onset of overt diabetes prolongs survival of type 1 diabetes model nod mice. Congenit. Anom. 2010, 50, 226–231. [Google Scholar] [CrossRef]
- Bi, X.; Li, F.; Liu, S.; Jin, Y.; Zhang, X.; Yang, T.; Dai, Y.; Li, X.; Zhao, A.Z. Ω-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J. Clin. Investig. 2017, 127, 1757–1771. [Google Scholar] [CrossRef] [Green Version]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A novel anti-inflammatory role of omega-3 pufas in prevention and treatment of atherosclerosis and vascular cognitive impairment and dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef] [Green Version]
- Sears, B. Anti-inflammatory diets. J. Am. Coll. Nutr. 2015, 34, 14–21. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Tutino, V.; De Nunzio, V.; Caruso, M.G.; Veronese, N.; Lorusso, D.; Di Masi, M.; Benedetto, M.L.; Notarnicola, M. Elevated aa/epa ratio represents an inflammatory biomarker in tumor tissue of metastatic colorectal cancer patients. Int. J. Mol. Sci. 2019, 20, 2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (jelis): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Yokoyama, M.; Saito, Y.; Origasa, H.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; Kita, T.; et al. Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ. J. 2009, 73, 1283–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germano, M.; Meleleo, D.; Montorfano, G.; Adorni, L.; Negroni, M.; Berra, B.; Rizzo, A.M. Plasma, red blood cells phospholipids and clinical evaluation after long chain omega-3 supplementation in children with attention deficit hyperactivity disorder (adhd). Nutr. Neurosci. 2007, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Sears, B.; Rizzo, A.M.; Mariani Cerati, D.; Caprio, M.; Ricordi, C.; Fabbri, A. Omega-3 pufas and vitamin d co-supplementation as a safe-effective therapeutic approach for core symptoms of autism spectrum disorder: Case report and literature review. Nutr. Neurosci. 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Boccuzzi, L.; Sears, B. The use of high-dose omega-3 pufas and vitamin-d co-supplementation as a therapeutic approach for ibd-related symptoms: Case report and literature review. CellR4 2019, 7, e2746. [Google Scholar]
- Laterza, L.; Gasbarrini, A. Commentary to: “The use of high-dose omega-3 pufas and vitamin-d co-supplementation as a therapeutic approach for ibd-related symptoms: Case report and literature review”. CellR4 2019, 7, e2741. [Google Scholar]
- Baidal, D.A.; Ricordi, C.; Garcia-Contreras, M.; Sonnino, A.; Fabbri, A. Combination high-dose omega-3 fatty acids and high-dose cholecalciferol in new onset type 1 diabetes: A potential role in preservation of beta-cell mass. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3313–3318. [Google Scholar]
- Cadario, F.; Savastio, S.; Rizzo, A.M.; Carrera, D.; Bona, G.; Ricordi, C. Can type 1 diabetes progression be halted? Possible role of high dose vitamin d and omega 3 fatty acids. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1604–1609. [Google Scholar]
- Cadario, F.; Savastio, S.; Ricotti, R.; Rizzo, A.M.; Carrera, D.; Maiuri, L.; Ricordi, C. Administration of vitamin d and high dose of omega 3 to sustain remission of type 1 diabetes. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 512–515. [Google Scholar]
- Baidal, D.A.; Sanchez, J.; Alejandro, R.; Blaschke, C.E.; Hirani, K.; Matheson, D.L.; Messinger, S.; Pugliese, A.; Rafkin, L.E.; Roque, L.A.; et al. Poseidon study: A pilot, safety and feasibility trial of high-dose omega 3 fatty acids and high-dose cholecalciferol supplementation in type 1 diabetes. CellR4 2018, 6, e2489. [Google Scholar]
- Mathieu, C.; Waer, M.; Laureys, J.; Rutgeerts, O.; Bouillon, R. Activated form of vitamin d [1,25(oh)2d3] and its analogs are dose-reducing agents for cyclosporine in vitro and in vivo. Transplant. Proc. 1994, 26, 3048–3049. [Google Scholar]
- Mathieu, C.; Laureys, J.; Waer, M.; Bouillon, R. Prevention of autoimmune destruction of transplanted islets in spontaneously diabetic nod mice by kh1060, a 20-epi analog of vitamin d: Synergy with cyclosporine. Transplant. Proc. 1994, 26, 3128–3129. [Google Scholar]
- Mathieu, C.; Casteels, K.; Waer, M.; Laureys, J.; Valckx, D.; Bouillon, R. Prevention of diabetes recurrence after syngeneic islet transplantation in nod mice by analogues of 1,25(oh)2d3 in combination with cyclosporin a: Mechanism of action involves an immune shift from th1 to th2. Transplant. Proc. 1998, 30, 541. [Google Scholar] [CrossRef]
- Casteels, K.; Waer, M.; Laureys, J.; Valckx, D.; Depovere, J.; Bouillon, R.; Mathieu, C. Prevention of autoimmune destruction of syngeneic islet grafts in spontaneously diabetic nonobese diabetic mice by a combination of a vitamin d3 analog and cyclosporine. Transplantation 1998, 65, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Van Etten, E.; Gysemans, C.; Verstuyf, A.; Bouillon, R.; Mathieu, C. Immunomodulatory properties of a 1,25(oh)(2) vitamin d(3) analog combined with ifnbeta in an animal model of syngeneic islet transplantation. Transplant. Proc. 2001, 33, 2319. [Google Scholar] [CrossRef]
- Gysemans, C.; Van Etten, E.; Overbergh, L.; Verstuyf, A.; Waer, M.; Bouillon, R.; Mathieu, C. Treatment of autoimmune diabetes recurrence in non-obese diabetic mice by mouse interferon-beta in combination with an analogue of 1alpha,25-dihydroxyvitamin-d3. Clin. Exp. Immunol. 2002, 128, 213–220. [Google Scholar] [CrossRef]
- Baeke, F.; Van Belle, T.L.; Takiishi, T.; Ding, L.; Korf, H.; Laureys, J.; Gysemans, C.; Mathieu, C. Low doses of anti-cd3, ciclosporin a and the vitamin d analogue, tx527, synergise to delay recurrence of autoimmune diabetes in an islet-transplanted nod mouse model of diabetes. Diabetologia 2012, 55, 2723–2732. [Google Scholar] [CrossRef] [Green Version]
- Leyssens, C.; Verlinden, L.; Verstuyf, A. The future of vitamin d analogs. Front. Physiol. 2014, 5, 122. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Z.Z.; Li, Y.; Fan, P.; Guo, J.; Xue, W.J.; Ding, X.M.; Tian, X.H.; Feng, X.S.; Zheng, J.; Tian, P.X.; et al. 1,25(oh)2d3 prolongs islet graft survival by inflammatory inhibition. Transplant. Proc. 2014, 46, 1615–1620. [Google Scholar] [CrossRef]
- Gregori, S.; Casorati, M.; Amuchastegui, S.; Smiroldo, S.; Davalli, A.; Adorini, L. Transplantation tolerance by 1,25-dihydroxyvitamin d(3)-induced costimulation blockade. Transplant. Proc. 2001, 33, 219–220. [Google Scholar] [CrossRef]
- Gregori, S.; Casorati, M.; Amuchastegui, S.; Smiroldo, S.; Davalli, A.M.; Adorini, L. Regulatory t cells induced by 1 alpha,25-dihydroxyvitamin d3 and mycophenolate mofetil treatment mediate transplantation tolerance. J. Immunol. 2001, 167, 1945–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurol, A.O.; Okten-Kursun, A.; Kasapoglu, P.; Suzergoz, F.; Kucuksezer, U.C.; Cevik, A.; Tutuncu, Y.; Yentur, S.P.; Gurol, S.D.; Kucuk, M.; et al. The synergistic effect of ω3 and vit d3 on glycemia and tnf-α in islet transplantation. Cell. Mol. Biol. 2016, 62, 90–98. [Google Scholar] [PubMed]
- Vassiliou, E.K.; Kesler, O.M.; Tadros, J.H.; Ganea, D. Bone marrow-derived dendritic cells generated in the presence of resolvin e1 induce apoptosis of activated cd4 + t cells. J. Immunol. 2008, 181, 4534–4544. [Google Scholar] [CrossRef] [Green Version]
- Lund, T.; Mangsbo, S.M.; Scholz, H.; Gjorstrup, P.; Tötterman, T.H.; Korsgren, O.; Foss, A. Resolvin e1 reduces proinflammatory markers in human pancreatic islets in vitro. Exp. Clin. Endocrinol. Diabetes 2010, 118, 237–244. [Google Scholar] [CrossRef]
- Stein, E.M.; Shane, E. Vitamin d in organ transplantation. Osteoporos. Int. 2011, 22, 2107–2118. [Google Scholar] [CrossRef]
- Stein, E.M.; Cohen, A.; Freeby, M.; Rogers, H.; Kokolus, S.; Scott, V.; Mancini, D.; Restaino, S.; Brown, R.; McMahon, D.J.; et al. Severe vitamin d deficiency among heart and liver transplant recipients. Clin. Transplant. 2009, 23, 861–865. [Google Scholar] [CrossRef] [Green Version]
- Querings, K.; Girndt, M.; Geisel, J.; Georg, T.; Tilgen, W.; Reichrath, J. 25-hydroxyvitamin d deficiency in renal transplant recipients. J. Clin. Endocrinol. Metab. 2006, 91, 526–529. [Google Scholar] [CrossRef]
- Segal, E.; Baruch, Y.; Kramsky, R.; Raz, B.; Ish-Shalom, S. Vitamin d deficiency in liver transplant patients in israel. Transplant. Proc. 2001, 33, 2955–2956. [Google Scholar] [CrossRef]
- Ewers, B.; Gasbjerg, A.; Moelgaard, C.; Frederiksen, A.M.; Marckmann, P. Vitamin d status in kidney transplant patients: Need for intensified routine supplementation. Am. J. Clin. Nutr. 2008, 87, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, S.S.; Gibney, E.M.; Gehr, T.W.; King, A.L.; Beckman, M.J. High prevalence of vitamin d deficiency in african american kidney transplant recipients. Transplantation 2008, 85, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Sadlier, D.M.; Magee, C.C. Prevalence of 25(oh) vitamin d (calcidiol) deficiency at time of renal transplantation: A prospective study. Clin. Transplant. 2007, 21, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Stavroulopoulos, A.; Cassidy, M.J.; Porter, C.J.; Hosking, D.J.; Roe, S.D. Vitamin d status in renal transplant recipients. Am. J. Transplant. 2007, 7, 2546–2552. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.T.; Eustace, J.A.; Plant, W.D.; Cashman, K.D.; O’Keefe, M.; Lordan, S.; Moloney, R. Inadequate dietary calcium and vitamin d intakes in renal-transplant recipients in ireland. J. Ren. Nutr. 2007, 17, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Colegio, O.R. Skin cancers in organ transplant recipients. Am. J. Transplant. 2017, 17, 2509–2530. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J. Dermatologic management, sun avoidance and vitamin d status in organ transplant recipients (otr). J. Photochem. Photobiol. B 2010, 101, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Akeno, N.; Matsunuma, A.; Maeda, T.; Kawane, T.; Horiuchi, N. Regulation of vitamin d-1alpha-hydroxylase and -24-hydroxylase expression by dexamethasone in mouse kidney. J. Endocrinol. 2000, 164, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Pascussi, J.M.; Robert, A.; Nguyen, M.; Walrant-Debray, O.; Garabedian, M.; Martin, P.; Pineau, T.; Saric, J.; Navarro, F.; Maurel, P.; et al. Possible involvement of pregnane x receptor-enhanced cyp24 expression in drug-induced osteomalacia. J. Clin. Investig. 2005, 115, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Courbebaisse, M.; Thervet, E.; Souberbielle, J.C.; Zuber, J.; Eladari, D.; Martinez, F.; Mamzer-Bruneel, M.F.; Urena, P.; Legendre, C.; Friedlander, G.; et al. Effects of vitamin d supplementation on the calcium-phosphate balance in renal transplant patients. Kidney Int. 2009, 75, 646–651. [Google Scholar] [CrossRef] [Green Version]
- Robien, K.; Oppeneer, S.J.; Kelly, J.A.; Hamilton-Reeves, J.M. Drug-vitamin d interactions: A systematic review of the literature. Nutr. Clin. Pract. 2013, 28, 194–208. [Google Scholar] [CrossRef] [Green Version]
- Boudville, N.C.; Hodsman, A.B. Renal function and 25-hydroxyvitamin d concentrations predict parathyroid hormone levels in renal transplant patients. Nephrol. Dial. Transplant. 2006, 21, 2621–2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannini, S.; Sella, S.; Silva Netto, F.; Cattelan, C.; Dalle Carbonare, L.; Lazzarin, R.; Marchini, F.; Rigotti, P.; Marcocci, C.; Cetani, F.; et al. Persistent secondary hyperparathyroidism and vertebral fractures in kidney transplantation: Role of calcium-sensing receptor polymorphisms and vitamin d deficiency. J. Bone Miner. Res. 2010, 25, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xie, X.B.; Peng, L.K.; Yu, S.J.; Peng, Y.T. Mechanism and treatment strategy of osteoporosis after transplantation. Int. J. Endocrinol. 2015, 2015, 280164. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, D.C.; Winkelmayer, W.C. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Executive summary of the 2017 kdigo chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update: What’s changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peric, M.; Koglin, S.; Kim, S.M.; Morizane, S.; Besch, R.; Prinz, J.C.; Ruzicka, T.; Gallo, R.L.; Schauber, J. Il-17a enhances vitamin d3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J. Immunol. 2008, 181, 8504–8512. [Google Scholar] [CrossRef] [Green Version]
- Schauber, J.; Oda, Y.; Büchau, A.S.; Yun, Q.C.; Steinmeyer, A.; Zügel, U.; Bikle, D.D.; Gallo, R.L. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and cd14 by 1,25-dihydroxyvitamin d3. J. Invest. Derm. 2008, 128, 816–824. [Google Scholar] [CrossRef] [Green Version]
- Hansdottir, S.; Monick, M.M.; Hinde, S.L.; Lovan, N.; Look, D.C.; Hunninghake, G.W. Respiratory epithelial cells convert inactive vitamin d to its active form: Potential effects on host defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar] [CrossRef] [Green Version]
- Cantorna, M.T.; Hullett, D.A.; Redaelli, C.; Brandt, C.R.; Humpal-Winter, J.; Sollinger, H.W.; Deluca, H.F. 1,25-dihydroxyvitamin d3 prolongs graft survival without compromising host resistance to infection or bone mineral density. Transplantation 1998, 66, 828–831. [Google Scholar] [CrossRef]
- Becker, B.N.; Hullett, D.A.; O’Herrin, J.K.; Malin, G.; Sollinger, H.W.; DeLuca, H. Vitamin d as immunomodulatory therapy for kidney transplantation. Transplantation 2002, 74, 1204–1206. [Google Scholar] [CrossRef]
- Redaelli, C.A.; Wagner, M.; Tien, Y.H.; Mazzucchelli, L.; Stahel, P.F.; Schilling, M.K.; Dufour, J.F. 1 alpha,25-dihydroxycholecalciferol reduces rejection and improves survival in rat liver allografts. Hepatology 2001, 34, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.B.; Zheng, S.S.; Jia, C.K.; Wang, Y. Effect of 1,25-dihydroxyvitamin d3 on preventing allograft from acute rejection following rat orthotopic liver transplantation. World J. Gastroenterol. 2003, 9, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Hullett, D.A.; Cantorna, M.T.; Redaelli, C.; Humpal-Winter, J.; Hayes, C.E.; Sollinger, H.W.; Deluca, H.F. Prolongation of allograft survival by 1,25-dihydroxyvitamin d3. Transplantation 1998, 66, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Tanaci, N.; Karakose, H.; Guvener, N.; Tutuncu, N.B.; Colak, T.; Haberal, M. Influence of 1,25-dihydroxyvitamin d3 as an immunomodulator in renal transplant recipients: A retrospective cohort study. Transplant. Proc. 2003, 35, 2885–2887. [Google Scholar] [CrossRef] [PubMed]
- Uyar, M.; Sezer, S.; Arat, Z.; Elsurer, R.; Ozdemir, F.N.; Haberal, M. 1,25-dihydroxyvitamin d(3) therapy is protective for renal function and prevents hyperparathyroidism in renal allograft recipients. Transplant. Proc. 2006, 38, 2069–2073. [Google Scholar] [CrossRef] [PubMed]
- Eyal, O.; Aharon, M.; Safadi, R.; Elhalel, M.D. Serum vitamin d levels in kidney transplant recipients: The importance of an immunosuppression regimen and sun exposure. Isr. Med. Assoc. J. 2013, 15, 628–633. [Google Scholar]
- Ahmadpoor, P.; Ilkhanizadeh, B.; Ghasemmahdi, L.; Makhdoomi, K.; Ghafari, A. Effect of active vitamin d on expression of co-stimulatory molecules and hla-dr in renal transplant recipients. Exp. Clin. Transplant. 2009, 7, 99–103. [Google Scholar]
- Froud, T.; Ricordi, C.; Baidal, D.A.; Hafiz, M.M.; Ponte, G.; Cure, P.; Pileggi, A.; Poggioli, R.; Ichii, H.; Khan, A.; et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am. J. Transplant. 2005, 5, 2037–2046. [Google Scholar] [CrossRef]
- Faradji, R.N.; Tharavanij, T.; Messinger, S.; Froud, T.; Pileggi, A.; Monroy, K.; Mineo, D.; Baidal, D.A.; Cure, P.; Ponte, G.; et al. Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation 2008, 86, 1658–1665. [Google Scholar] [CrossRef]
- Kuo, Y.T.; Kuo, C.H.; Lam, K.P.; Chu, Y.T.; Wang, W.L.; Huang, C.H.; Hung, C.H. Effects of vitamin d3 on expression of tumor necrosis factor-alpha and chemokines by monocytes. J. Food Sci. 2010, 75, H200–H204. [Google Scholar] [CrossRef]
- Davalli, A.M.; Scaglia, L.; Zangen, D.H.; Hollister, J.; Bonner-Weir, S.; Weir, G.C. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes 1996, 45, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Riachy, R.; Vandewalle, B.; Kerr Conte, J.; Moerman, E.; Sacchetti, P.; Lukowiak, B.; Gmyr, V.; Bouckenooghe, T.; Dubois, M.; Pattou, F. 1,25-dihydroxyvitamin d3 protects rinm5f and human islet cells against cytokine-induced apoptosis: Implication of the antiapoptotic protein a20. Endocrinology 2002, 143, 4809–4819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riachy, R.; Vandewalle, B.; Moerman, E.; Belaich, S.; Lukowiak, B.; Gmyr, V.; Muharram, G.; Kerr Conte, J.; Pattou, F. 1,25-dihydroxyvitamin d3 protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the fas receptor. Apoptosis 2006, 11, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Society, E. Evaluation, treatment and prevention of vitamin d deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
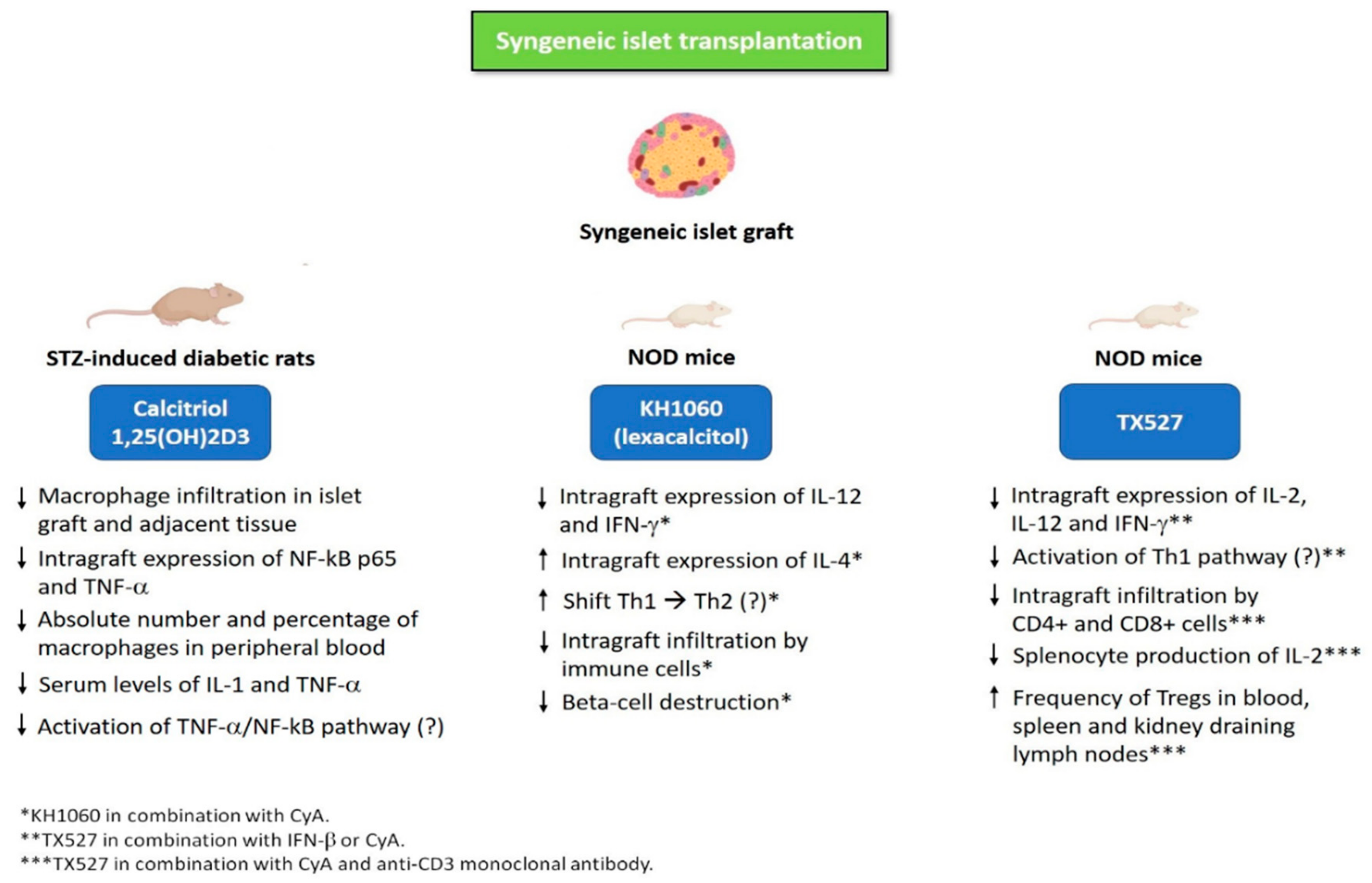

| Study Treatment | Study Treatment Duration | Animal Model | Main Findings | References |
|---|---|---|---|---|
| KH1060 (Lexacalcitol) * | Treatment was initiated the day before transplantation and continued until 60 days after transplantation | NOD mice receiving syngeneic islets under the kidney capsule | Low doses of KH1060 (0.5 μg/kg/twice daily) and CyA (7.5 mg/kg/day) were well tolerated and more effective compared to high doses of KH1060 (1 μg/kg/twice daily) or CyA (15 mg/kg/day) administered as monotherapies. MST of graft after islet transplantation: KH1060 + CyA group, 62 days; high dose KH1060, 55 days; high dose CyA, 58 days. Cytokine profile expression in islet grafts revealed significantly lower levels of IL-12 and IFN-γ, along with significantly higher levels of IL-4 in NOD mice treated with KH1060 plus CyA compared to those treated with KH1060 or CyA alone. | [73,74,75] |
| KH1060 (Lexacalcitol) * | Treatment was initiated the day before transplantation and continued until disease recurrence or 60 days after transplantation | NOD mice receiving syngeneic islets under the kidney capsule | MST of graft after islet transplantation: high dose CyA (15 mg/kg/day), 60 ± 26 days; high dose KH1060 (1 μg/kg/2 days), 50 ±15 days; low doses of CyA (7.5 mg/kg/day) plus KH1060 (0.5 μg/kg/2 days), 48 ± 28 days. MST of graft was significantly longer in all the three groups compared to vehicle (peanut oil)-treated controls. | [76] |
| TX527 ** | Treatment was initiated the day before transplantation and continued until day 20 (CyA and IFN-β) or day 30 (TX527) after transplantation | NOD mice receiving syngeneic islets under the kidney capsule | MST of graft after islet transplantation: TX527 (5 μg/kg/day) plus IFN-β (1 × 105 IU/day), 62 ± 20 days; TX527 (5 μg/kg/day) plus CyA (7.5 mg/kg/day), 31 ± 12 days. MST of graft was significantly longer in mice on TX527 plus IFN-β or CyA compared to mice treated with vehicle, monotherapy or IFN-β plus CyA. Mice treated with TX527 plus IFN-β or CyA exhibited significantly reduced graft levels of IL-2, IL-12 and IFN-γ compared to vehicle (peanut oil)-treated controls, as assessed by cytokine mRNA analysis of islet grafts performed 6 days after transplantation. | [77,78] |
| TX527 ** | TX527 and CyA were administered from day 1 until day 60 after transplantation, whereas anti-CD3 monoclonal antibody was administered from day 0 until day 4 after transplantation | NOD mice receiving syngeneic islets under the kidney capsule | Mice receiving triple-combination therapy with TX527 (10 μg/kg every 2 days, day 1 until day 60) plus CyA (5 mg/kg per day, day 1 until day 60) and anti-CD3 monoclonal antibody (2.5 μg/day, days 0–4) showed a significantly longer islet graft survival (MST, 79.5 ± 18.6 days) compared to those receiving anti-CD3 monotherapy (MST, 24.8 ± 7.3 days) and dual therapy with anti-CD3 plus CyA (MST, 25.5 ± 12.4 days). Histology of the transplanted islets revealed that grafts of mice treated with triple-combination therapy were more preserved and less infiltrated by CD4+ cells and effector/memory phenotype CD8+ T cells (on day 21 after transplantation). Mice receiving triple-combination therapy showed significantly increased frequency of Tregs in blood, spleen and kidney draining lymph nodes, compared to untreated (control mice) and anti-CD3-treated mice. Importantly, Tregs isolated from mice receiving triple-combination therapy maintained intact suppressive capacity in vivo, as supported by the fact that they significantly delayed diabetes in the NOD Scid transfer model. Anti-CD3 monotherapy led to increased production of TNF-α, IL-5, IL-21 and IL-10, but the upregulation of these cytokines was abrogated by the triple-combination therapy with anti-CD3 plus TX527 and CyA. Moreover, triple-combination therapy significantly reduced IL-2 production by splenocytes compared to anti-CD3 monotherapy. | [79] |
| Calcitriol | Calcitriol was administered from day 1 until day 20 after transplantation | Sprague-Dawley STZ-induced diabetic rats receiving syngeneic intraportal islet transplantation | Rats receiving calcitriol (5 mg/day by intraperitoneal injection) exhibited a significantly improved islet graft survival compared to control group (propylene glycol administered by intraperitoneal injection): 50% of recipients in the control group maintained a functioning graft for 14 days, whereas 80% of calcitriol-treated recipients remained euglycemic for at least 14 days. Histology revealed that calcitriol-treated mice exhibited a reduced macrophage infiltration in both islet graft and adjacent tissue 7 days after transplantation. At day 7 after transplantation, the absolute number and percentage of macrophages in peripheral blood were significantly lower in calcitriol group compared to control group. Moreover, calcitriol down-regulated the increase in serum levels of IL-1 and TNF-α compared to the control group. Western blot showed that graft expression of NF-kB p65 and TNF-α was significantly lower in calcitriol-treated mice compared to the control group. | [81] |
| Study Treatment | Study Treatment Duration | Animal Model | Main Findings | References |
|---|---|---|---|---|
| Calcitriol in combination with MMF | MMF and/or calcitriol were administered from the day before transplantation and continued until day 30 after transplantation | BALB/c STZ-induced diabetic mice receiving allogeneic islets under the kidney capsule (pancreatic islets were isolated from C57BL/6 (B6) mice) | MMF (100 mg/kg/day) and calcitriol (5 μg/kg/three times a week) combination therapy was associated with significantly longer islet graft survival compared to MMF or calcitriol alone (% of graft survival 70 days after transplantation: 85%, 52%, 48%, respectively). MMF and calcitriol combination therapy was associated with significantly higher resistance to islet graft rejection in comparison to MMF or calcitriol alone (% of graft survival 100 days after transplantation: 72.2%, 33.3%, 52.7%, respectively). | [82] |
| Calcitriol in combination with MMF | MMF and/or calcitriol were administered from the day before transplantation and continued until day 30 after transplantation | BALB/c STZ-induced diabetic mice receiving allogeneic islets under the kidney capsule (pancreatic islets were isolated from C57BL/6 (B6) mice) | MMF (100 mg/kg/day) and calcitriol (5 μg/kg/three times a week) combination therapy inhibited the peri-graft recruitment of macrophages and DCs and decreased IL-12 secretion. MMF plus calcitriol increased the frequency of CD4+CD25+ regulatory T cells in the spleen and in the kidney lymph nodes draining the islet graft. These cells were able to transfer long-term transplant tolerance in naïve syngeneic recipient mice (up to 40 days). | [83] |
| Calcitriol-modulated DCs | Transplant recipient mice received three intravenous transfers of calcitriol-modulated murine DCs on days −10, −3 and 0 before transplantation | C57BL/6 alloxan-induced diabetic mice receiving allogeneic islets under the kidney capsule (pancreatic islets were isolated from BALB/c donor mice). Bone marrow cells were harvested from C57BL/6 mice and subsequently induced to differentiate into mature DCs (10 day-culture). The in vitro DC generation was performed in the absence (control DCs) or presence (10−8 M) of calcitriol (calcitriol-modulated DCs). In order to perform the DC transfer experiment in the islet allotransplantation model, DCs were pulsed during the last 48 hours of culture with BALB/c islet antigen (BALB/c islet antigen-loaded control DCs). | 5 out of 7 recipient mice receiving calcitriol-modulated DCs before islet allotransplantation did not experience hyperacute graft rejection, that was instead observed in all 4 mice receiving BALB/c islet antigen-loaded control DCs. Islet allograft survival was not consistently prolonged in mice receiving calcitriol-modulated DCs compared to mice who did not receive any immunomodulatory treatment (untreated group): MST, 11.4 ± 2.2 days vs. 9.0 ± 1.0 days, respectively. | [39] |
| Vitamin D3 plus omega-3 PUFAs (EPA and DHA) | Vitamin D3 and/or omega-3 PUFAs were administered on days 0, 1 and 2 after transplantation. Daclizumab was administered intravenously for induction immunosuppression, at a dose of 0.05 mg/kg body weight before transplantation (day 0) and on days 1 and 2 after transplantation. | STZ-induced diabetic Wistar albino rats receiving allogeneic intraportal islet transplantation | Vitamin D3 (5 μg/kg) plus EPA and DHA (7 mg/kg) significantly reduced the increase in serum levels of TNF-α at days 1 and 2 after transplantation compared to control group and rats treated with vitamin D3 or omega-3 PUFAs alone. | [84] |
| n | 17 |
|---|---|
| Gender | 12 females, 5 males |
| Mean age ± SD (years) | 55.2 ± 4.9 |
| Mean BMI ± SD (kg/m2) | 22.1 ± 0.4 |
| Mean duration of graft function ± SD (years) | 11.1 ± 8.4 |
| Mean serum 25(OH)D levels ± SD (ng/mL) | 51.0 ±7.0 |
| Mean plasma AA/EPA ratio ± SD | 31.3 ± 12.3 |
| Vitamin D users * | n = 15 |
| Mean total daily dose of vitamin D (IU/day) | 1626 IU |
| Mean daily dose of vitamin D (IU/kg/day) | 28.4 |
| Omega-3 PUFA users (EPA and DHA) ** | n = 5 *** |
| Mean total daily dose of omega-3 PUFAs (mg/day) | 2000 |
| Mean daily dose of omega-3 PUFAs (mg/kg/day) | 33.5 |
| Mean plasma AA/EPA ratio values ± SD among omega-3 PUFA users | 3.7 ± 1.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Infante, M.; Ricordi, C.; Padilla, N.; Alvarez, A.; Linetsky, E.; Lanzoni, G.; Mattina, A.; Bertuzzi, F.; Fabbri, A.; Baidal, D.; et al. The Role of Vitamin D and Omega-3 PUFAs in Islet Transplantation. Nutrients 2019, 11, 2937. https://doi.org/10.3390/nu11122937
Infante M, Ricordi C, Padilla N, Alvarez A, Linetsky E, Lanzoni G, Mattina A, Bertuzzi F, Fabbri A, Baidal D, et al. The Role of Vitamin D and Omega-3 PUFAs in Islet Transplantation. Nutrients. 2019; 11(12):2937. https://doi.org/10.3390/nu11122937
Chicago/Turabian StyleInfante, Marco, Camillo Ricordi, Nathalia Padilla, Ana Alvarez, Elina Linetsky, Giacomo Lanzoni, Alessandro Mattina, Federico Bertuzzi, Andrea Fabbri, David Baidal, and et al. 2019. "The Role of Vitamin D and Omega-3 PUFAs in Islet Transplantation" Nutrients 11, no. 12: 2937. https://doi.org/10.3390/nu11122937
APA StyleInfante, M., Ricordi, C., Padilla, N., Alvarez, A., Linetsky, E., Lanzoni, G., Mattina, A., Bertuzzi, F., Fabbri, A., Baidal, D., & Alejandro, R. (2019). The Role of Vitamin D and Omega-3 PUFAs in Islet Transplantation. Nutrients, 11(12), 2937. https://doi.org/10.3390/nu11122937









