Association of DASH and Depressive Symptoms with BMI over Adulthood in Racially and Socioeconomically Diverse Adults Examined in the HANDLS Study
Abstract
1. Introduction
2. Methods
2.1. Background on Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) Study
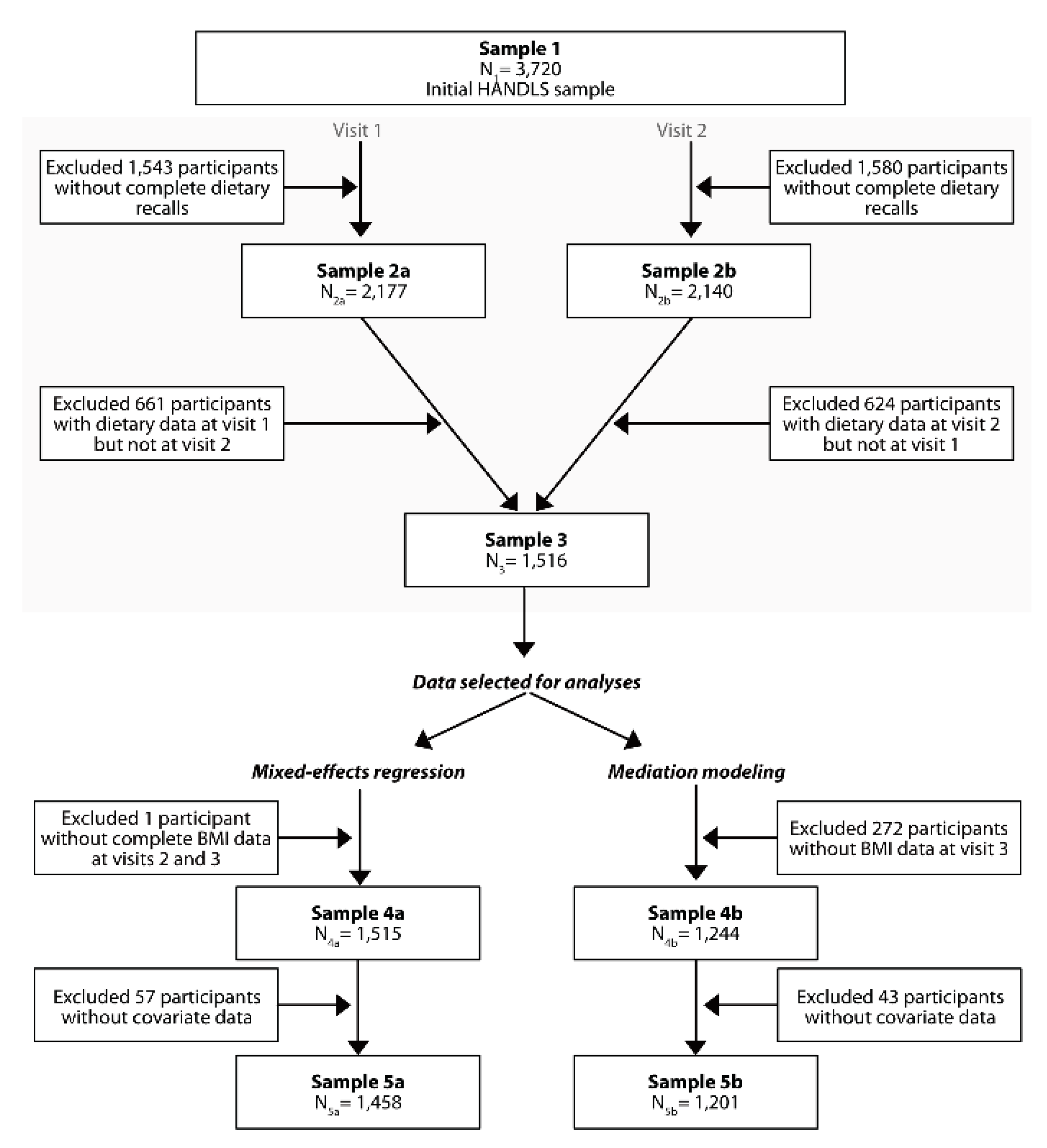
2.2. Study Sample
2.3. Dietary Methods and Quality
2.4. Demographic and Health-Related Measures
2.5. Depressive Symptoms
2.6. Outcome Measure
2.7. Data Handling and Statistical Analysis
3. Results
3.1. Characteristics of Study Participants by CES-D Tertiles
3.2. DASH Diet Score by CES-D Tertiles: Energy-Adjusted and Full Model
3.3. DASH Diet Score, CES-D, and BMI Over Time: Mixed-Effects Regression Models
3.4. DASH Diet Score as a Mediator between CES-D and BMI at Last Visit: RAA Mediation Models
3.5. CES-D Score as a Mediator between DASH and BMI at Last Visit: RAA Mediation Model
4. Discussion
4.1. The Link between DASH Score and Depressive Symptoms
4.2. The Link between DASH Score and BMI
4.3. The Link between Depressive Symptoms and BMI
4.4. Strengths, Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asghari, G.; Mirmiran, P.; Yuzbashian, E.; Azizi, F. A systematic review of diet quality indices in relation to obesity. Br. J. Nutr. 2017, 117, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, K.M.; McNaughton, S.A. Diet quality is associated with obesity and hypertension in Australian adults: A cross sectional study. BMC Public Health 2016, 16, 1037. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Pan, A.; Hou, T.; Chiuve, S.E.; Tobias, D.K.; Mozaffarian, D.; Willett, W.C.; Hu, F.B. Long-Term Change in Diet Quality Is Associated with Body Weight Change in Men and Women. J. Nutr. 2015, 145, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.T.F.; Chan, R.S.M.; Ko, G.T.C.; Lau, E.S.H.; Chow, F.C.C.; Kong, A.P.S. Diet quality is inversely associated with obesity in Chinese adults with type 2 diabetes. Nutr. J. 2018, 17, 63. [Google Scholar] [CrossRef]
- Cespedes Feliciano, E.M.; Tinker, L.; Manson, J.E.; Allison, M.; Rohan, T.; Zaslavsky, O.; Waring, M.E.; Asao, K.; Garcia, L.; Rosal, M.; et al. Change in Dietary Patterns and Change in Waist Circumference and DXA Trunk Fat Among Postmenopausal Women. Obesity 2016, 24, 2176–2184. [Google Scholar] [CrossRef]
- US Department of Agriculture ARS, Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture; US Department of Agriculture ARS, Dietary Guidelines Advisory Committee: Washington, DC, USA, 2015.
- Champagne, C.M.; Broyles, S.T.; Moran, L.D.; Cash, K.C.; Levy, E.J.; Lin, P.H.; Batch, B.C.; Lien, L.F.; Funk, K.L.; Dalcin, A.; et al. Dietary Intakes Associated with Successful Weight Loss and Maintenance during the Weight Loss Maintenance Trial. J. Am. Diet. Assoc. 2011, 111, 1826–1835. [Google Scholar] [CrossRef]
- Soltani, S.; Shirani, F.; Chitsazi, M.J.; Salehi-Abargouei, A. The effect of dietary approaches to stop hypertension (DASH) diet on weight and body composition in adults: A systematic review and meta-analysis of randomized controlled clinical trials. Obes. Rev. 2016, 17, 442–454. [Google Scholar] [CrossRef]
- Boggs, D.A.; Rosenberg, L.; Rodríguez-Bernal, C.L.; Palmer, J.R. Long-term diet quality is associated with lower obesity risk in young African American women with normal BMI at baseline. J. Nutr. 2013, 143, 1636–1641. [Google Scholar] [CrossRef]
- Wang, T.; Heianza, Y.; Sun, D.; Huang, T.; Ma, W.; Rimm, E.B.; Manson, J.E.; Hu, F.B.; Willett, W.C.; Qi, L. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: Gene-diet interaction analysis in two prospective cohort studies. BMJ Clin. Res. Ed. 2018, 360, j5644. [Google Scholar] [CrossRef]
- Zheng, Y.; Manson, J.E.; Yuan, C.; Liang, M.H.; Grodstein, F.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Associations of Weight Gain from Early to Middle Adulthood With Major Health Outcomes Later in Life. JAMA 2017, 318, 255–269. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Jee, Y.H.; Chang, H.; Jung, K.J.; Jee, S.H. Cohort study on the effects of depression on atherosclerotic cardiovascular disease risk in Korea. BMJ Open 2019, 9, e026913. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.; Rosenbaum, S.; Rebar, A.; Happell, B. Prevalence of Chronic Health Conditions in Australian Adults with Depression and/or Anxiety. Issues Ment. Health Nurs. 2019, 40, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Preiss, K.; Brennan, L.; Clarke, D. A systematic review of variables associated with the relationship between obesity and depression. Obes. Rev. 2013, 14, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornago, A.; Sanchez-Villegas, A.; Bes-Rastrollo, M.; Gea, A.; Molero, P.; Lahortiga-Ramos, F.; Martinez-Gonzalez, M.Á. Relationship between adherence to Dietary Approaches to Stop Hypertension (DASH) diet indices and incidence of depression during up to 8 years of follow-up. Public Health Nutr. 2017, 20, 2383–2392. [Google Scholar] [CrossRef]
- Florez, K.R.; Dubowitz, T.; Ghosh-Dastidar, M.B.; Beckman, R.; Collins, R.L. Associations between depressive symptomatology, diet, and body mass index among participants in the supplemental nutrition assistance program. J. Acad. Nutr. Diet. 2015, 115, 1102–1108. [Google Scholar] [CrossRef]
- Evans, M.K.; Lepkowski, J.M.; Powe, N.R.; LaVeist, T.; Kuczmarski, M.F.; Zonderman, A.B. Healthy aging in neighborhoods of diversity across the life span (HANDLS): Overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn. Dis. 2010, 20, 267–275. [Google Scholar]
- Healthy Aging in Neighborhoods of Diversity across the Life Span. Available online: https://handls.nih.gov/06Coll-dataDoc.htm (accessed on 13 November 2019).
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- Raper, N.; Perloff, B.; Ingwersen, L.; Steinfeldt, L.; Anand, J. An Overview of USDA’s Dietary Intake Data System. Food Compos. Anal. 2004, 17, 545–555. [Google Scholar] [CrossRef]
- Food Surveys Research Group. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group (accessed on 13 November 2019).
- Mellen, P.B.; Gao, S.K.; Vitolins, M.Z.; Goff, D.C., Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch. Int. Med. 2008, 168, 308–314. [Google Scholar] [CrossRef]
- The 2004 HHS Poverty Guidelines. Available online: https://aspe.hhs.gov/2004-hhs-poverty-guidelines (accessed on 13 November 2019).
- Wilkinson, G.S. Wide Range Achievement Test—Revision 3; Jastak Association: Wilmington, DE, USA, 1993. [Google Scholar]
- Radloff, L. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Myers, J.K.; Weissman, M.M. Use of a self-report symptom scale to detect depression in a community sample. Am. J. Psychiatry 1980, 137, 1081–1084. [Google Scholar] [PubMed]
- STATA: Statistics/Data Analysis: Release 16.0; Stata Corporation: College Station, TX, USA, 2019.
- Valeri, L.; Vanderweele, T.J. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: Theoretical assumptions and implementation with SAS and SPSS macros. Psychol. Methods 2013, 18, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Heckman, J.J. Sample selection bias as a specification error. Econometrica 1979, 47, 153–161. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Hossain, S.; Fanelli-Kuczmarski, M.T.; Beydoun, H.A.; Canas, J.A.; Evans, M.K.; Zonderman, A.B. Vitamin D Status and Intakes and Their Association With Cognitive Trajectory in a Longitudinal Study of Urban Adults. J. Clin. Endocrinol. Metab. 2018, 103, 1654–1668. [Google Scholar] [CrossRef] [PubMed]
- Fanelli Kuczmarski, M.; Bodt, B.A.; Stave Shupe, E.; Zonderman, A.B.; Evans, M.K. Dietary Patterns Associated with Lower 10-Year Atherosclerotic Cardiovascular Disease Risk among Urban African-American and White Adults Consuming Western Diets. Nutrients 2018, 10, 158. [Google Scholar] [CrossRef]
- Nicolaou, M.; Colpo, M.; Vermeulen, E.; Elstgeest, L.E.M.; Cabout, M.; Gibson-Smith, D.; Knuppel, A.; Sini, G.; Schoenaker, D.; Mishra, G.D.; et al. Association of a priori dietary patterns with depressive symptoms: A harmonised meta-analysis of observational studies. Psychol. Med. 2019, 1–12. [Google Scholar] [CrossRef]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sanchez-Villegas, A.; Kivimaki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef]
- Elstgeest, L.E.M.; Winkens, L.H.H.; Penninx, B.; Brouwer, I.A.; Visser, M. Associations of depressive symptoms and history with three a priori diet quality indices in middle-aged and older adults. J. Affect. Disord. 2019, 249, 394–403. [Google Scholar] [CrossRef]
- Barak, F.; Falahi, E.; Keshteli, A.H.; Yazdannik, A.; Esmaillzadeh, A. Adherence to the Dietary Approaches to Stop Hypertension (DASH) diet in relation to obesity among Iranian female nurses. Public Health Nutr. 2015, 18, 705–712. [Google Scholar] [CrossRef]
- Phillips, C.M.; Harrington, J.M.; Perry, I.J. Relationship between dietary quality, determined by dash score, and cardiometabolic health biomarkers: A cross-sectional analysis in adults. Clin. Nutr. 2019, 38, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Whitton, C.; Rebello, S.A.; Lee, J.; Tai, E.S.; van Dam, R.M. A Healthy Asian A Posteriori Dietary Pattern Correlates with A Priori Dietary Patterns and Is Associated with Cardiovascular Disease Risk Factors in a Multiethnic Asian Population. J. Nutr. 2018, 148, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Nazare, J.A.; Smith, J.; Borel, A.L.; Alméras, N.; Tremblay, A.; Bergeron, J.; Poirier, P.; Després, J.P. Changes in Both Global Diet Quality and Physical Activity Level Synergistically Reduce Visceral Adiposity in Men with Features of Metabolic Syndrome. J. Nutr. 2013, 143, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Ledikwe, J.H.; Rolls, B.J.; Smiciklas-Wright, H.; Mitchell, D.C.; Ard, J.D.; Champagne, C.; Karanja, N.; Lin, P.H.; Stevens, V.J.; Appel, L.J. Reductions in dietary energy density are associated with weight loss in overweight and obese participants in the PREMIER trial. Am. J. Clin. Nutr. 2007, 85, 1212–1221. [Google Scholar] [CrossRef]
- Mertens, E.; Markey, O.; Geleijnse, J.M.; Lovegrove, J.A.; Givens, D.I. Adherence to a healthy diet in relation to cardiovascular incidence and risk markers: Evidence from the Caerphilly prospective study. Eur. J. Nutr. 2018, 57, 1245–1258. [Google Scholar] [CrossRef]
- Murakami, K.; Livingstone, M.B.E.; Sasaki, S. Diet quality scores in relation to metabolic risk factors in Japanese adults: A cross-sectional analysis from the 2012 National Health and Nutrition Survey, Japan. Eur. J. Nutr. 2019, 58, 2037–2050. [Google Scholar] [CrossRef]
- Sahrai, M.S.; Huybrechts, I.; Biessy, C.; Gunter, M.J.; Romieu, I.; Torres-Mejía, G.; Dossus, L. Association of a Priori-Defined Dietary Patterns with Anthropometric Measurements: A Cross-Sectional Study in Mexican Women. Nutrients 2019, 11, 603. [Google Scholar] [CrossRef]
- Park, Y.M.M.; Steck, S.E.; Fung, T.T.; Zhang, J.; Hazlett, L.J.; Han, K.; Lee, S.H.; Kwon, H.S.; Merchant, A.T. Mediterranean diet, Dietary Approaches to Stop Hypertension (DASH) style diet, and metabolic health in US adults. Clin. Nutr. 2017, 36, 1301–1309. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Fanelli-Kuczmarski, M.T.; Shaked, D.; Dore, G.A.; Beydoun, H.A.; Rostant, O.S.; Evans, M.K.; Zonderman, A.B. Alternative Pathway Analyses Indicate Bidirectional Relations between Depressive Symptoms, Diet Quality, and Central Adiposity in a Sample of Urban US Adults. J. Nutr. 2016, 146, 1241–1249. [Google Scholar] [CrossRef]
- Sutin, A.R.; Zonderman, A.B. Depressive symptoms are associated with weight gain among women. Psychol. Med. 2012, 42, 2351–2360. [Google Scholar] [CrossRef]
- Henriksen, C.A.; Mather, A.A.; Mackenzie, C.S.; Bienvenu, O.J.; Sareen, J. Longitudinal associations of obesity with affective disorders and suicidality in the Baltimore epidemiologic catchment area follow-up study. J. Nerv. Ment. Dis. 2014, 202, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Sachs-Ericsson, N.; Burns, A.B.; Gordon, K.H.; Eckel, L.A.; Wonderlich, S.A.; Crosby, R.D.; Blazer, D.G. Body mass index and depressive symptoms in older adults: The moderating roles of race, sex, and socioeconomic status. Am. J. Geriatr. Psychiatry 2007, 15, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Vogelzangs, N.; Kritchevsky, S.B.; Beekman, A.T.; Brenes, G.A.; Newman, A.B.; Satterfield, S.; Yaffe, K.; Harris, T.B.; Penninx, B.W. Health ABCS: Obesity and onset of significant depressive symptoms: Results from a prospective community-based cohort study of older men and women. J. Clin. Psychiatry 2010, 71, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Wild, B.; Herzog, W.; Lechner, S.; Niehoff, D.; Brenner, H.; Muller, H.; Rothenbacher, D.; Stegmaier, C.; Raum, E. Gender specific temporal and cross-sectional associations between BMI-class and symptoms of depression in the elderly. J. Psychosom. Res. 2012, 72, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, Q.; Czernichow, S.; Kivimaki, M.; Okereke, O.I.; Lucas, M.; Manson, J.E.; Ascherio, A.; Hu, F.B. Bidirectional association between depression and obesity in middle-aged and older women. Int. J. Obes. 2012, 36, 595–602. [Google Scholar] [CrossRef]
- Singh, G.; Jackson, C.A.; Dobson, A.; Mishra, G.D. Bidirectional association between weight change and depression in mid-aged women: A population-based longitudinal study. Int. J. Obes. 2014, 38, 591–596. [Google Scholar] [CrossRef]
- Naska, A.; Lagiou, A.; Lagiou, P. Dietary Assessment Methods in Epidemiological Research: Current State of the Art and Future Prospects. F1000Research 2017, 6, 926. [Google Scholar] [CrossRef]
| Characteristics | Depressive Symptoms (Mean) Tertiles 1 | |||
|---|---|---|---|---|
| T1 | T2 | T3 | p2 | |
| (N = 519) | (N = 473) | (N = 466) | ||
| Demographic and Socio-economic | ||||
| Sex at V1, % male | 42.4 | 44.6 | 34.7 | 0.004 |
| Age at V1, years (X ± SE) | 49.0 ± 0.4 | 48.0 ± 0.4 | 48.1 ± 0.4 | 0.15 |
| Age at V2, years (X ± SE) | 53.7 ± 0.4 | 52.7 ± 0.4 | 52.9 ± 0.4 | 0.18 |
| Age at V3, years (X ± SE) | 57.9 ± 0.4 | 56.5 ± 0.5 | 56.4 ± 0.4 | 0.022 |
| African American at V 1, % | 60.3 | 61.3 | 59.2 | 0.808 |
| Poverty status at V1, % (<125% poverty) | 31.4 | 39.1 | 53.2 | <0.0001 |
| Education at V1, years Completed, % | ||||
| <High School | 3.7 | 6.1 | 10.0 | <0.0001 |
| High School | 45.7 | 62.8 | 65.4 | |
| >High School | 50.7 | 31.1 | 24.7 | |
| Literacy at V1, WRAT-3 score | <0.0001 | |||
| <36, % | 11.4 | 22.0 | 29.2 | |
| 37–40, % | 13.7 | 16.3 | 17.2 | |
| 41–46, % | 27.9 | 29.2 | 28.4 | |
| ≥47, % | 47.0 | 32.6 | 25.3 | |
| Unemployed in last month at V1, % yes | 23.3 | 31.3 | 45.3 | <0.0001 |
| Unemployment in last month, % missing | 17.0 | 17.8 | 19.1 | |
| Health-Related | ||||
| Self-rated health at V1 | <0.0001 | |||
| Poor/Average, % | 11.9 | 19.2 | 39.7 | |
| Good, % | 39.3 | 44.6 | 39.5 | |
| Very good/Excellent % | 48.7 | 36.2 | 20.8 | |
| CES-D score (mean of V1& V2) (X ± SE) | 5.15 ± 0.11 | 13.59 ± 0.12 | 26.85 ± 0.33 | <0.0001 |
| Drug and tobacco use at V1 | ||||
| Any drug, current user, % 3 | 43.4 | 46.3 | 48.1 | 0.306 |
| Any drug, missing, % | 8.3 | 6.6 | 9.0 | |
| Tobacco, current user, % | 28.7 | 45.2 | 49.6 | <0.0001 |
| Tobacco, missing, % | 10.2 | 7.4 | 11.2 | |
| BMI at V2, kg/m2 (X ± SE) | 30.6 ± 0.3 | 30.2 ± 0.4 | 30.8 ± 0.4 | 0.542 |
| BMI at V3, kg/m2 (X ± SE) | 31.1 ± 0.4 | 30.2 ± 0.4 | 31.0 ± 0.4 | 0742 |
| BMI (mean of V2 & vV3), kg/m2 (X ± SE) | 31.0 ± 0.3 | 30.7 ± 0.4 | 30.9 ± 0.4 | 0.671 |
| BMI annual rate of change (ΔBMI), (X ± SE) 4 | 0.01 ± 0.04 | 0.056 ± 0.04 | 0.08 ± 0.05 | 0.596 |
| Diet-Related | ||||
| DASH total score at V1 (X ± SE) | 1.89 ± 0.07 | 1.62 ± 0.06 | 1.64 ± 0.06 | 0.0020 |
| DASH total score at V2 (X ± SE) | 1.83 ± 0.06 | 1.69 ± 0.06 | 1.76 ± 0.06 | 0.211 |
| DASH total score (mean) (X ± SE) 5 | 1.86 ± 0.05 | 1.65 ± 0.05 | 1.70 ± 0.05 | 0.0046 |
| Total Energy intake at V1, kcal/d (X ± SE) | 2039 ± 43 | 2023 ± 40 | 1941 ± 44 | 0.218 |
| Total Energy intake at V2, kcal/d (X ± SE) | 2118 ± 39 | 2079 ± 39 | 1950 ± 37 | 0.0060 |
| Total Energy intake (mean), kcal/d (X ± SE) 5 | 2079 ± 36 | 2051 ± 34 | 1945 ± 33 | 0.018 |
| Energy Intake from Grocery Store | ||||
| Energy intake at V1, kcal/d (X ± SE) | 1526 ± 38 | 1552 ± 38 | 1535 ± 41 | 0.893 |
| Energy intake at V2, kcal/d (X ± SE) | 1551 ± 36 | 1608 ± 37 | 1536 ± 35 | 0.333 |
| Energy intake (mean), kcal/d (X ± SE) 5 | 1539 ± 32 | 1580 ± 32 | 1535 ± 31 | 0.546 |
| Model 1: CES-D (Mean) Tertiles 1 | Model 2: CES-D (Mean) Tertiles 1 | ||||||
|---|---|---|---|---|---|---|---|
| β ± SE (T2 vs. T1) | β ± SE (T3 vs. T1) | p-Trend 2 | β ± SE (T2 vs. T1) | β ± SE (T3 vs. T1) | p-Trend 2 | ||
| DASH (mean) total score | |||||||
| Overall | N = 1458 | −0.22 ± 0.06 | −0.21 ± 0.06 | 0.001 | −0.15 ± 0.06 | −0.10 ± 0.07 | 0.116 |
| Men | N = 592 | −0.18 ± 0.09 | −0.23 ± 0.10 | 0.013 | −0.12 ± 0.09 | −0.12 ± 0.10 | 0.188 |
| Women | N = 866 | −0.25 ± 0.09 | −0.20 ± 0.09 | 0.023 | −0.21 ± 0.09 | −0.11 ± 0.09 | 0.224 |
| Whites | N = 579 | −0.27 ± 0.11 | −0.32 ± 0.11 | 0.004 | −0.17 ± 0.11 | −0.13 ± 0.12 | 0.263 |
| AA | N = 879 | −0.17 ± 0.07 | −0.14 ± 0.08 | 0.057 | −0.16 ± 0.08 | −0.10 ± 0.08 | 0.189 |
| Above poverty | N = 862 | −0.24 ± 0.08 | −0.29 ± 0.09 | 0.001 | −0.16 ± 0.08 | −0.19 ± 0.09 | 0.024 |
| Below poverty | N = 596 | −0.13 ± 0.10 | −0.01 ± 0.10 | 0.768 | −0.14 ± 0.10 | +0.02 ± 0.10 | 0.706 |
| Overall | Men | Women | Whites | African Americans | Below Poverty | Above Poverty | |
|---|---|---|---|---|---|---|---|
| BMI | (n = 1458; k = 1.8) | (n = 592; k = 1.8) | (n = 866; k = 1.8) | (n = 579; k = 1.8) | (n = 879; k = 1.8) | (n = 596; k = 1.8) | (n = 862; k = 1.8) |
| Model 1: | |||||||
| TIME | +0.32 ± 0.15 * | +0.29 ± 0.20 | +0.51 ± 0.20 * | +0.42 ± 0.18 | +0.28 ± 0.39 | +0.38 ± 0.23 | +0.36 ± 0.34 |
| CES-D(mean) | +0.01 ± 0.02 | −0.01 ± 0.03 | +0.02 ± 0.03 | −0.02 ± 0.03 | +0.03 ± 0.03 | −0.01 ± 0.03 | +0.02 ± 0.03 |
| CES-D(mean) × TIME | +0.001 ± 0.003 | −0.007 ± 0.004 | +0.004 ± 0.003 | +0.002 ± 0.003 | +0.001 ± 0.003 | +0.005 ± 0.004 | −0.004 ± 0.003 |
| DASH(mean) | −0.55 ± 0.20 **,2 | −0.13 ± 0.29 | −0.75 ± 0.27 **,2 | −0.94 ± 0.28 **,2 | −0.05 ± 0.27 | −0.96 ± 0.34 **,2 | −0.27 ± 0.24 |
| DASH(mean) × TIME | −0.04 ± 0.02 | −0.02 ± 0.03 | −0.04 ± 0.03 | −0.04 ± 0.03 | −0.05 ± 0.03 | −0.09 ± 0.04 *,2 | −0.02 ± 0.02 |
| Model 2: | |||||||
| TIME | +0.31 ± 0.15 * | +0.29 ± 0.20 | +0.50 ± 0.20 * | +0.42 ± 0.18 * | +0.30 ± 0.39 | +0.38 ± 0.23 | +0.37 ± 0.34 |
| CES-D(mean) | +0.01 ± 0.02 | −0.01 ± 0.03 2 | +0.02 ± 0.03 | −0.02 ± 0.03 | +0.03 ± 0.03 | −0.01 ± 0.03 | +0.02 ± 0.03 |
| CES-D (mean) × TIME | +0.001 ± 0.003 | −0.007 ± 0.004 2 | +0.005 ± 0.003 | +0.003 ± 0.003 | +0.001 ± 0.003 | +0.005 ± 0.004 2 | −0.005 ± 0.003 |
| Model 3: | |||||||
| TIME | +0.32 ± 0.15 * | +0.22 ± 0.20 | +0.56 ± 0.19 ** | +0.45 ± 0.18 * | +0.29 ± 0.39 | +0.44 ± 0.22 | +0.32 ± 0.34 |
| DASH(mean) | −0.55 ± 0.20 **,2 | −0.13 ± 0.29 | −0.76 ± 0.27 **,2 | −0.93 ± 0.28 **,2 | −0.05 ± 0.27 | −0.96 ± 0.34 ** | −0.28 ± 0.24 |
| DASH(mean) × TIME | −0.04 ± 0.02 | −0.02 ± 0.04 | −0.04 ± 0.03 | −0.04 ± 0.03 | −0.05 ± 0.03 | −0.09 ± 0.04 * | −0.02 ± 0.02 |
| Controlled Direct Effect | Natural Direct Effect | Natural Indirect Effect | Marginal Total Effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | (SEE) | Pwald | β | (SEE) | Pwald | β | (SEE) | Pwald | β | (SEE) | Pwald | |
| CES-D→DASH→BMI 2 | ||||||||||||
| Overall sample, n = 1201 | −0.007 | 0.030 | 0.82 | −0.007 | 0.030 | 0.82 | +0.003 | 0.005 | 0.59 | −0.004 | 0.030 | 0.89 |
| Men, n = 469 | −0.060 | 0.050 | 0.22 | −0.060 | 0.050 | 0.18 | +0.001 | 0.003 | 0.76 | −0.060 | 0.050 | 0.19 |
| Women, n = 732 | +0.030 | 0.040 | 0.46 | +0.024 | 0.042 | 0.57 | +0.003 | 0.008 | 0.72 | +0.027 | 0.042 | 0.53 |
| Whites, n = 587 | −0.010 | 0.047 | 0.82 | −0.030 | 0.046 | 0.52 | +0.009 | 0.012 | 0.44 | −0.020 | 0.047 | 0.67 |
| African Americans, n = 714 | +0.017 | 0.042 | 0.68 | +0.017 | 0.042 | 0.69 | +0.001 | 0.002 | 0.74 | +0.017 | 0.042 | 0.68 |
| Below poverty, n = 483 | +0.013 | 0.047 | 0.79 | +0.018 | 0.047 | 0.70 | −0.003 | 0.008 | 0.66 | +0.014 | 0.047 | 0.76 |
| Above poverty, n = 718 | −0.034 | 0.043 | 0.42 | −0.035 | 0.043 | 0.41 | +0.004 | 0.005 | 0.35 | −0.031 | 0.043 | 0.47 |
| DASH→CES-D→BMI 3 | ||||||||||||
| Overall sample, n = 1201 | −0.14 | 0.03 | <0.001 | −0.14 | 0.03 | <0.001 | +0.001 | 0.001 | 0.63 | −0.14 | 0.03 | <0.001 |
| Men, n = 469 | −0.05 | 0.05 | 0.26 | −0.06 | 0.05 | 0.22 | +0.001 | 0.002 | 0.73 | −0.06 | 0.05 | 0.23 |
| Women, n = 732 | −0.14 | 0.04 | <0.001 | −0.15 | 0.04 | <0.001 | +0.001 | 0.002 | 0.75 | −0.15 | 0.04 | <0.001 |
| Whites, n = 587 | −0.18 | 0.04 | <0.001 | −0.18 | 0.04 | <0.001 | +0.003 | 0.004 | 0.48 | −0.18 | 0.04 | <0.001 |
| African Americans, n = 714 | −0.03 | 0.04 | 0.43 | −0.03 | 0.04 | 0.43 | −0.001 | 0.002 | 0.76 | −0.04 | 0.04 | 0.42 |
| Below poverty, n = 483 | −0.19 | 0.05 | 0.001 | −0.19 | 0.05 | <0.0001 | −0.001 | 0.002 | 0.76 | −0.20 | 0.05 | <0.0001 |
| Above poverty, n = 718 | −0.07 | 0.04 | 0.071 | −0.07 | 0.04 | 0.066 | +0.001 | 0.002 | 0.48 | −0.07 | 0.04 | 0.072 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanelli Kuczmarski, M.; Hossain, S.; Beydoun, M.A.; Maldonando, A.; Evans, M.K.; Zonderman, A.B. Association of DASH and Depressive Symptoms with BMI over Adulthood in Racially and Socioeconomically Diverse Adults Examined in the HANDLS Study. Nutrients 2019, 11, 2934. https://doi.org/10.3390/nu11122934
Fanelli Kuczmarski M, Hossain S, Beydoun MA, Maldonando A, Evans MK, Zonderman AB. Association of DASH and Depressive Symptoms with BMI over Adulthood in Racially and Socioeconomically Diverse Adults Examined in the HANDLS Study. Nutrients. 2019; 11(12):2934. https://doi.org/10.3390/nu11122934
Chicago/Turabian StyleFanelli Kuczmarski, Marie, Sharmin Hossain, May A. Beydoun, Ana Maldonando, Michele K. Evans, and Alan B. Zonderman. 2019. "Association of DASH and Depressive Symptoms with BMI over Adulthood in Racially and Socioeconomically Diverse Adults Examined in the HANDLS Study" Nutrients 11, no. 12: 2934. https://doi.org/10.3390/nu11122934
APA StyleFanelli Kuczmarski, M., Hossain, S., Beydoun, M. A., Maldonando, A., Evans, M. K., & Zonderman, A. B. (2019). Association of DASH and Depressive Symptoms with BMI over Adulthood in Racially and Socioeconomically Diverse Adults Examined in the HANDLS Study. Nutrients, 11(12), 2934. https://doi.org/10.3390/nu11122934





