Vitamin D Deficiency and Sarcopenia in Older Persons
Abstract
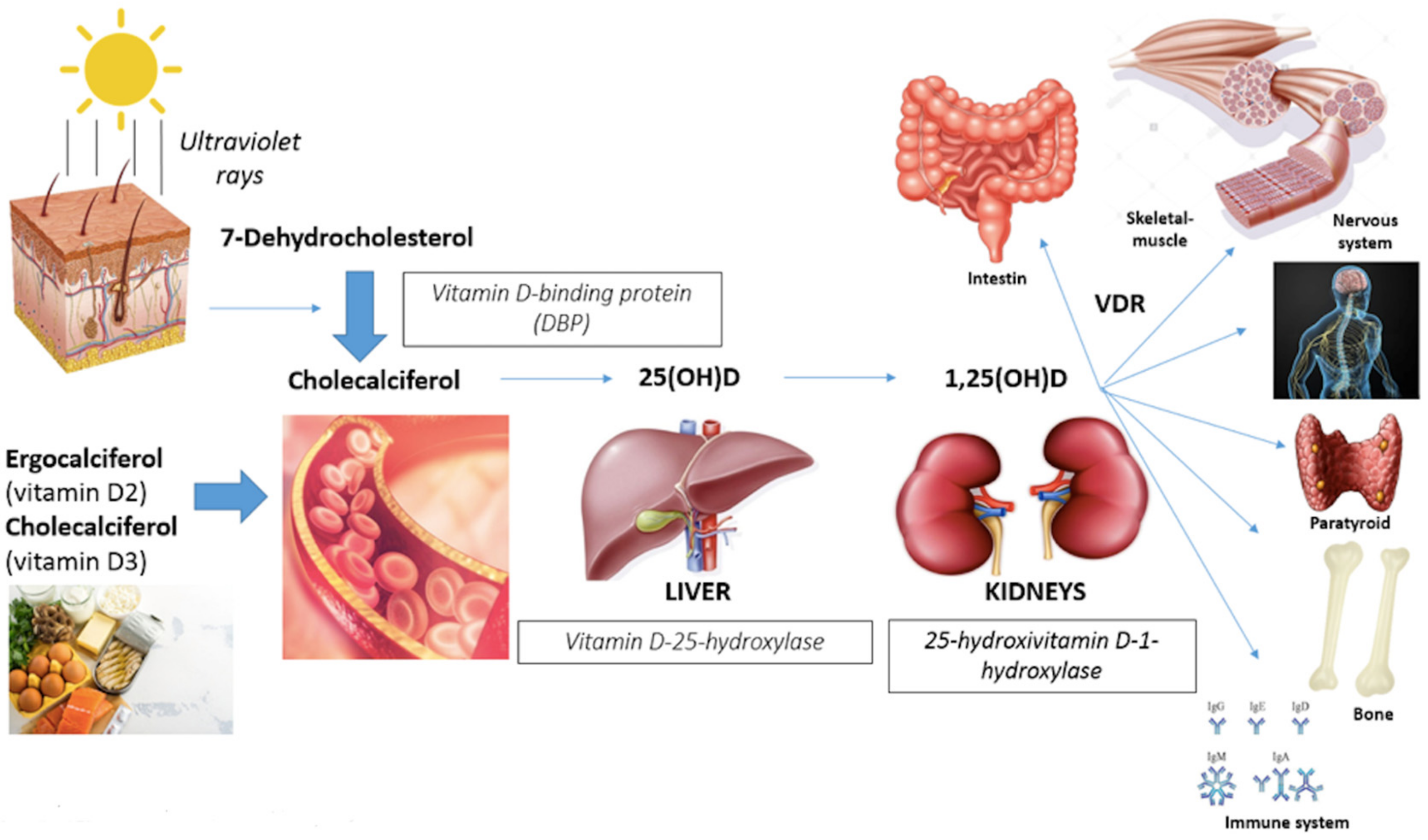
1. Introduction
- Gastrointestinal track (calcium absorption);
- Bone (induction of bone remodeling turnover with calcium deposition in newly-formed bone);
- Immune cells system (anti-inflammatory effects with suppression of interleukine-6 and neoplastic cells proliferation);
- Myocardium, vascular smooth muscles and endothelium (remodeling cardiac muscle and improving in flow-mediated dilatation and blood pressure);
- Nervous system (affecting neuronal differentiation, maturation and growth, neuroplasticity and neurotransmission);
2. Role of Vitamin D on the Skeletal Muscle System
2.1. Effects Vitamin D on Muscle Cell Types
- -
- Type I muscle cells are considered slow twitch, characterized by aerobic metabolism with low power production and high endurance capacity. They present a thick network of capillaries, important for carrying more oxygen, and a large quantity of myoglobin and mitochondria, for fat and carbohydrates’ oxidative phosphorylation. For these reasons, they have red color. Because of their lower strength and slow speed of contraction, they are essential for endurance exercise.
- -
- Type II muscle cells are defined as fast twitch, characterized by anaerobic metabolism with high speed and strength contraction, important for sprinting exercises. There are two major subtypes, divided according to speed and force generated: IIA identified as “fast twitch oxidative” with intermediate characteristics between type I and II (also these, in fact, have the red phenotype) and IIB defined as “fast twitch glicolytic” characterized by high power and low endurance. Only the latter have a pale color, due to a low number of mitochondria, lower amount of myoglobin and fewer capillaries.
2.2. Epidemiological Association between Vitamin D and Muscle Strength and Physical Performance
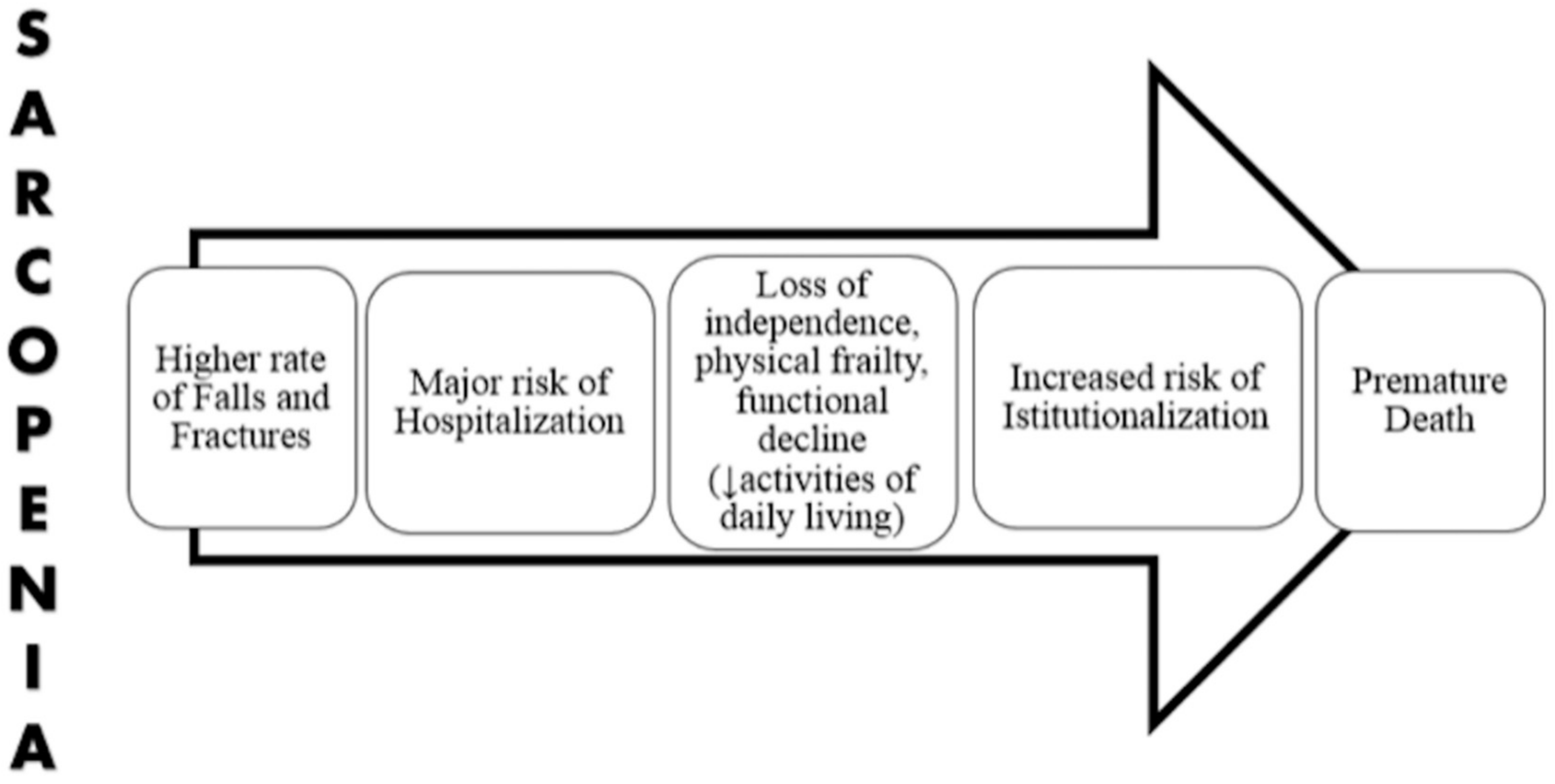
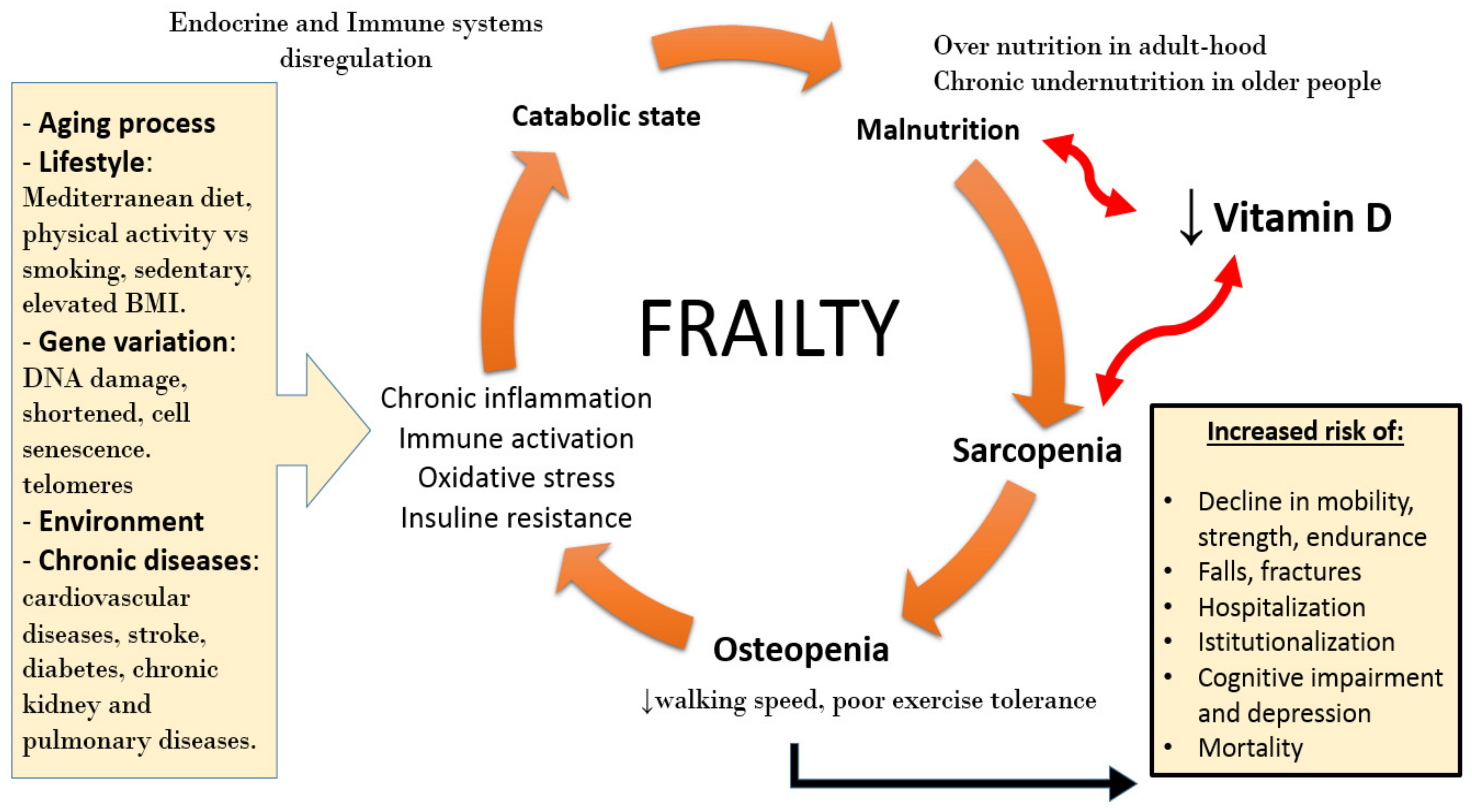
3. Sarcopenia and Frailty
4. Vitamin D Deficiency and Sarcopenia
5. Nutritional Intervention
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1,25(OH)D | 1,25-dihydroxyvitamin D |
| 25(OH)D | 25-dihydroxyvitamin D |
| 1-OHase | 25-hydroxyvitamin D-1-hydroxylase |
| VDR | Vitamin D receptor |
| VDREs | Vitamin D response elements |
| ASM | Appendicular skeletal muscle mass |
| BMI | Body mass index |
| BIA | Bioimpedentiometry |
| HMB | Hydroxymethylbutyrate |
| FI | Frailty Index |
| SPPB | Short Physical Performance Battery |
| TUG | Get Up and Go Test |
| PF&S | Physical frailty and sarcopenia |
References
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, P.; Lombardi, G.; Colombini, A.; Banfi, G. Vitamin D in exercise: Physiologic and analytical concerns. Clin. Chim. Acta 2013, 415, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.H.; He, D.H.; Zhou, B.; Zhu, Y.B.; Zhao, D.; Huang, L.C.; Ding, G.Q. Analysis of vitamin D status in men highly exposed to sunlight. Biomed. Environ. Sci. 2015, 28, 913–916. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D: A d-lightful solution for health. J. Investig. Med. 2011, 59, 872–880. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stöckle, U.; Ochs, G.; de Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial Role of Vitamin D in the Musculoskeletal System. Nutrients 2016, 8, 319. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.; Borchers, M.; Gudat, F.; Dürmüller, U.; Stähelin, H.; Dick, W. Vitamin D Receptor Expression in Human Muscle Tissue Decreases with Age. J. Bone Min. Res. 2004, 19, 265–269. [Google Scholar] [CrossRef]
- Ispoglou, T.; Deighton, K.; King, R.F.; White, H.; Lees, M. Novel essential amino acid supplements enriched with L-leucine facilitate increased protein and energy intakes in older women: A randomised controlled trial. Nutr. J. 2017, 16, 75. [Google Scholar] [CrossRef]
- Heath, K.M.; Elovic, E.P. Vitamin D deficiency—Implications in the rehabilitation setting. Am. J. Phys. Med. Rehab. 2006, 85, 916–923. [Google Scholar] [CrossRef]
- Shinchuck, L.; Holick, M.F. Vitamin D and rehabilitation: Improving functional outcomes. Nutr. Clin. Pract. 2007, 22, 297–304. [Google Scholar] [CrossRef]
- Sutton, A.L.M.; MacDonald, P.N. Vitamin D: More than a bone-a-fide hormone. Mol. Endocrinol. 2003, 17, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Deluca, H.F. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014, 3, 479. [Google Scholar] [CrossRef] [PubMed]
- Ceglia, L. Vitamin D and its role in skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Pojednic, R.M.; Ceglia, L. The emerging biomolecular role of vitamin D in skeletal muscle. Exerc. Sport Sci. Rev. 2014, 42, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A. Relevance of vitamin D in muscle health. Rev. Endocr. Metab. Disord. 2012, 13, 71–77. [Google Scholar] [CrossRef]
- Agergaard, J.; Trøstrup, J.; Uth, J.; Iversen, J.V.; Boesen, A.; Andersen, J.L.; Schjerling, P.; Langberg, H. Does vitamin D intake during resistance training improve the skeletal muscle hypertrophic and strength response in young and elderly men? A randomized controlled trial. Nutr. Metab. 2015, 30, 12–32. [Google Scholar] [CrossRef]
- Granic, A.; Hill, T.R.; Davies, K.; Jagger, C.; Adamson, A.; Siervo, M.; Kirkwood, T.B.L.; Mathers, J.C.; Sayer, A.A. Vitamin D status, Muscle Strenght and Physical Performance Decline in Very Old Adults: A Prospective study. Nutrients 2017, 9, 379. [Google Scholar] [CrossRef]
- Wang, Y.; DeLuca, H.F. Is the Vitamin D Receptor found in Muscle? Endocrinology 2010, 152, 354–363. [Google Scholar] [CrossRef]
- Olsson, K.; Saini, A.; Strömberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for vitamin D receptor expression and direct effects of 1α,25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology 2016, 157, 98–111. [Google Scholar] [CrossRef]
- Bischoff, H.A.; Borchers, M.; Gudat, F.; Duermueller, U.; Theiler, R.; Stähelin, H.B.; Dick, W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem. J. 2001, 33, 19–24. [Google Scholar] [CrossRef]
- Hassan-Smith, Z.K.; Jenkinson, C.; Smith, D.J.; Hernandez, I.; Morgan, S.A.; Crabtree, N.J.; Gittoes, N.J.; Keevil, B.G.; Stewart, P.M.; Hewison, M. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 exert distinct effects on human skeletal muscle function and gene expression. PLoS ONE 2017, 12, e0170665. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Seelander, M.; Sotiropoulos, A.; Coletti, D.; Lancha, A.H. Vitamin D, muscle recovery, sarcopenia, cachexia and muscle atrophy. Nutrition 2018, 60. [Google Scholar] [CrossRef] [PubMed]
- Lösel, R.; Wehling, M. Non genomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 2003, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Sharples, A.P.; Polydorou, I.; Alwan, N.; Donovan, T.; Tang, J.; Fraser, W.D.; Cooper, R.G.; Morton, J.P.; Stewart, C.; et al. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Jpn. J. Phys. Fit. Sports Med. 2015, 309, 1019–1031. [Google Scholar] [CrossRef]
- Garcia, L.A.; King, K.K.; Ferrini, M.G.; Norris, K.C.; Artaza, J.N. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 2011, 152, 2976–2986. [Google Scholar] [CrossRef]
- Angeline, M.E.; Gee, A.O.; Shindle, M.; Warren, R.F.; Rodeo, S.A. The effect of vitamin D deficiency in athletes. Am. J. Sports Med. 2013, 41, 461–464. [Google Scholar] [CrossRef]
- Birge, S.J.; Haddad, J.G. 25-hydroxycholecalciferol stimulation of muscle metabolism. J. Clin. Investig. 1975, 56, 1100–1107. [Google Scholar] [CrossRef]
- Girgis, C.M.; Cha, K.M.; So, B.; Tsang, M.; Chen, J.; Houweling, P.J.; Schindeler, A.; Stokes, R.; Swarbrick, M.M.; Evesson, F.J.; et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J. Cachexia Sarcopenia Muscle 2019. [Google Scholar] [CrossRef]
- Koundourakis, N.E.; Avgoustinaki, P.D.; Malliaraki, N.; Margioris, A.N. Muscular effects of Vitamin D in young athletes and non-athletes and in the elderly. Hormones 2016, 15, 471–488. [Google Scholar] [CrossRef]
- Shuler, F.D.; Wingate, M.K.; Moore, G.H.; Giangarra, C. Sports health benefits of vitamin D. Sports Health 2012, 4, 496–501. [Google Scholar] [CrossRef]
- Knutsen, K.V.; Brekke, M.; Gjelstad, S.; Lagerlov, P. Vitamin D status in patients with musculoskeletal pain, fatigue and headache: A cross-sectional descriptive study in a multi-ethnic general practice in Norway. Scand. J. Prim. Health 2010, 28, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Cesari, M.; Ferrucci, L.; Cherubini, A.; Maggio, D.; Bartali, B.; Johnson, M.A.; Schwartz, G.G.; Kritchevsky, S.B. Association between vitamin D status and physical performance: The InCHIANTI study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Brouwer-Brolsma, E.M.; Nienaber-Rousseau, C.; van Loon, L.J.C.; De Groot, L.C.P.G.M. Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur. J. Clin. Nutr. 2013, 67, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Tooze, J.A.; Hausman, D.B.; Johnson, M.A.; Nicklas, B.J.; Miller, M.E.; Neiberg, R.H.; Marsh, A.P.; Newman, A.B.; Blair, S.N.; et al. Change in 25-hydroxyvitamin D and physical performance in older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Tooze, J.A.; Neiberg, R.H.; Hausman, D.B.; Johnson, M.A.; Cauley, J.A.; Bauer, D.C.; Cawthon, P.M.; Shea, M.K.; Schwartz, G.G.; et al. 25-hydroxyvitamin D status and change in physical performance and strength in older adults: The Health, Aging, and Body Composition Study. Am. J. Epidemiol. 2012, 176, 1025–1034. [Google Scholar] [CrossRef]
- Annweiler, C.; Beauchet, O.; Berrut, G.; Fantino, B.; Bonnefoy, M.; Herrmann, F.R.; Schott, A.M. Is there an association between serum 25-hydroxyvitamin D concentration and muscle strength among older women? Results from baseline assessment of the EPIDOS study. J. Nutr. Health Aging 2009, 13, 90–95. [Google Scholar] [CrossRef]
- Matheï, C.; Pottelbergh, G.V.; Vaes, B.; Adriaensen, W.; Gruson, D.; Degryse, J.M. No relation between vitamin D status and physical performance in the oldest old: Results from the Belfrail study. Age Ageing 2013, 42, 186–190. [Google Scholar] [CrossRef]
- Sohl, E.; van Schoor, N.M. Vitamin D status is associated with functional limitations and functional decline in older individuals. J. Clin. Endocrinol. Metab. 2013, 98, 1483–1490. [Google Scholar] [CrossRef]
- Dam, T.T.; von Mühlen, D.; Barrett-Connor, E.L. Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos. Int. 2009, 20, 751–760. [Google Scholar] [CrossRef]
- Wicherts, I.S.; van Schoor, N.M.; Boeke, A.J.P.; Visser, M.; Deeg, D.J.H.; Smit, J.; Knol, D.L.; Lips, P. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007, 92, 2058–2065. [Google Scholar] [CrossRef]
- Houston, D.K.; Tooze, J.A.; Davis, C.C.; Chaves, P.H.M.; Hirsch, C.H.; Robbins, J.A.; Arnold, A.M.; Newman, A.B.; Kritchevsky, S.B. Serum 25-hydroxyvitamin D and physical function in older adults: The Cardiovascular Health Study All Stars. J. Am. Geriatr. Soc. 2011, 59, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.M.; Granic, A.; Davies, K.; Kirkwood, T.B.L.; Jagger, C.; Sayer, A.A. Prevalence and incidence of sarcopenia in the very old: Findings from the Newcastle 85+ study. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.R.; Granic, A.; Davies, K.; Collerton, J.; Martin-Ruiz, C.; Siervo, M.; Mathers, J.C.; Adamson, A.J.; Francis, R.M.; Pearce, S.H.; et al. Serum 25-hydroxyvitamin D concentration and its determinants in the very old: The Newcastle 85 + Study. Osteoporos. Int. 2016, 27, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J. What is sarcopenia? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1995, 50, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Bellelli, G.; Zambon, A.; Volpato, S.; Abete, P.; Bianchi, L.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; et al. The association between delirium and sarcopenia in older adult patients admit-ted to acute geriatrics units: Results from the GLISTEN multicenter observational study. Clin. Nutr. 2018, 37, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Ferrucci, L.; Cherubini, A.; Maggio, M.; Bandinelli, S.; Savino, E.; Brombo, G.; Zuliani, G.; Guralnik, J.M.; Landi, F.; et al. The Predictive Value of the EWGSOP Definition of Sarcopenia: Results from the InCHIANTI Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 259–264. [Google Scholar] [CrossRef]
- Bianchi, L.; Abete, P.; Bellelli, G.; Bo, O.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Manca, G.M.; Rizzo, M.R.; et al. Prevalence and Clinical Correlates of Sarcopenia, Identified According to the EWGSOP Definition and Diagnostic Algorithm, in Hospitalized Older People: The GLISTEN Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1575–1581. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.; Thompson, W.; James, L.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. IOF Committee of Scientific Advisors (CSA) Nutrition Working Group. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Bally, M.R.; Blaser Yildirim, P.Z.; Bounoure, L. Nutritional Support and Outcomes in Malnourished Medical Inpatients: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2016, 176, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Custureri, R.; Puntoni, M.; Bianchi, L.; Daragjati, J.; Garaboldi, S.; Simonato, M.; Greco, A.; Rizzo, E.; Santo, P.D.; et al. Effects of oral amino acid supplementation on Prognostic Index in hospitalized older patients: A multicenter randomized, double-blind, placebo-controlled pilot study. Clin. Interv. Aging 2018, 13, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Hirani, V.; Cumming, R.G.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Hsu, B.; Handelsman, D.J.; Waite, L.M.; Seibel, M.J. Longitudinal Associations Between Vitamin D Metabolites and Sarcopenia in Older Australian men: The Concord Health and Aging in Men Project. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, 146–156. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Cesari, M.; Calvani, R.; Msihid, J.; Tosato, M.; Rodriguez-Mañas, L.; Lattanzio, F.; Cherubini, A.; Bejuit, R.; Di Bari, M.; et al. The “Sarcopenia and Physical fRailty IN older people: Multi-componenT Treatment strategies” (SPRINTT) randomized controlled trial: Case finding, screening and characteristics of eligible participants. Exp. Gerontol. 2018, 113, 48–57. [Google Scholar] [CrossRef]
- Wagatsuma, A.; Sakuma, K. Vitamin D Signaling in Myogenesis: Potential for Treatment of Sarcopenia. Biomed. Res. Int. 2014, 2014, 121254. [Google Scholar] [CrossRef]
- Steffl, M.; Bohannon, R.W.; Sontakova, L.; Tufano, J.J.; Shiells, K.; Holmerova, I. Relationship between sarcopenia and physical activity in older people: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 835–845. [Google Scholar] [CrossRef]
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26. [Google Scholar] [CrossRef]
- Cummings, S.R.; Kiel, D.P.; Black, D.M. Vitamin D supplementation and increased risk of falling: A cautionary tale of vitamin supplements retold. JAMA Intern. Med. 2016, 176, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Endo, I.; Inoue, D.; Mitsui, T.; Umaki, Y.; Akaike, M.; Yoshizawa, T.; Kato, S.; Matsumoto, T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003, 144, 5138–5144. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W.; Johnson, C.A.; McCabe, G.P.; Carnell, N.S. Dietary protein requirements of younger and older adults. Am. J. Clin. Nutr. 2008, 88, 1322–1329. [Google Scholar] [PubMed]
- Huo, Y.R.; Suriyaarachchi, P.; Gomez, F.; Curcio, C.L.; Boersma, D.; Muir, S.W.; Montero-Odasso, M.; Gunawardene, P.; Demontiero, O.; Duque, G. Phenotype of osteosarcopenia in older individuals with a history of falling. J. Am. Med. Dir. Assoc. 2015, 16, 290–295. [Google Scholar] [CrossRef]
- Roubenoff, R. Sarcopenia: A Major modifiable cause of frailty in the Elderly. J. Nutr. Health Aging 1999, 4, 140–142. [Google Scholar]
- Latham, N.K.; Anderson, C.S.; Lee, A.; Bennett, D.A.; Moseley, A.; Cameron, I.D. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: The Frailty Interventions Trial in Elderly Subjects (FITNESS). J. Am. Geriatr. Soc. 2003, 51, 291–299. [Google Scholar] [CrossRef]
- Dawson-Hughes, B. Vitamin D and muscle function. J. Steroid Biochem. Mol. Biol. 2017, 173, 313–316. [Google Scholar] [CrossRef]
- Moreira-Pfrimer, L.D.F.; Pedrosa, M.A.C.; Teixeira, L.; Lazaretti-Castro, M. Treatment of Vitamin D Deficiency Increases Lower Limb Muscle Strength in Institutionalized Older People Independently of Regular Physical Activity: A Randomized Double-Blind Controlled Trial. Ann. Nutr. Metab. 2009, 54, 291–300. [Google Scholar] [CrossRef]
- Pfeifer, M.; Begerow, B.; Minne, H.; Suppan, K.; Fahrleitner-Pammer, A.; Dobnig, H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos. Int. 2009, 20, 315–322. [Google Scholar] [CrossRef]
- Iolascon, G.; Moretti, A.; de Sire, A.; Calafiore, D.; Gimigliano, F. Effectiveness of calcifediol in improving muscle function in post-menopausal women: A prospective cohort study. Adv. Ther. 2017, 34, 744–752. [Google Scholar] [CrossRef]
- Verlaan, S.; Maier, A.B.; Andrea, B.; Bauer, J.M.; Bautmans, I.; Brandt, K.; Donini, L.; Maggio, M.; McMurdoj, M.E.T.; Mets, T.; et al. Sufficient levels of 25-hydroxyvitamin D and protein intake required to increase muscle mass in sarcopenic older adults—The PROVIDE study. Clin. Nutr. 2018, 37, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Levis, S.; Gòmez-Marìn, O. Vitamin D and Physical Function in Sedentary Older Men. JAGS 2016, 65, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Fielding, R.A.; Dawson-Hughes, B. The effect of vitamin D supplementation on lower-extremity power and function in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Uusi-Rasi, K.; Patil, R.; Karinkanta, S.; Kannus, P.; Tokola, K.; Lamberg-Allardt, C.; Sievänen, H. Exercise and vitamin D in fall prevention among older women: A randomized clinical trial. JAMA 2015, 175, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Brolsma, E.M.; Bischoff-Ferrari, H.A.; Bouillon, R.; Feskens, E.J.; Gallagher, C.J.; Hypponen, E.; Llewellyn, D.J.; Stoecklin, E.; Dierkes, J.; Kies, A.K.; et al. Vitamin D: Do we get enough? A discussion between vitamin D experts in order to make a step towards the harmonisation of dietary reference intakes for vitamin D across Europe. Osteoporos. Int. 2013, 24, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv. Exp. Med. Biol. 2014, 810, 500–525. [Google Scholar]
| Author/Year | Study Design | Patient Characteristics | N Included in Analyses | Intervention | Control Group | Duration | Conclusions |
|---|---|---|---|---|---|---|---|
| Moreira-Primer et al. 2009 [74] | RCT * | Institutionalized people ≥ 60 y | 56 | 1000 mg calcium/day + 150.000 IU vitamin D/month (after 2 months: 90.000 IU/month) | 1000 mg calcium/day + placebo/month | 6 months | Strength muscle: improvement of hip flexors and knee extensors strength |
| Pfeifer et al. 2009 [75] | RCT | Community-dwelling people ≥ 70 y with 25(OH)D ≤ 78 nmol/L | 242 | 1000 mg calcium/day + 800 IU vitamin D/day | 1000 mg calcium/day | 12 months | Muscle strength and physical performance: improvement of hand grip strength and knee isometric extension strength, SPPB, TUG and 4-m walking speed |
| Iolascon et al. 2017 [76] | PCS | Post-menopausal women ≥ 50 y with osteoporosis and/or vitamin D deficiency | 113 | 20 μg vitamin D/day | - | 6 months | Muscle strength and physical performance: improvement of isometric leg extension strength and TUG |
| Verlaan et al. 2018 [61] | RCT | Sarcopenic older adults | 380 | 20 g protein (3 g leucine) + 3 g fat + 9 g carbohydrates + 800 IU vitamin D twice daily | Iso-caloric control product twice daily | 13 weeks | Muscle mass and physical performance: improvement of BIA and chair-stand test |
| Latham et al. 2003 [66] | RCT | Frail older people, after hospital discharge | 243 | Single dose of 300.000 IU | Placebo (single dose) | 10 weeks | Physical performance: no improvement of quadriceps resistance exercise |
| Levis et al. 2017 [72] | RCT | Sedentary men 65–90 y with 25(OH)D < 30 ng/mL and SPPB ≤ 9 | 130 | 4.000 IU vitamin D/day | Placebo/day | 9 months | Physical performance: no improvement of SPPB or gait speed |
| Shea et al. 2019 [73] | RCT | Community-dwelling people ≥ 60 y with 25(OH)D ≤ 20 ng/mL | 100 | 858 (+800) IU vitamin D/day | Placebo/day | 1 year | Lower-extremity power, strength and lean mass: no improvement of Keiser pneumatic leg press, backward tandem walk test, SPPB, dual X-ray |
| Uusi-Rasi et al. 2015 [74] | RCT | Home-dwelling women 70–80 y with at least 1 fall in the previous year and no use of vitamin D supplements | 409 | 800 IU vitamin D/day ± exercise | Placebo/day ± exercise | 2 years | Mass muscle, muscle strength and physical performance: no improvement of BIA and SPPB, TUG, 4-m walking speed and 5 times chair stand |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. https://doi.org/10.3390/nu11122861
Remelli F, Vitali A, Zurlo A, Volpato S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients. 2019; 11(12):2861. https://doi.org/10.3390/nu11122861
Chicago/Turabian StyleRemelli, Francesca, Aurora Vitali, Amedeo Zurlo, and Stefano Volpato. 2019. "Vitamin D Deficiency and Sarcopenia in Older Persons" Nutrients 11, no. 12: 2861. https://doi.org/10.3390/nu11122861
APA StyleRemelli, F., Vitali, A., Zurlo, A., & Volpato, S. (2019). Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients, 11(12), 2861. https://doi.org/10.3390/nu11122861





