Stimulatory Response of Celiac Disease Peripheral Blood Mononuclear Cells Induced by RNAi Wheat Lines Differing in Grain Protein Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) Quantification of SSP Proteins
2.3. Total Protein and NGP Quantification
2.4. Liquid Chromatography–Tandem Mass Spectrometry Analysis
2.5. Gluten Content Determination by Competitive Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Peripheral Blood Mononuclear Cell Proliferation and interferon (IFN)-γ Production Analysis
2.7. Statistics
3. Results
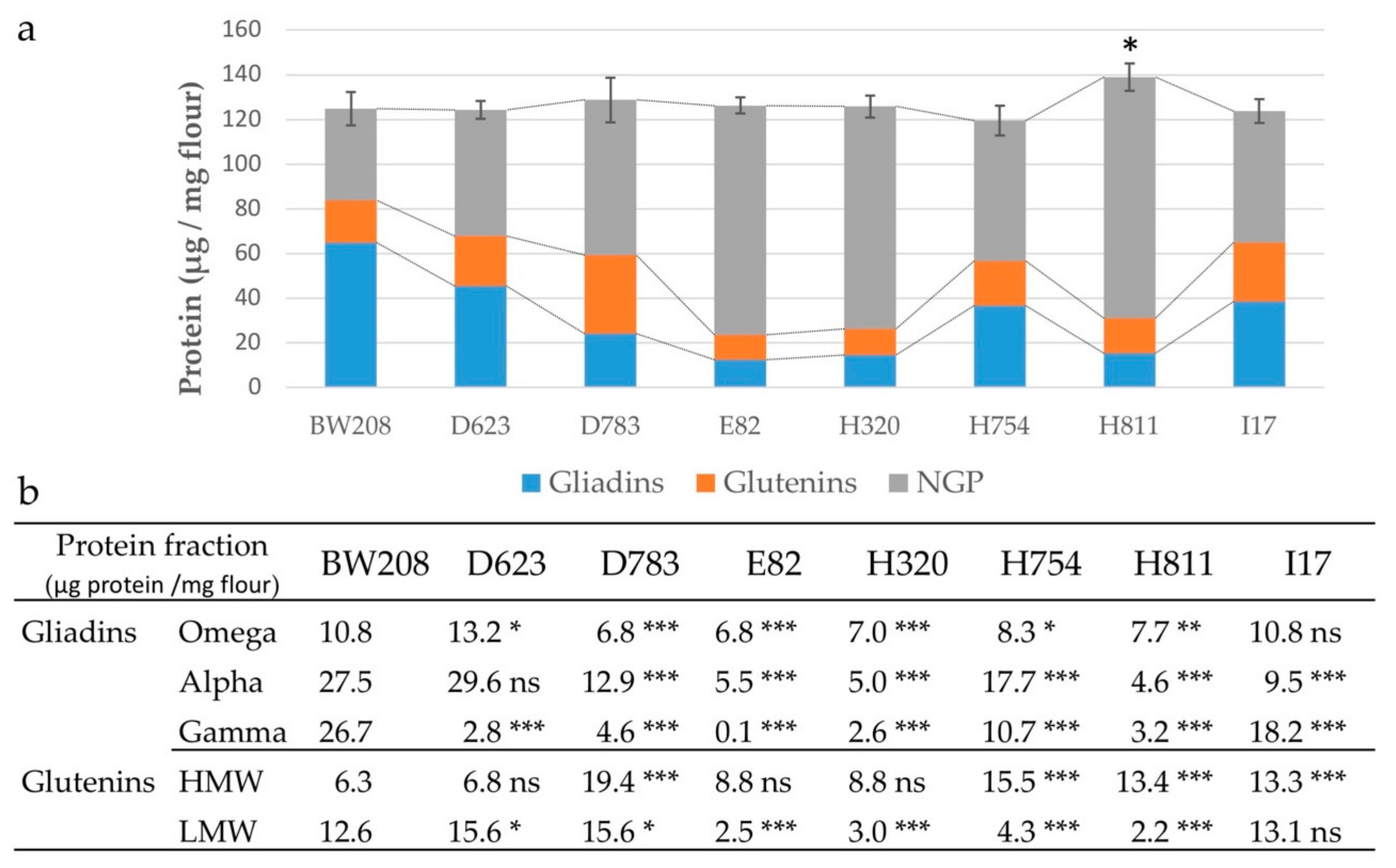
3.1. Grain Protein Composition of RNAi Lines
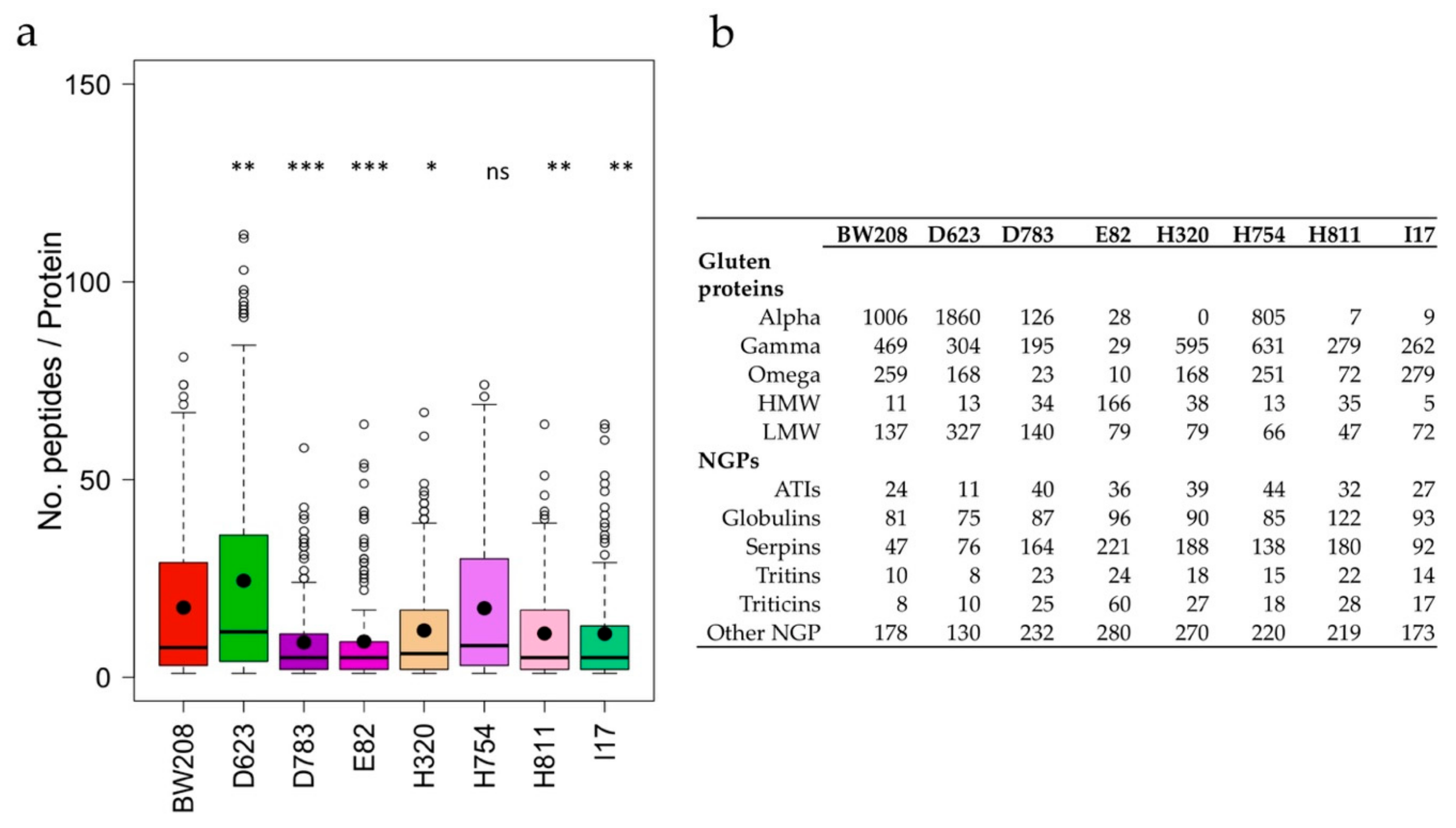
3.2. Analysis of Pepsin and Trypsin Protein Extract Digestion (PT-Digestion)
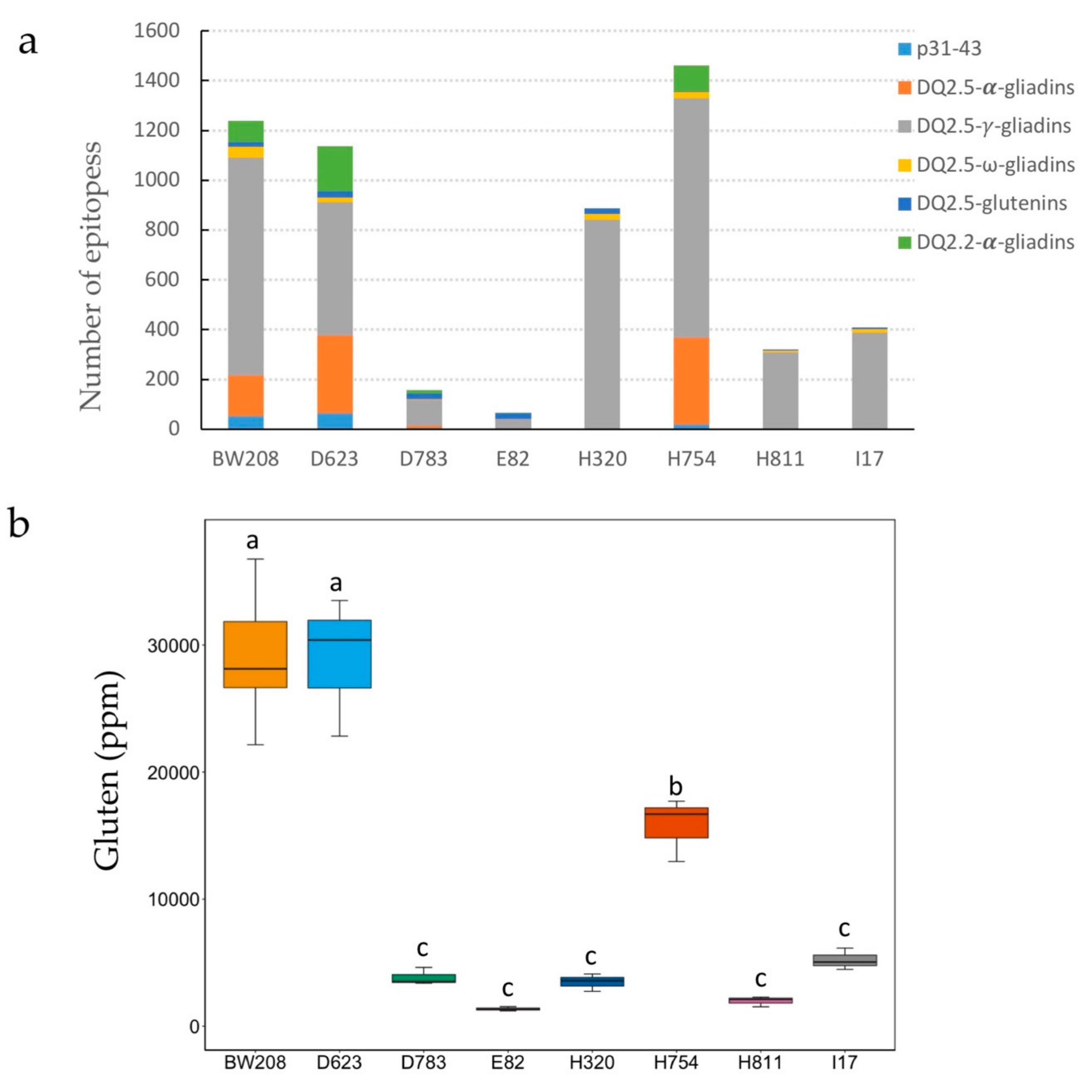
3.3. Gluten Immunogenicity by G12 moAb
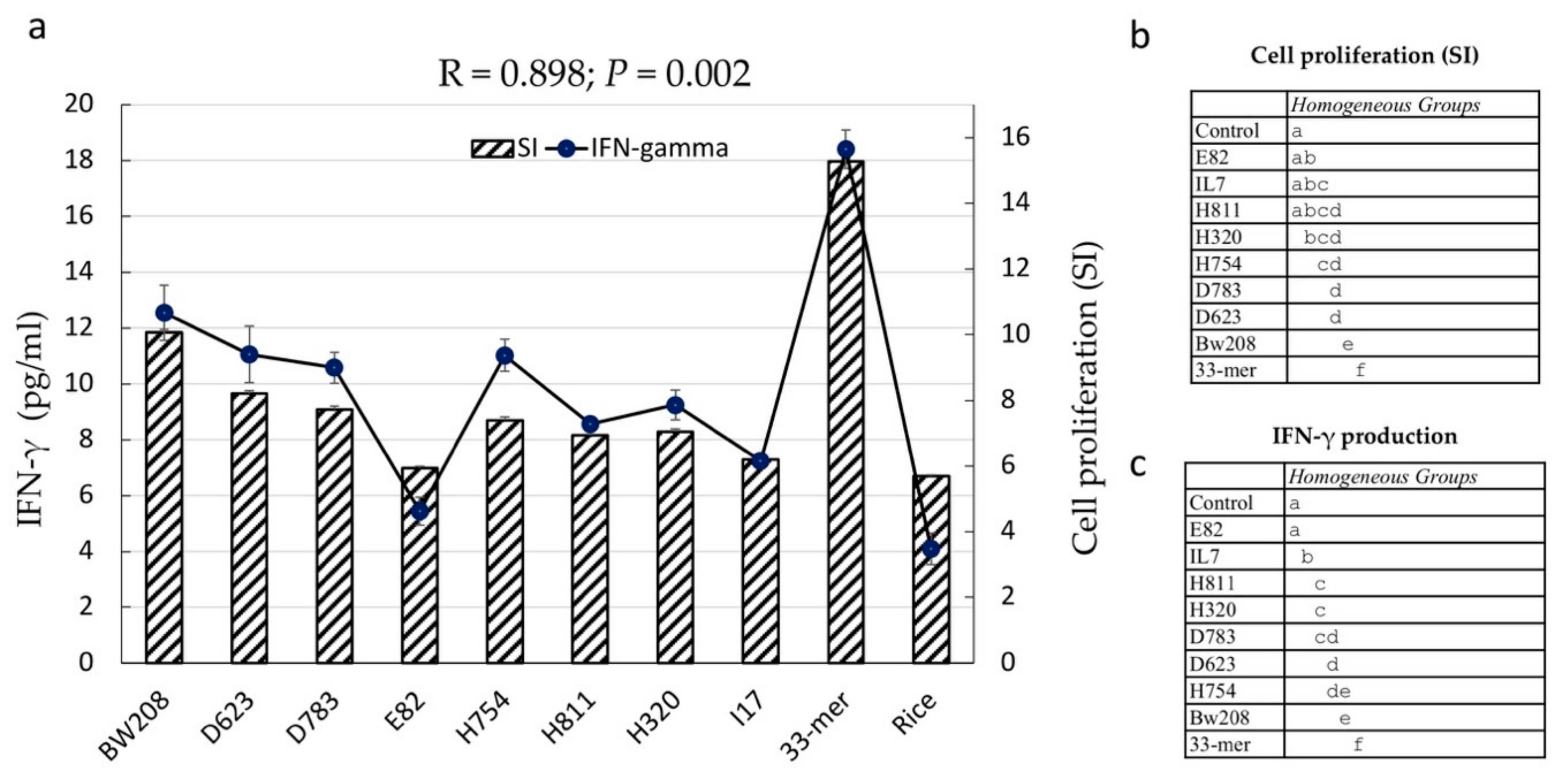
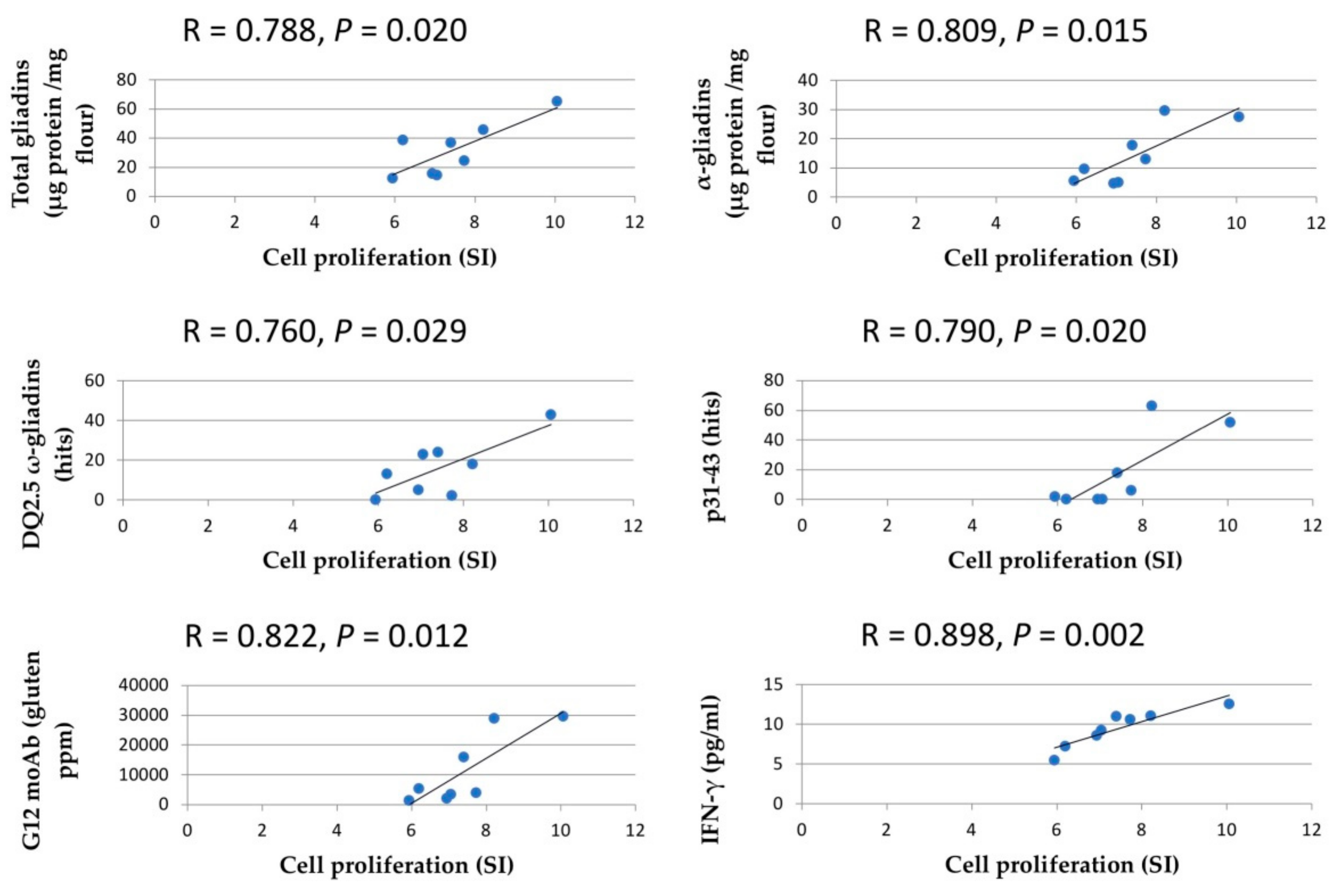
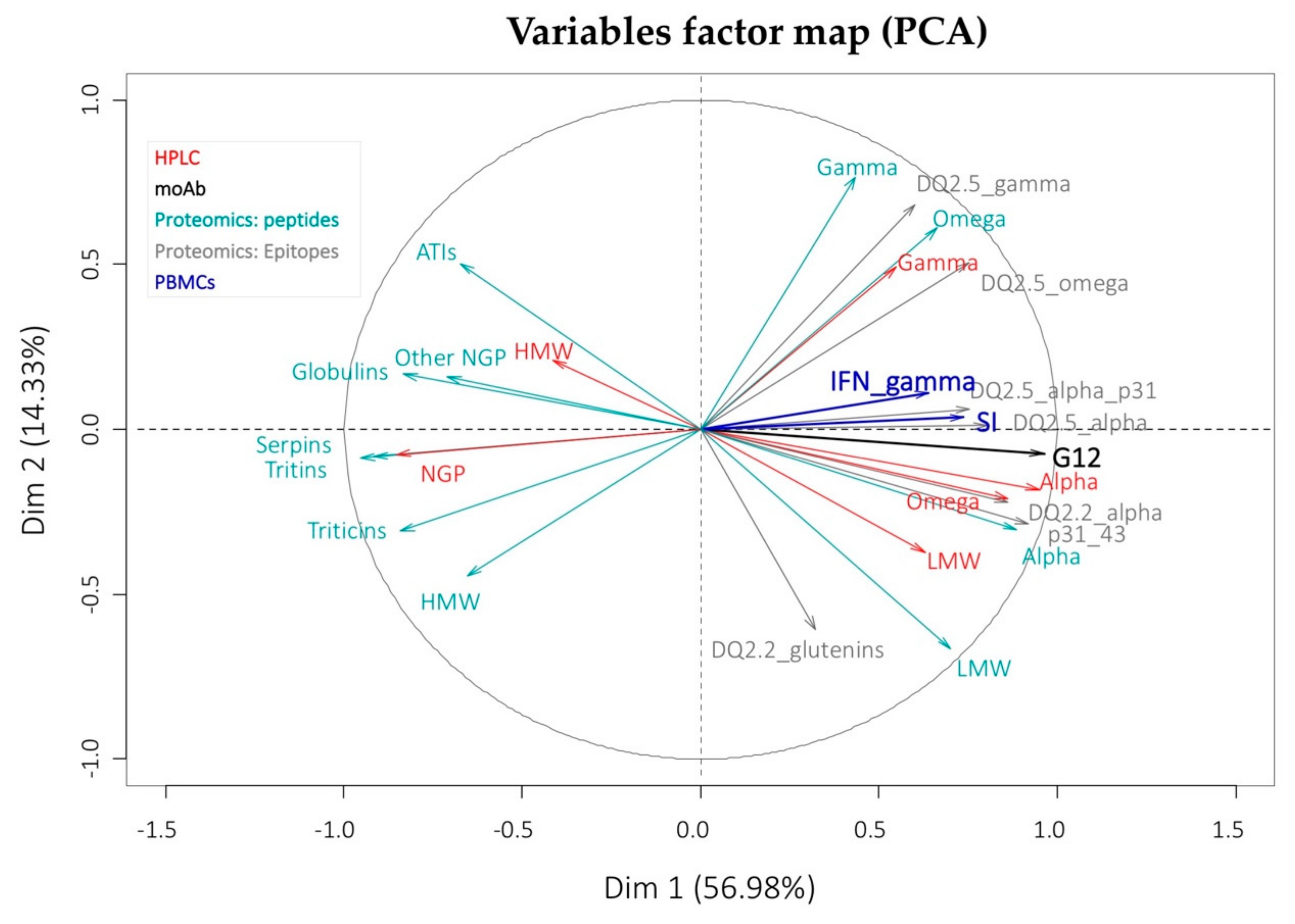
3.4. Stimulatory Response of PBMCs from CD Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- Choung, R.S.; Larson, S.A.; Khaleghi, S.; Rubio-Tapia, A.; Ovsyannikova, I.G.; King, K.S.; Larson, J.J.; Lahr, B.D.; Poland, G.A.; Camilleri, M.J.; et al. Prevalence and Morbidity of Undiagnosed Celiac Disease From a Community-Based Study. Gastroenterology 2017, 152, 830–839.e835. [Google Scholar] [CrossRef]
- Junker, Y.; Zeissig, S.; Kim, S.-J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Tjon, J.M.; van Bergen, J.; Koning, F. Celiac disease: How complicated can it get? Immunogenetics 2010, 62, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. The changing immunological paradigm in coeliac disease. Immunol. Lett. 2006, 105, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, L.; Ciacci, C.; Auricchio, S.; Brown, V.; Quaratino, S.; Londei, M. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology 2000, 119, 996–1006. [Google Scholar] [CrossRef]
- Maiuri, L.; Ciacci, C.; Ricciardelli, I.; Vacca, L.; Raia, V.; Auricchio, S.; Picard, J.; Osman, M.; Quaratino, S.; Londei, M. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet 2003, 362, 30–37. [Google Scholar] [CrossRef]
- Tollefsen, S.; Arentz-Hansen, H.; Fleckenstein, B.; Molberg, Ø.; Ráki, M.; Kwok, W.W.; Jung, G.; Lundin, K.E.A.; Sollid, L.M. HLA-DQ2 and -DQ8 signatures of gluten T cell epitopes in celiac disease. J. Clin. Investig. 2006, 116, 2226–2236. [Google Scholar] [CrossRef]
- Holtmeier, W.; Caspary, W.F. Celiac disease. Orphanet J. Rare Dis. 2006, 1, 3. [Google Scholar] [CrossRef]
- Arentz–Hansen, H.; McAdam, S.N.; Molberg, Ø.; Fleckenstein, B.; Lundin, K.E.A.; Jørgensen, T.J.D.; Jung, G.; Roepstorff, P.; Sollid, L.M. Celiac lesion T cells recognize epitopes that cluster in regions of gliadins rich in proline residues. Gastroenterology 2002, 123, 803–809. [Google Scholar] [CrossRef]
- Shan, L.; Molberg, Ø.; Parrot, I.; Hausch, F.; Filiz, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural Basis for Gluten Intolerance in Celiac Sprue. Science 2002, 297, 2275. [Google Scholar] [CrossRef] [PubMed]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.B.S.; Roager, H.M.; Søndertoft, N.B.; Gøbel, R.J.; Kristensen, M.; Vallès-Colomer, M.; Vieira-Silva, S.; Ibrügger, S.; Lind, M.V.; Mærkedahl, R.B.; et al. A low-gluten diet induces changes in the intestinal microbiome of healthy Danish adults. Nat. Commun. 2018, 9, 4630. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Denli, A.M.; Hannon, G.J. The rest is silence. RNA 2001, 7, 1509–1521. [Google Scholar] [PubMed]
- Hammond, S.M.; Bernstein, E.; Beach, D.; Hannon, G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000, 404, 293–296. [Google Scholar] [CrossRef]
- Gil-Humanes, J.; Pistón, F.; Hernando, A.; Alvarez, J.B.; Shewry, P.R.; Barro, F. Silencing of γ-gliadins by RNA interference (RNAi) in bread wheat. J. Cereal Sci. 2008, 48, 565–568. [Google Scholar] [CrossRef]
- Barro, F.; Iehisa, J.C.M.; Giménez, M.J.; García-Molina, M.D.; Ozuna, C.V.; Comino, I.; Sousa, C.; Gil-Humanes, J. Targeting of prolamins by RNAi in bread wheat: Effectiveness of seven silencing-fragment combinations for obtaining lines devoid of coeliac disease epitopes from highly immunogenic gliadins. Plant Biotechnol. J. 2016, 14, 986–996. [Google Scholar] [CrossRef]
- Gil-Humanes, J.; Pistón, F.; Tollefsen, S.; Sollid, L.M.; Barro, F. Effective shutdown in the expression of celiac disease-related wheat gliadin T-cell epitopes by RNA interference. Proc. Natl. Acad. Sci. USA 2010, 107, 7023–17028. [Google Scholar] [CrossRef]
- García-Molina, M.D.; Muccilli, V.; Saletti, R.; Foti, S.; Masci, S.; Barro, F. Comparative proteomic analysis of two transgenic low-gliadin wheat lines and non-transgenic wheat control. J. Proteom. 2017, 165, 102–112. [Google Scholar] [CrossRef]
- Pistón, F.; Gil-Humanes, J.; Barro, F. Integration of promoters, inverted repeat sequences and proteomic data into a model for high silencing efficiency of coeliac disease related gliadins in bread wheat. BMC Plant Biol. 2013, 13, 136. [Google Scholar] [CrossRef]
- Volta, U.; Caio, G.; Tovoli, F.; De Giorgio, R. Non-celiac gluten sensitivity: Questions still to be answered despite increasing awareness. Cell. Mol. Immunol. 2013, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Pistón, F.; Gil-Humanes, J.; Rodríguez-Quijano, M.; Barro, F. Down-regulating γ-gliadins in bread wheat leads to non-specific increases in other gluten proteins and has no major effect on dough gluten strength. PLoS ONE 2011, 6, e24754. [Google Scholar] [CrossRef] [PubMed]
- Pistón, F.; León, E.; Lazzeri, P.A.; Barro, F. Isolation of two storage protein promoters from Hordeum chilense and characterization of their expression patterns in transgenic wheat. Euphytica 2008, 162, 371–379. [Google Scholar] [CrossRef]
- Sollid, L.M.; Qiao, S.-W.; Anderson, R.P.; Gianfrani, C.; Koning, F. Nomenclature and listing of celiac disease relevant gluten T-cell epitopes restricted by HLA-DQ molecules. Immunogenetics 2012, 64, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.N. Gluten, major histocompatibility complex, and the small intestine: A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992, 102, 330–354. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185. [Google Scholar] [CrossRef]
- Morón, B.; Cebolla, Á.; Manyani, H.; Álvarez-Maqueda, M.; Megías, M.; Thomas, M.d.C.; López, M.C.; Sousa, C. Sensitive detection of cereal fractions that are toxic to celiac disease patients by using monoclonal antibodies to a main immunogenic wheat peptide. Am. J. Clin. Nutr. 2008, 87, 405–414. [Google Scholar] [CrossRef]
- Altenbach, S.B.; Chang, H.-C.; Yu, X.B.; Seabourn, B.W.; Green, P.H.; Alaedini, A. Elimination of Omega-1,2 Gliadins From Bread Wheat (Triticum aestivum) Flour: Effects on Immunogenic Potential and End-Use Quality. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Wen, S.; Wen, N.; Pang, J.; Langen, G.; Brew-Appiah, R.A.T.; Mejias, J.H.; Osorio, C.; Yang, M.; Gemini, R.; Moehs, C.P.; et al. Structural genes of wheat and barley 5-methylcytosine DNA glycosylases and their potential applications for human health. Proc. Natl. Acad. Sci. USA 2012, 109, 20543–20548. [Google Scholar] [CrossRef]
- Gil-Humanes, J.; Pistón, F.; Altamirano-Fortoul, R.; Real, A.; Comino, I.; Sousa, C.; Rosell, C.M.; Barro, F. Reduced-Gliadin Wheat Bread: An Alternative to the Gluten-Free Diet for Consumers Suffering Gluten-Related Pathologies. PLoS ONE 2014, 9, e90898. [Google Scholar] [CrossRef]
- Gil-Humanes, J.; Pistón, F.; Shewry, P.R.; Tosi, P.; Barro, F. Suppression of gliadins results in altered protein body morphology in wheat. J. Exp. Bot. 2011, 62, 4203–4213. [Google Scholar] [CrossRef] [PubMed]
- Larré, C.; Lupi, R.; Gombaud, G.; Brossard, C.; Branlard, G.; Moneret-Vautrin, D.A.; Rogniaux, H.; Denery-Papini, S. Assessment of allergenicity of diploid and hexaploid wheat genotypes: Identification of allergens in the albumin/globulin fraction. J. Proteom. 2011, 74, 1279–1289. [Google Scholar] [CrossRef]
- Matsuo, H.; Yokooji, T.; Taogoshi, T. Common food allergens and their IgE-binding epitopes. Allergol. Int. 2015, 64, 332–343. [Google Scholar] [CrossRef]
- Huebener, S.; Tanaka, C.K.; Uhde, M.; Zone, J.J.; Vensel, W.H.; Kasarda, D.D.; Beams, L.; Briani, C.; Green, P.H.R.; Altenbach, S.B.; et al. Specific Nongluten Proteins of Wheat Are Novel Target Antigens in Celiac Disease Humoral Response. J. Proteome Res. 2015, 14, 503–511. [Google Scholar] [CrossRef]
- Fallang, L.-E.; Bergseng, E.; Hotta, K.; Berg-Larsen, A.; Kim, C.-Y.; Sollid, L.M. Differences in the risk of celiac disease associated with HLA-DQ2.5 or HLA-DQ2.2 are related to sustained gluten antigen presentation. Nat. Immunol. 2009, 10, 1096. [Google Scholar] [CrossRef]
- Ozuna, C.V.; Iehisa, J.C.M.; Giménez, M.J.; Alvarez, J.B.; Sousa, C.; Barro, F. Diversification of the celiac disease α-gliadin complex in wheat: A 33-mer peptide with six overlapping epitopes, evolved following polyploidization. Plant J. 2015, 82, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Tye-Din, J.A.; Stewart, J.A.; Dromey, J.A.; Beissbarth, T.; van Heel, D.A.; Tatham, A.; Henderson, K.; Mannering, S.I.; Gianfrani, C.; Jewell, D.P.; et al. Comprehensive, Quantitative Mapping of T Cell Epitopes in Gluten in Celiac Disease. Sci. Transl. Med. 2010, 2, 41ra51. [Google Scholar] [CrossRef]
- Comino, I.; Real, A.; de Lourdes Moreno, M.; Montes, R.; Cebolla, Á.; Sousa, C. Immunological determination of gliadin 33-mer equivalent peptides in beers as a specific and practical analytical method to assess safety for celiac patients. J. Sci. Food Agric. 2013, 93, 933–943. [Google Scholar] [CrossRef]
- Morón, B.; Bethune, M.T.; Comino, I.; Manyani, H.; Ferragud, M.; López, M.C.; Cebolla, Á.; Khosla, C.; Sousa, C. Toward the Assessment of Food Toxicity for Celiac Patients: Characterization of Monoclonal Antibodies to a Main Immunogenic Gluten Peptide. PLoS ONE 2008, 3, e2294. [Google Scholar] [CrossRef] [PubMed]
- Real, A.; Comino, I.; Moreno, M.d.L.; López-Casado, M.Á.; Lorite, P.; Torres, M.I.; Cebolla, Á.; Sousa, C. Identification and In Vitro Reactivity of Celiac Immunoactive Peptides in an Apparent Gluten-Free Beer. PLoS ONE 2014, 9, e100917. [Google Scholar] [CrossRef]
- Moreno, M.d.L.; Muñoz-Suano, A.; López-Casado, M.Á.; Torres, M.I.; Sousa, C.; Cebolla, Á. Selective capture of most celiac immunogenic peptides from hydrolyzed gluten proteins. Food Chem. 2016, 205, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, N.; Tye-Din, J.A.; Hardy, M.Y.; Anderson, R.P. Ex-vivo whole blood secretion of interferon (IFN)-γ and IFN-γ-inducible protein-10 measured by enzyme-linked immunosorbent assay are as sensitive as IFN-γ enzyme-linked immunospot for the detection of gluten-reactive T cells in human leucocyte antigen (HLA)-DQ2·5+-associated coeliac disease. Clin. Exp. Immunol. 2014, 175, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Villatoro, M.; Vaquero, L.; Pastor, J.; Giménez, M.J.; Ozuna, C.V.; Sánchez-León, S.; García-Molina, M.D.; Segura, V.; Comino, I.; et al. The Dietary Intervention of Transgenic Low-Gliadin Wheat Bread in Patients with Non-Celiac Gluten Sensitivity (NCGS) Showed No Differences with Gluten Free Diet (GFD) but Provides Better Gut Microbiota Profile. Nutrients 2018, 10, 1964. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Vojdani, E. Gluten and non-gluten proteins of wheat as target antigens in autism, Crohn’s and celiac disease. J. Cereal Sci. 2017, 75, 252–260. [Google Scholar] [CrossRef]
- Østergaard, H.; Rasmussen, S.K.; Roberts, T.H.; Hejgaard, J. Inhibitory Serpins from Wheat Grain with Reactive Centers Resembling Glutamine-rich Repeats of Prolamin Storage Proteins: Cloning and characterization of five major molecular forms. J. Biol. Chem. 2000, 275, 33272–33279. [Google Scholar] [CrossRef]
| Line | Plasmid 1 | Plasmid 2 | Prolamin Target |
|---|---|---|---|
| BW208 | NA 1 | NA 1 | NA 1 |
| D623 | pghpg8.1 | NA 1 | γ-gliadin |
| D783 | pDhp_ω/α | NA 1 | ω-, α-, and γ-gliadin |
| E82 | pghpg8.1 | pDhp_ω/α | ω-, α-, and γ-gliadin |
| H320 | pDhp_α/βZR | pDhp_ω4ZR | ω- and α-gliadin |
| H754 | pDhp_ω8ZR | NA 1 | ω-gliadin |
| H811 | pDhp_α/βZR | pDhp_ω8ZR | LMW, ω- and α-gliadin |
| I17 | pDhp_α/βZR | NA 1 | α-gliadin |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-León, S.; Giménez, M.J.; Comino, I.; Sousa, C.; López Casado, M.Á.; Torres, M.I.; Barro, F. Stimulatory Response of Celiac Disease Peripheral Blood Mononuclear Cells Induced by RNAi Wheat Lines Differing in Grain Protein Composition. Nutrients 2019, 11, 2933. https://doi.org/10.3390/nu11122933
Sánchez-León S, Giménez MJ, Comino I, Sousa C, López Casado MÁ, Torres MI, Barro F. Stimulatory Response of Celiac Disease Peripheral Blood Mononuclear Cells Induced by RNAi Wheat Lines Differing in Grain Protein Composition. Nutrients. 2019; 11(12):2933. https://doi.org/10.3390/nu11122933
Chicago/Turabian StyleSánchez-León, Susana, María José Giménez, Isabel Comino, Carolina Sousa, Miguel Ángel López Casado, María Isabel Torres, and Francisco Barro. 2019. "Stimulatory Response of Celiac Disease Peripheral Blood Mononuclear Cells Induced by RNAi Wheat Lines Differing in Grain Protein Composition" Nutrients 11, no. 12: 2933. https://doi.org/10.3390/nu11122933
APA StyleSánchez-León, S., Giménez, M. J., Comino, I., Sousa, C., López Casado, M. Á., Torres, M. I., & Barro, F. (2019). Stimulatory Response of Celiac Disease Peripheral Blood Mononuclear Cells Induced by RNAi Wheat Lines Differing in Grain Protein Composition. Nutrients, 11(12), 2933. https://doi.org/10.3390/nu11122933






