Exercise Mitigates the Loss of Muscle Mass by Attenuating the Activation of Autophagy during Severe Energy Deficit
Abstract
:1. Introduction
2. Materials and Methods
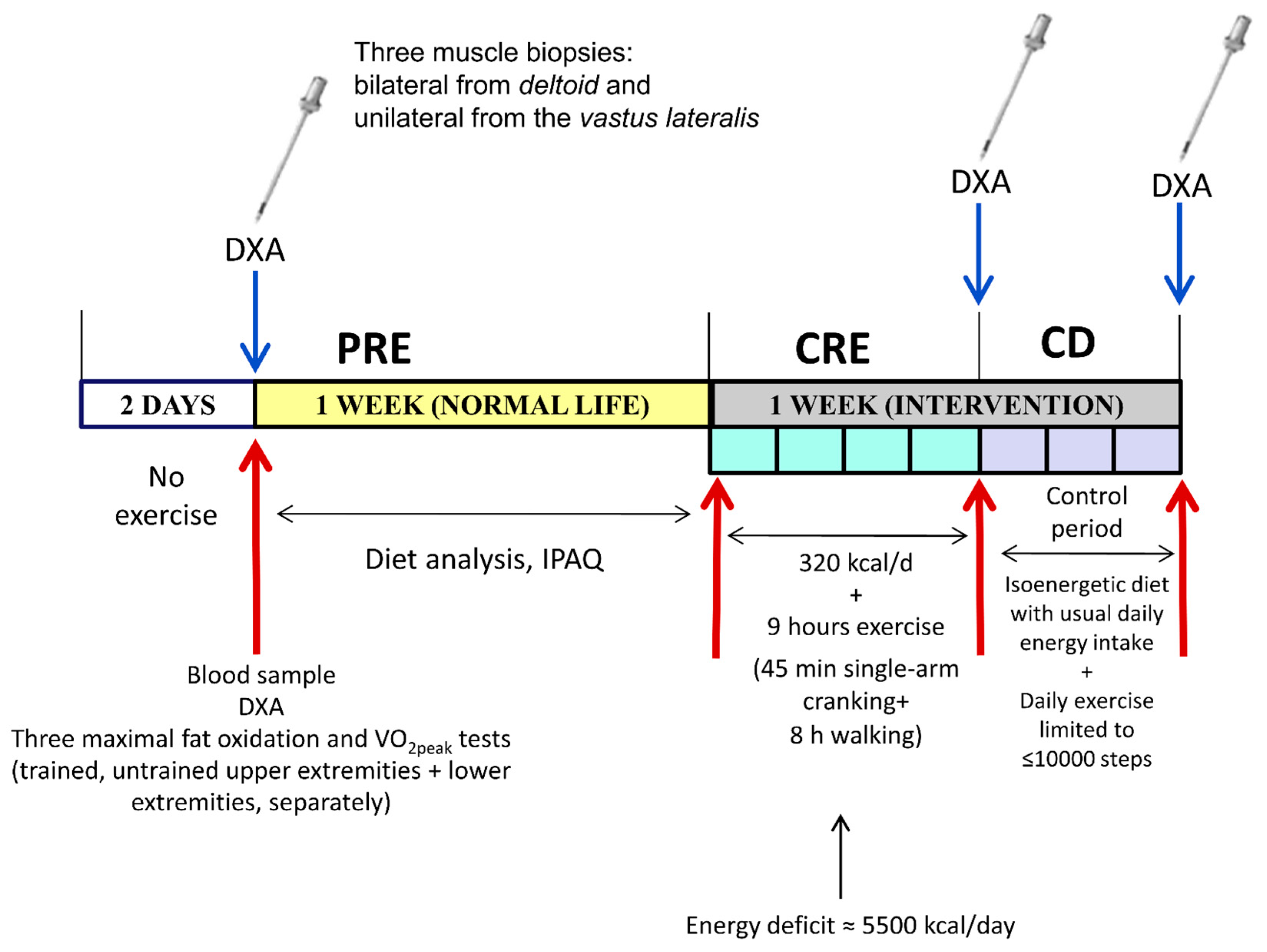
2.1. Experimental Protocol
2.2. Assessment of Physical Activity, Nutrition, and Body Composition
2.3. Hormonal and Biochemical Analyses
2.4. Biopsy Sampling
2.5. Protein Extraction and Western Blotting
2.6. Materials
2.7. Statistical Analysis
3. Results
3.1. Exercise Preserved Muscle Mass in a Dose-Dependent Fashion without Significant Changes in Protein Synthesis Signaling
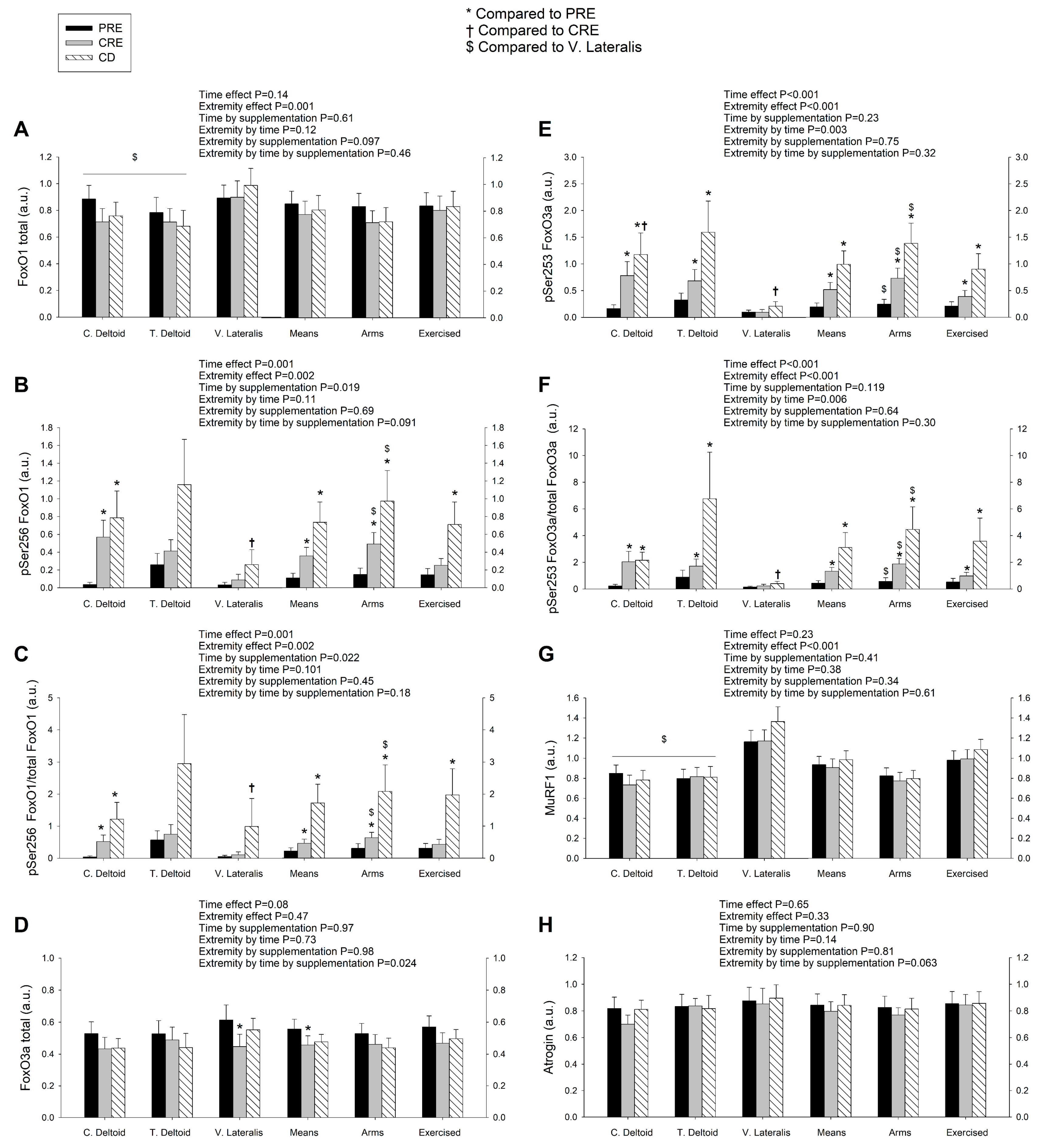
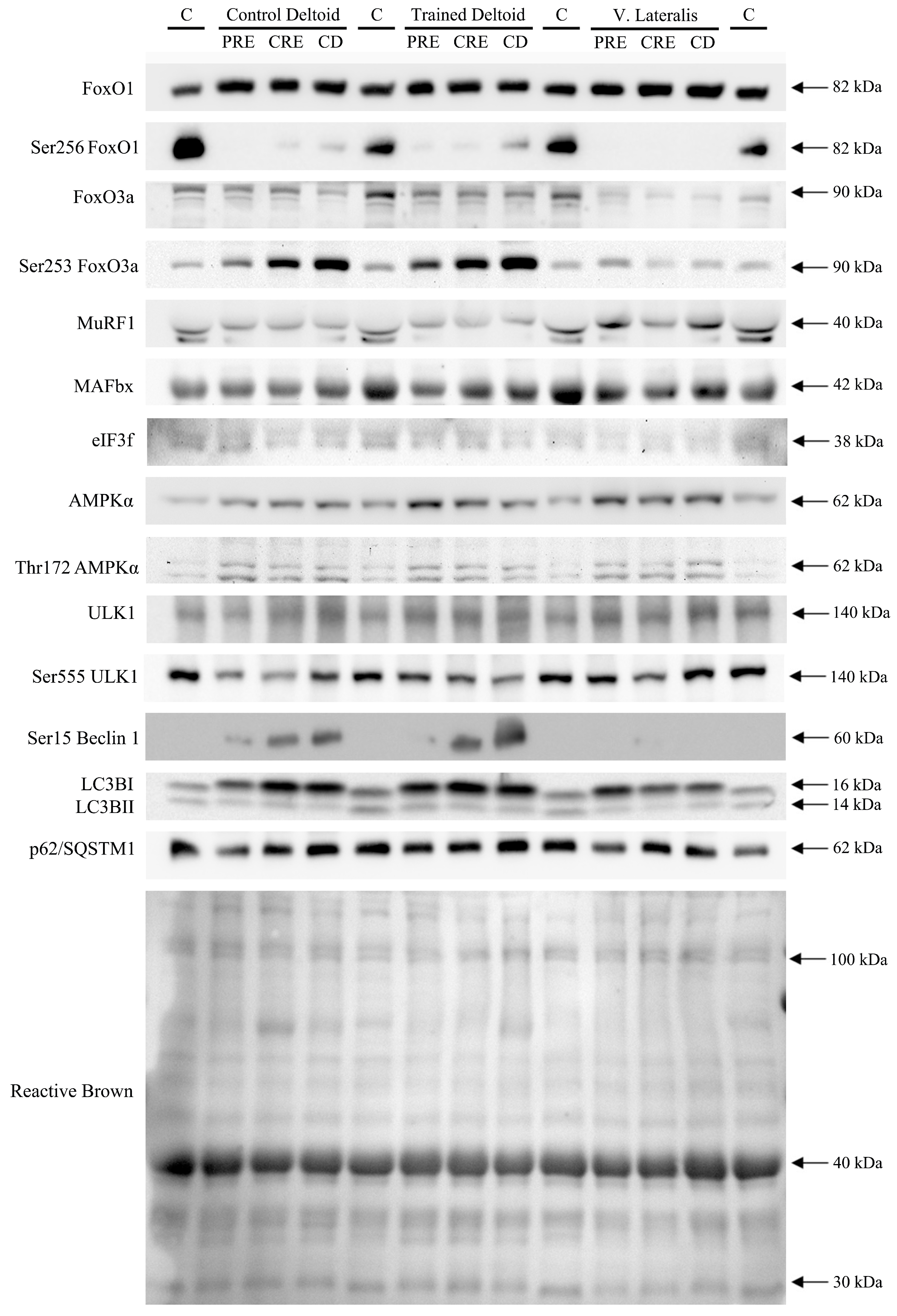
3.2. Exercise Attenuated the Increase of Ser256 FoxO1 and Ser253 FoxO3a Elicited by the Negative Energy Balance, but Had No Significant Effect on the Levels of MuRF1, MAFbx, and Eif3f Protein in Skeletal Muscle, Regardless of Protein Supplementation
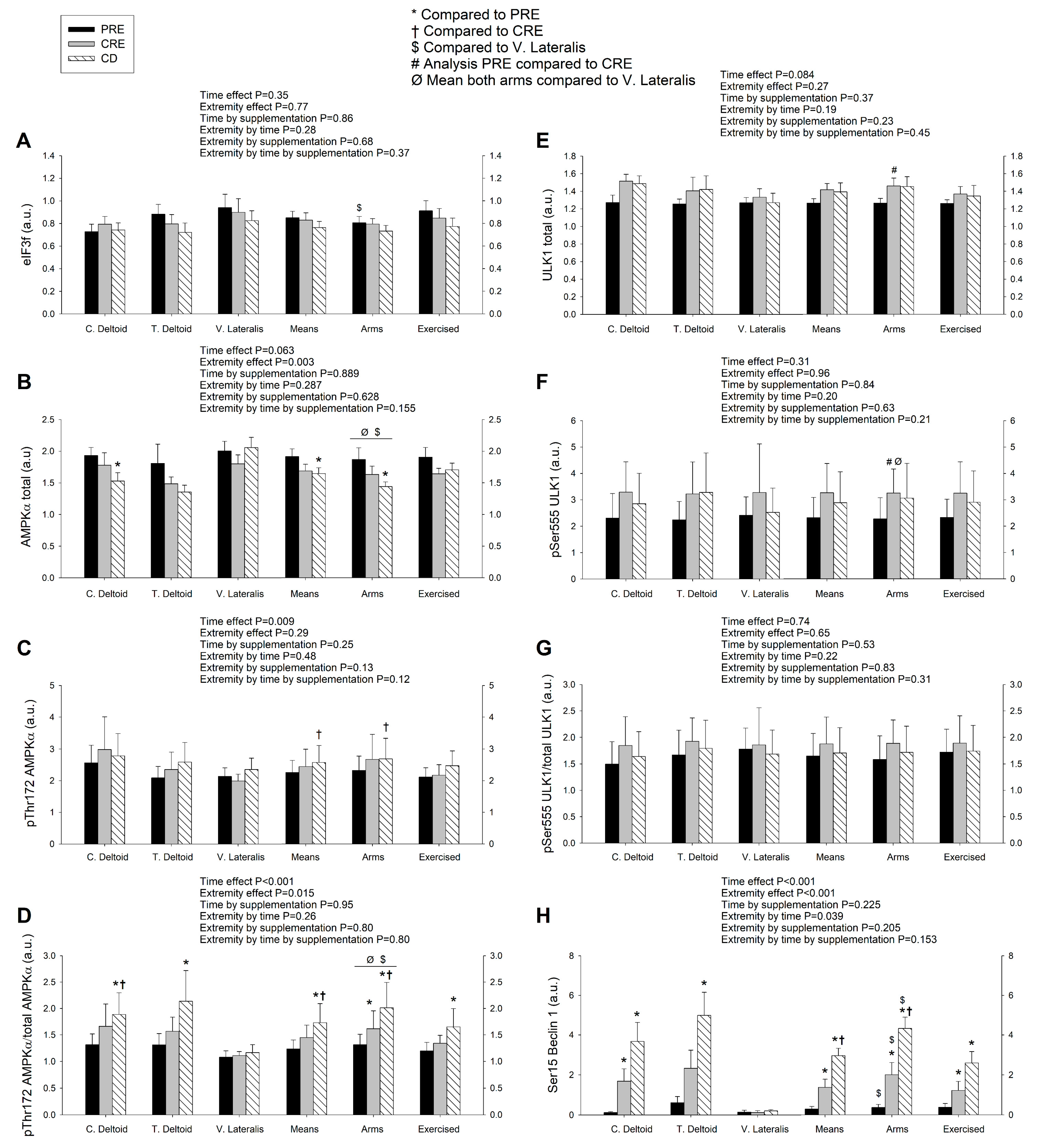
3.3. A High Volume of Exercise Prevents the Phosphorylation of Thr172 AMPK, Ser555 ULK1, and Ser15 Beclin 1 in Skeletal Muscle, Blunting the Autophagic Response and Preserving Lean Mass during a Severe Energy Deficit
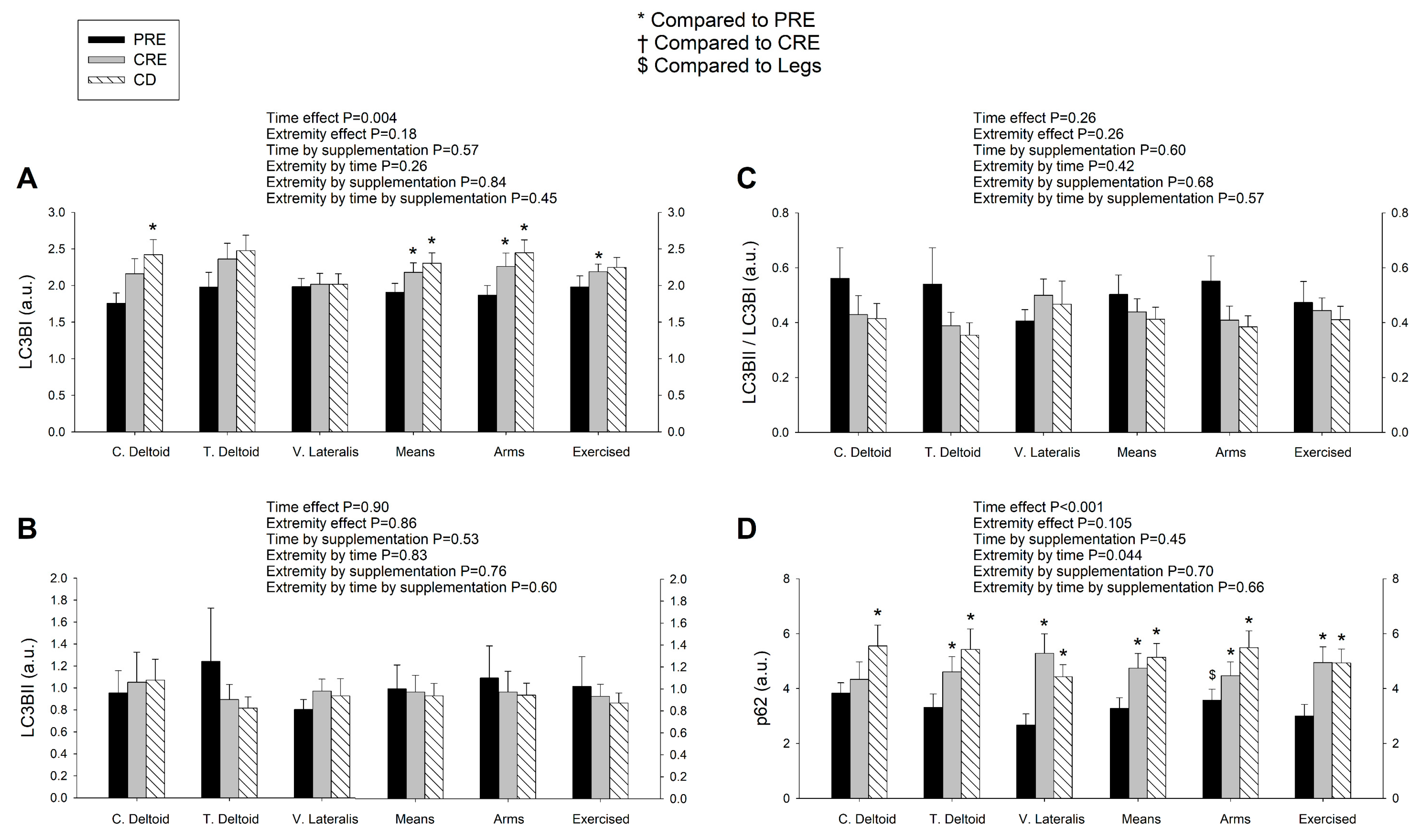
3.4. LC3BI Expression in Skeletal Muscle Increases with a Severe Energy Deficit, without Significant Changes in LC3BII nor the LC3BII/LCBI Ratio, Regardless of the Ingestion of Proteins
3.5. p62/SQSTM1 Expression Increases with a Negative Energy Balance and this Response Is Amplified by Exercise in a Dose-Dependent Fashion
3.6. The Autophagic Response to a Severe Energy Deficit Is Positively Associated with the Increase in the Catabolic Index (Cortisol/Testosterone), Negatively Associated with Insulin Changes, and Partly Modulated by Circulating Essential Amino Acids
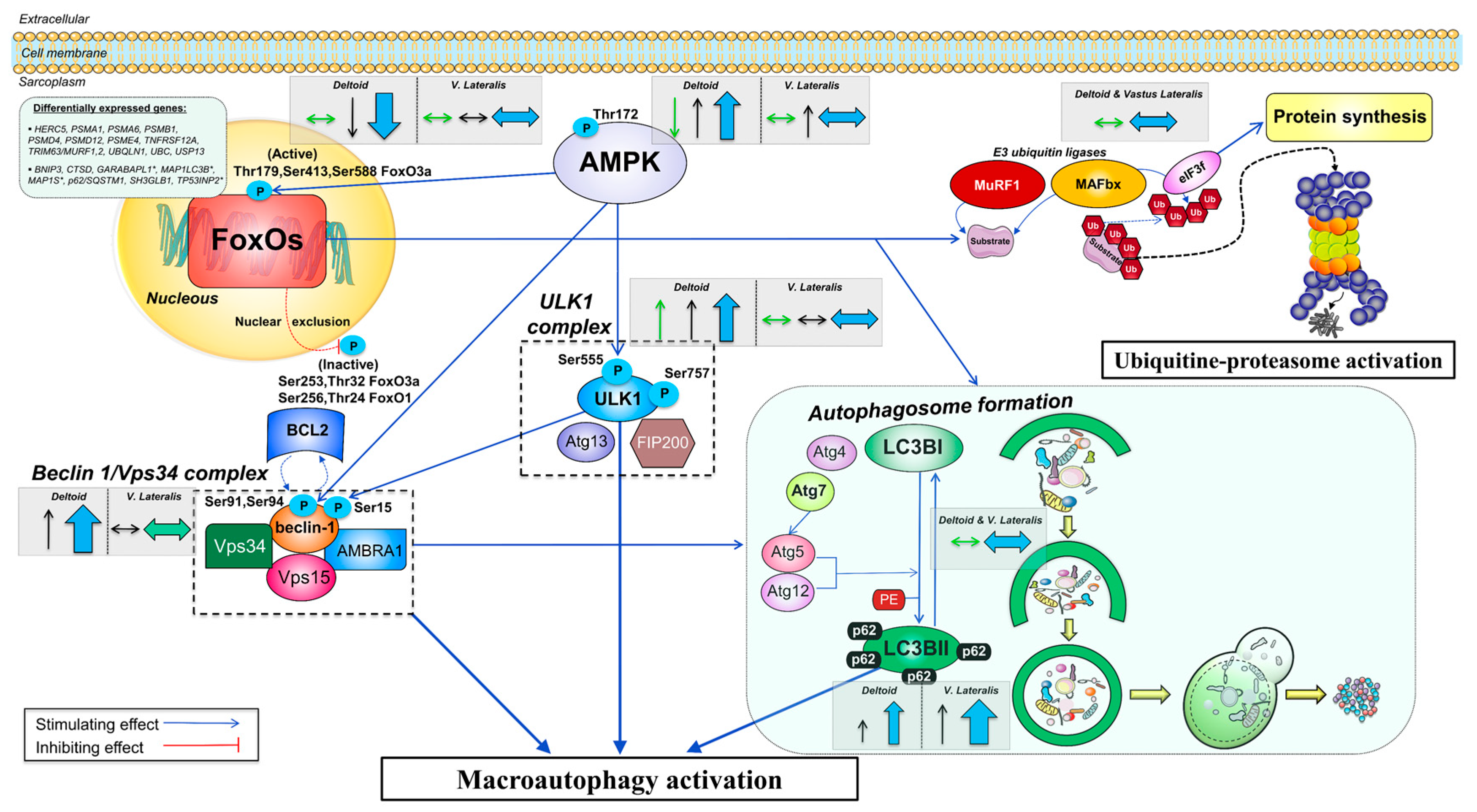
4. Discussion
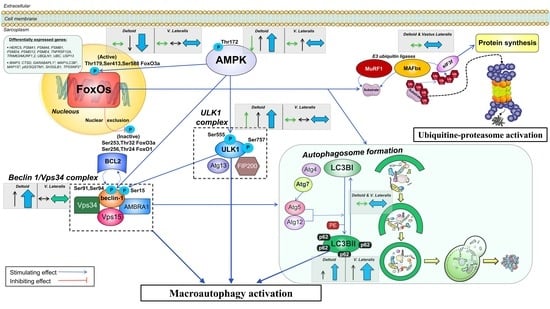
4.1. The Activation of the Signaling Cascades Regulating Protein Degradation Is Primarily Driven By AMPK and Not FoxOs under a Severe Energy Deficit
4.2. The Changes in Circulating EAA, BCAA, Leucine, and Insulin Are Inversely Associated with the Changes in Autophagy Induction in the Muscles Submitted to a Large Exercise Volume
4.3. The Ubiquitin-Proteasome System Is not Primarily Mediating Skeletal Muscle Protein Degradation during a Severe Energy Deficit
4.4. Signaling Data Indicate that the Autophagy-Lysosome-Mediated Protein Degradation Is Attenuated by High-Volume Low-Intensity Exercise, Likely Contributing to the Preservation of Muscle Mass in the Lower Extremities
4.5. Skeletal Muscle Seems Unresponsive to the Anticatabolic Effects of EAA during a Severe Energy Deficit
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weinheimer, E.M.; Sands, L.P.; Campbell, W.W. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: Implications for sarcopenic obesity. Nutr. Rev. 2010, 68, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.A.; Plank, L.D.; Finn, P.J.; Whalley, G.A.; Sharpe, N.; Clark, M.A.; Hill, G.L. Massive nitrogen loss in critical surgical illness: Effect on cardiac mass and function. Ann. Surg. 1997, 226, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Lambell, K.J.; King, S.J.; Forsyth, A.K.; Tierney, A.C. Association of energy and protein delivery on skeletal muscle mass changes in critically ill adults: A a systematic review. JPEN J. Parenter. Enter. Nutr. 2018, 42, 1112–1122. [Google Scholar] [CrossRef]
- Hessels, L.; Koopmans, N.; Gomes Neto, A.W.; Volbeda, M.; Koeze, J.; Lansink-Hartgring, A.O.; Bakker, S.J.; Oudemans-van Straaten, H.M.; Nijsten, M.W. Urinary creatinine excretion is related to short-term and long-term mortality in critically ill patients. Intensive Care Med. 2018, 44, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Marks, B.L.; Rippe, J.M. The importance of fat free mass maintenance in weight loss programmes. Sports Med. 1996, 22, 273–281. [Google Scholar] [CrossRef]
- Hickmann, C.E.; Castanares-Zapatero, D.; Deldicque, L.; Van den Bergh, P.; Caty, G.; Robert, A.; Roeseler, J.; Francaux, M.; Laterre, P.F. Impact of very early physical therapy during septic shock on skeletal muscle: A randomized controlled trial. Crit. Care Med. 2018, 46, 1436–1443. [Google Scholar] [CrossRef]
- Carbone, J.W.; McClung, J.P.; Pasiakos, S.M. Recent Advances in the Characterization of Skeletal Muscle and Whole-Body Protein Responses to Dietary Protein and Exercise during Negative Energy Balance. Adv. Nutr. 2019, 10, 70–79. [Google Scholar] [CrossRef]
- Knapik, J.; Meredith, C.; Jones, B.; Fielding, R.; Young, V.; Evans, W. Leucine metabolism during fasting and exercise. J. Appl. Physiol. 1991, 70, 43–47. [Google Scholar] [CrossRef]
- Pikosky, M.A.; Smith, T.J.; Grediagin, A.; Castaneda-Sceppa, C.; Byerley, L.; Glickman, E.L.; Young, A.J. Increased protein maintains nitrogen balance during exercise-induced energy deficit. Med. Sci. Sports Exerc. 2008, 40, 505–512. [Google Scholar] [CrossRef]
- Tsalikian, E.; Howard, C.; Gerich, J.E.; Haymond, M.W. Increased leucine flux in short-term fasted human subjects: Evidence for increased proteolysis. Am. J. Physiol. 1984, 247, E323–E327. [Google Scholar] [CrossRef]
- Areta, J.L.; Burke, L.M.; Camera, D.M.; West, D.W.; Crawshay, S.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M.; Hawley, J.A.; Coffey, V.G. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E989–E997. [Google Scholar] [CrossRef] [PubMed]
- Carbone, J.W.; Pasiakos, S.M.; Vislocky, L.M.; Anderson, J.M.; Rodriguez, N.R. Effects of short-term energy deficit on muscle protein breakdown and intramuscular proteolysis in normal-weight young adults. Appl. Physiol. Nutr. Metab. 2014, 39, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.J.; McGlory, C.; Damas, F.; Mazara, N.; Baker, S.K.; Phillips, S.M. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB J. 2018, 32, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.M.; Carbone, J.W.; Berryman, C.E.; Carrigan, C.T.; Murphy, N.E.; Ferrando, A.A.; Young, A.J.; Pasiakos, S.M. Severe energy deficit at high altitude inhibits skeletal muscle mTORC1-mediated anabolic signaling without increased ubiquitin proteasome activity. FASEB J. 2018. [Google Scholar] [CrossRef]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Lecker, S.H.; Jagoe, R.T.; Gilbert, A.; Gomes, M.; Baracos, V.; Bailey, J.; Price, S.R.; Mitch, W.E.; Goldberg, A.L. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004, 18, 39–51. [Google Scholar] [CrossRef]
- Sandri, M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef]
- Sanchez, A.M.J.; Csibi, A.; Raibon, A.; Docquier, A.; Lagirand-Cantaloube, J.; Leibovitch, M.-P.; Leibovitch, S.A.; Bernardi, H. eIF3f: A central regulator of the antagonism atrophy/hypertrophy in skeletal muscle. Int. J. Biochem. Cell Biol. 2013, 45, 2158–2162. [Google Scholar] [CrossRef]
- Masiero, E.; Agatea, L.; Mammucari, C.; Blaauw, B.; Loro, E.; Komatsu, M.; Metzger, D.; Reggiani, C.; Schiaffino, S.; Sandri, M. Autophagy is required to maintain muscle mass. Cell Metab. 2009, 10, 507–515. [Google Scholar] [CrossRef]
- Sin, J.; Andres, A.M.; Taylor, D.J.; Weston, T.; Hiraumi, Y.; Stotland, A.; Kim, B.J.; Huang, C.; Doran, K.S.; Gottlieb, R.A. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy 2016, 12, 369–380. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Bassik, M.C.; Moresi, V.; Sun, K.; Wei, Y.; Zou, Z.; An, Z.; Loh, J.; Fisher, J.; Sun, Q.; et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 2012, 481, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.A.; Okutsu, M.; Zhang, M.; Greene, N.P.; Laker, R.C.; Breen, D.S.; Hoehn, K.L.; Yan, Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013, 27, 4184–4193. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, C.; Jamart, C.; Benoit, N.; Naslain, D.; Prémont, C.; Prévet, J.; Van Thienen, R.; Deldicque, L.; Francaux, M. Activation of autophagy in human skeletal muscle is dependent on exercise intensity and AMPK activation. FASEB J. 2015, 29, 3515–3526. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.B.; Vendelbo, M.H.; Christensen, B.; Clasen, B.F.; Bak, A.M.; Jørgensen, J.O.L.; Møller, N.; Jessen, N. Physical exercise increases autophagic signaling through ULK1 in human skeletal muscle. J. Appl. Physiol. 2015, 118, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rincon, M.; Morales-Alamo, D.; Calbet, J.A.L. Exercise-mediated modulation of autophagy in skeletal muscle. Scand. J. Med. Sci. Sports 2018, 28, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.-Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.-L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Smiles, W.J.; Areta, J.L.; Coffey, V.G.; Phillips, S.M.; Moore, D.R.; Stellingwerff, T.; Burke, L.M.; Hawley, J.A.; Camera, D.M. Modulation of autophagy signaling with resistance exercise and protein ingestion following short-term energy deficit. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R603–R612. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Jamart, C.; Francaux, M.; Millet, G.Y.; Deldicque, L.; Frere, D.; Feasson, L. Modulation of autophagy and ubiquitin-proteasome pathways during ultra-endurance running. J. Appl. Physiol. 2012, 112, 1529–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Jamart, C.; Deldicque, L.; An, G.L.; Lee, Y.H.; Kim, C.K.; Raymackers, J.M.; Francaux, M. Endoplasmic reticulum stress markers and ubiquitin-proteasome pathway activity in response to a 200-km run. Med. Sci. Sports Exerc. 2011, 43, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, E.A.; Vepkhvadze, T.F.; Lednev, E.M.; Vinogradova, O.L.; Popov, D.V. Branched-chain amino acids administration suppresses endurance exercise-related activation of ubiquitin proteasome signaling in trained human skeletal muscle. J. Physiol. Sci. 2018, 68, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Borgenvik, M.; Apró, W.; Blomstrand, E. Intake of branched-chain amino acids influences the levels of MAFbx mRNA and MuRF-1 total protein in resting and exercising human muscle. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E510–E521. [Google Scholar] [CrossRef]
- Fritzen, A.M.; Madsen, A.B.; Kleinert, M.; Treebak, J.T.; Lundsgaard, A.-M.; Jensen, T.E.; Richter, E.A.; Wojtaszewski, J.; Kiens, B.; Frøsig, C. Regulation of autophagy in human skeletal muscle: Effects of exercise, exercise training and insulin stimulation. J. Physiol. (Lond.) 2016, 594, 745–761. [Google Scholar] [CrossRef]
- Rittig, N.; Bach, E.; Thomsen, H.H.; Møller, A.B.; Hansen, J.; Johannsen, M.; Jensen, E.; Serena, A.; Jørgensen, J.O.; Richelsen, B.; et al. Anabolic effects of leucine-rich whey protein, carbohydrate, and soy protein with and without β-hydroxy-β-methylbutyrate (HMB) during fasting-induced catabolism: A human randomized crossover trial. Clin. Nutr. 2017, 36, 697–705. [Google Scholar] [CrossRef]
- Perez-Suarez, I.; Ponce-Gonzalez, J.G.; de La Calle-Herrero, J.; Losa-Reyna, J.; Martin-Rincon, M.; Morales-Alamo, D.; Santana, A.; Holmberg, H.C.; Calbet, J.A.L. Severe energy deficit upregulates leptin receptors, leptin signaling, and PTP1B in human skeletal muscle. J. Appl. Physiol. 2017, 123, 1276–1287. [Google Scholar] [CrossRef]
- Martin-Rincon, M.; Perez-Suarez, I.; Perez-Lopez, A.; Ponce-Gonzalez, J.G.; Morales-Alamo, D.; de Pablos-Velasco, P.; Holmberg, H.C.; Calbet, J.A.L. Protein synthesis signaling in skeletal muscle is refractory to whey protein ingestion during a severe energy deficit evoked by prolonged exercise and caloric restriction. Int. J. Obes. (Lond.) 2019, 43, 872–882. [Google Scholar] [CrossRef]
- Ström, K.; Morales-Alamo, D.; Ottosson, F.; Edlund, A.; Hjort, L.; Jörgensen, S.W.; Almgren, P.; Zhou, Y.; Martin-Rincon, M.; Ekman, C.; et al. N1-methylnicotinamide is a signalling molecule produced in skeletal muscle coordinating energy metabolism. Sci. Rep. 2018, 8, 3016. [Google Scholar] [CrossRef] [Green Version]
- Calbet, J.A.; Ponce-Gonzalez, J.G.; Perez-Suarez, I.; de la Calle Herrero, J.; Holmberg, H.C. A time-efficient reduction of fat mass in 4 days with exercise and caloric restriction. Scand. J. Med. Sci. Sports 2015, 25, 223–233. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; Ponce-Gonzalez, J.G.; Calle-Herrero, J.; Perez-Suarez, I.; Martin-Rincon, M.; Santana, A.; Morales-Alamo, D.; Holmberg, H.C. Exercise preserves lean mass and performance during severe energy deficit: The role of exercise volume and dietary protein content. Front. Physiol. 2017, 8, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ara, I.; Larsen, S.; Stallknecht, B.; Guerra, B.; Morales-Alamo, D.; Andersen, J.L.; Ponce-Gonzalez, J.G.; Guadalupe-Grau, A.; Galbo, H.; Calbet, J.A.; et al. Normal mitochondrial function and increased fat oxidation capacity in leg and arm muscles in obese humans. Int. J. Obes. (Lond.) 2011, 35, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnaiger, E.; Boushel, R.; Sondergaard, H.; Munch-Andersen, T.; Damsgaard, R.; Hagen, C.; Diez-Sanchez, C.; Ara, I.; Wright-Paradis, C.; Schrauwen, P.; et al. Mitochondrial coupling and capacity of oxidative phosphorylation in skeletal muscle of Inuit and Caucasians in the arctic winter. Scand. J. Med. Sci. Sports 2015, 25, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Zinner, C.; Morales-Alamo, D.; Ortenblad, N.; Larsen, F.J.; Schiffer, T.A.; Willis, S.J.; Gelabert-Rebato, M.; Perez-Valera, M.; Boushel, R.; Calbet, J.A.; et al. The physiological mechanisms of performance enhancement with sprint interval training differ between the upper and lower extremities in humans. Front. Physiol. 2016, 7, 426. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Elia, M.; Stratton, R.; Stubbs, J. Techniques for the study of energy balance in man. Proc. Nutr. Soc. 2003, 62, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Guerra, B.; Ponce-Gonzalez, J.G.; Morales-Alamo, D.; Guadalupe-Grau, A.; Kiilerich, K.; Fuentes, T.; Ringholm, S.; Bienso, R.S.; Santana, A.; Lundby, C.; et al. Leptin signaling in skeletal muscle after bed rest in healthy humans. Eur. J. Appl. Physiol. 2014, 114, 345–357. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Ponce-Gonzalez, J.G.; Guadalupe-Grau, A.; Rodriguez-Garcia, L.; Santana, A.; Cusso, R.; Guerrero, M.; Dorado, C.; Guerra, B.; Calbet, J.A. Critical role for free radicals on sprint exercise-induced CaMKII and AMPKalpha phosphorylation in human skeletal muscle. J. Appl. Physiol. 2013, 114, 566–577. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Bjørkøy, G.; Lamark, T.; Pankiv, S.; Øvervatn, A.; Brech, A.; Johansen, T. Monitoring autophagic degradation of p62/SQSTM1. Meth. Enzymol. 2009, 452, 181–197. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.S.; Ueno, T.; Hara, T.; Mizushima, N.; Iwata, J.; Ezaki, J.; Murata, S.; et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131, 1149–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, A.M.; Csibi, A.; Raibon, A.; Cornille, K.; Gay, S.; Bernardi, H.; Candau, R. AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. J. Cell. Biochem. 2012, 113, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Jamart, C.; Benoit, N.; Raymackers, J.M.; Kim, H.J.; Kim, C.K.; Francaux, M. Autophagy-related and autophagy-regulatory genes are induced in human muscle after ultraendurance exercise. Pflug. Arch. 2012, 112, 3173–3177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Heide, L.P.; Hoekman, M.F.M.; Smidt, M.P. The ins and outs of FoxO shuttling: Mechanisms of FoxO translocation and transcriptional regulation. Biochem. J. 2004, 380, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Ye, M.; Bucur, O.; Zhu, S.; Tanya Santos, M.; Rabinovitz, I.; Wei, W.; Gao, D.; Hahn, W.C.; Khosravi-Far, R. Protein phosphatase 2A reactivates FOXO3a through a dynamic interplay with 14-3-3 and AKT. Mol. Biol. Cell 2010, 21, 1140–1152. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef] [Green Version]
- Furuyama, T.; Kitayama, K.; Yamashita, H.; Mori, N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem. J. 2003, 375, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Pilegaard, H.; Neufer, P.D. Transcriptional regulation of pyruvate dehydrogenase kinase 4 in skeletal muscle during and after exercise. Proc. Nutr. Soc. 2004, 63, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, A.M.; Candau, R.B.; Bernardi, H. FoxO transcription factors: Their roles in the maintenance of skeletal muscle homeostasis. Cell. Mol. Life Sci. 2014, 71, 1657–1671. [Google Scholar] [CrossRef]
- Helge, J.W. Arm and leg substrate utilization and muscle adaptation after prolonged low-intensity training. Acta Physiol. (Oxf.) 2010, 199, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, R.A.; Shaw, L.M. An autophagy-independent function for Beclin 1 in cancer. Mol. Cell Oncol. 2015, 3, e1030539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.L.; Xia, H.G.; Kim, M.; Xu, L.H.; Li, Y.; Zhang, L.H.; Cai, Y.; Norberg, H.V.; Zhang, T.; Furuya, T.; et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 2011, 147, 223–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, J.W.; Margolis, L.M.; McClung, J.P.; Cao, J.J.; Murphy, N.E.; Sauter, E.R.; Combs, G.F.; Young, A.J.; Pasiakos, S.M. Effects of energy deficit, dietary protein, and feeding on intracellular regulators of skeletal muscle proteolysis. FASEB J. 2013, 27, 5104–5111. [Google Scholar] [CrossRef]
- Lagirand-Cantaloube, J.; Offner, N.; Csibi, A.; Leibovitch, M.P.; Batonnet-Pichon, S.; Tintignac, L.A.; Segura, C.T.; Leibovitch, S.A. The initiation factor eIF3-f is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. Embo J. 2008, 27, 1266–1276. [Google Scholar] [CrossRef] [Green Version]
- Rubinsztein, D.C.; Cuervo, A.M.; Ravikumar, B.; Sarkar, S.; Korolchuk, V.; Kaushik, S.; Klionsky, D.J. In search of an “autophagomometer”. Autophagy 2009, 5, 585–589. [Google Scholar] [CrossRef]
- Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Dreyer, H.C.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R533–R540. [Google Scholar] [CrossRef] [Green Version]
- Moberg, M.; Hendo, G.; Jakobsson, M.; Mattsson, C.M.; Ekblom-Bak, E.; Flockhart, M.; Ponten, M.; Soderlund, K.; Ekblom, B. Increased autophagy signaling but not proteasome activity in human skeletal muscle after prolonged low-intensity exercise with negative energy balance. Physiol. Rep. 2017, 5, e13518. [Google Scholar] [CrossRef] [Green Version]
- Fritzen, A.M.; Frosig, C.; Jeppesen, J.; Jensen, T.E.; Lundsgaard, A.M.; Serup, A.K.; Schjerling, P.; Proud, C.G.; Richter, E.A.; Kiens, B. Role of AMPK in regulation of LC3 lipidation as a marker of autophagy in skeletal muscle. Cell. Signal. 2016, 28, 663–674. [Google Scholar] [CrossRef]
- Botti-Millet, J.; Nascimbeni, A.C.; Dupont, N.; Morel, E.; Codogno, P. Fine-tuning autophagy: From transcriptional to posttranslational regulation. Am. J. Physiol. Cell Physiol. 2016, 311, C351–C362. [Google Scholar] [CrossRef]
- Hoffer, L.J.; Forse, R.A. Protein metabolic effects of a prolonged fast and hypocaloric refeeding. Am. J. Physiol. 1990, 258, E832–E840. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Margolis, L.M.; McClung, J.P.; Cao, J.J.; Whigham, L.D.; Combs, G.F.; Young, A.J. Whole-body protein turnover response to short-term high-protein diets during weight loss: A randomized controlled trial. Int. J. Obes. (Lond.) 2014, 38, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.P.; Rumpler, W.V.; Leskiw, M.J.; Schluter, M.D.; Staples, R.; Bodwell, C.E. Effect of reduced dietary intake on energy expenditure, protein turnover, and glucose cycling in man. Metab. Clin. Exp. 1991, 40, 478–483. [Google Scholar] [CrossRef]
- Keys, A. Experimental studies of starvation on men. Bull. Chic. Med. Soc. 1946, 49, 42–46. [Google Scholar]
- Louard, R.J.; Barrett, E.J.; Gelfand, R.A. Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism 1995, 44, 424–429. [Google Scholar] [CrossRef]
- Rooyackers, O.E.; Nair, K.S. Hormonal regulation of human muscle protein metabolism. Annu. Rev. Nutr. 1997, 17, 457–485. [Google Scholar] [CrossRef]
- Biolo, G.; Tipton, K.D.; Klein, S.; Wolfe, R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. Endocrinol. Metab. 1997, 273, E122–E129. [Google Scholar] [CrossRef]
- Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Timmerman, K.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J. Nutr. 2010, 140, 1970–1976. [Google Scholar] [CrossRef]
- Tipton, K.D.; Ferrando, A.A.; Phillips, S.M.; Doyle, D.; Wolfe, R.R. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am. J. Physiol. 1999, 276, E628–E634. [Google Scholar] [CrossRef]
- Bifari, F.; Nisoli, E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: A pharmacological point of view. Br. J. Pharmacol. 2017, 174, 1366–1377. [Google Scholar] [CrossRef] [Green Version]
- Herningtyas, E.H.; Okimura, Y.; Handayaningsih, A.E.; Yamamoto, D.; Maki, T.; Iida, K.; Takahashi, Y.; Kaji, H.; Chihara, K. Branched-chain amino acids and arginine suppress MaFbx/atrogin-1 mRNA expression via mTOR pathway in C2C12 cell line. Biochim. Biophys. Acta 2008, 1780, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Baptista, I.L.; Leal, M.L.; Artioli, G.G.; Aoki, M.S.; Fiamoncini, J.; Turri, A.O.; Curi, R.; Miyabara, E.H.; Moriscot, A.S. Leucine attenuates skeletal muscle wasting via inhibition of ubiquitin ligases. Muscle Nerve 2010, 41, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Ito, Y.; Nishizawa, N.; Nagasawa, T. Regulation of muscle protein degradation, not synthesis, by dietary leucine in rats fed a protein-deficient diet. Amino Acids 2009, 37, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Reitelseder, S.; Agergaard, J.; Doessing, S.; Helmark, I.C.; Schjerling, P.; van Hall, G.; Kjaer, M.; Holm, L. Positive muscle protein net balance and differential regulation of atrogene expression after resistance exercise and milk protein supplementation. Eur. J. Nutr. 2014, 53, 321–333. [Google Scholar] [CrossRef]
- Dalbo, V.J.; Roberts, M.D.; Hassell, S.; Kerksick, C.M. Effects of pre-exercise feeding on serum hormone concentrations and biomarkers of myostatin and ubiquitin proteasome pathway activity. Eur. J. Nutr. 2013, 52, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.M.; Reidy, P.T.; Gundermann, D.M.; Borack, M.S.; Walker, D.K.; D’Lugos, A.C.; Volpi, E.; Rasmussen, B.B. The impact of postexercise essential amino acid ingestion on the ubiquitin proteasome and autophagosomal-lysosomal systems in skeletal muscle of older men. J. Appl. Physiol. 2017, 122, 620–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenhaff, P.L.; Karagounis, L.G.; Peirce, N.; Simpson, E.J.; Hazell, M.; Layfield, R.; Wackerhage, H.; Smith, K.; Atherton, P.; Selby, A.; et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E595–E604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchis-Moysi, J.; Idoate, F.; Olmedillas, H.; Guadalupe-Grau, A.; Alayon, S.; Carreras, A.; Dorado, C.; Calbet, J.A. The upper extremity of the professional tennis player: Muscle volumes, fiber-type distribution and muscle strength. Scand. J. Med. Sci. Sports 2010, 20, 524–534. [Google Scholar] [CrossRef]
- Ørtenblad, N.; Nielsen, J.; Boushel, R.; Söderlund, K.; Saltin, B.; Holmberg, H.-C. The muscle fiber profiles, mitochondrial content, and enzyme activities of the exceptionally well-trained arm and leg muscles of elite cross-country skiers. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Nielsen, J.; Gejl, K.D.; Hey-Mogensen, M.; Holmberg, H.-C.; Suetta, C.; Krustrup, P.; Elemans, C.P.H.; Ørtenblad, N. Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J. Physiol. 2017, 595, 2839–2847. [Google Scholar] [CrossRef]
| Variable | Diet | |
|---|---|---|
| Sucrose | Whey Protein | |
| (n = 7) | (n = 8) | |
| Age (years) | 38.7 ± 8.2 | 43.0 ± 8.0 |
| Height (cm) | 181 ± 5.5 | 180 ± 4.2 |
| Body mass (kg) | 98 ± 12.0 | 100 ± 14.9 |
| BMI (kg/m2) | 29.9 ± 3.1 | 30.9 ± 4.2 |
| Lean mass (kg) | 63.1 ± 3.1 | 65.4 ± 6.0 |
| Fat mass (kg) | 31.5 ± 9.1 | 31.4 ± 9.2 |
| Body fat (%) | 31.6 ± 5.3 | 30.9 ± 4.1 |
| VO2max (L/min) | 3.8 ± 0.3 | 3.9 ± 0.3 |
| Daily energy intake (kcal) | 2256 ± 513 | 2086 ± 489 |
| Physical activity (IPAQ) (kcal/d) | 612 ± 315 | 601 ± 289 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Rincon, M.; Pérez-López, A.; Morales-Alamo, D.; Perez-Suarez, I.; de Pablos-Velasco, P.; Perez-Valera, M.; Perez-Regalado, S.; Martinez-Canton, M.; Gelabert-Rebato, M.; Juan-Habib, J.W.; et al. Exercise Mitigates the Loss of Muscle Mass by Attenuating the Activation of Autophagy during Severe Energy Deficit. Nutrients 2019, 11, 2824. https://doi.org/10.3390/nu11112824
Martin-Rincon M, Pérez-López A, Morales-Alamo D, Perez-Suarez I, de Pablos-Velasco P, Perez-Valera M, Perez-Regalado S, Martinez-Canton M, Gelabert-Rebato M, Juan-Habib JW, et al. Exercise Mitigates the Loss of Muscle Mass by Attenuating the Activation of Autophagy during Severe Energy Deficit. Nutrients. 2019; 11(11):2824. https://doi.org/10.3390/nu11112824
Chicago/Turabian StyleMartin-Rincon, Marcos, Alberto Pérez-López, David Morales-Alamo, Ismael Perez-Suarez, Pedro de Pablos-Velasco, Mario Perez-Valera, Sergio Perez-Regalado, Miriam Martinez-Canton, Miriam Gelabert-Rebato, Julian William Juan-Habib, and et al. 2019. "Exercise Mitigates the Loss of Muscle Mass by Attenuating the Activation of Autophagy during Severe Energy Deficit" Nutrients 11, no. 11: 2824. https://doi.org/10.3390/nu11112824
APA StyleMartin-Rincon, M., Pérez-López, A., Morales-Alamo, D., Perez-Suarez, I., de Pablos-Velasco, P., Perez-Valera, M., Perez-Regalado, S., Martinez-Canton, M., Gelabert-Rebato, M., Juan-Habib, J. W., Holmberg, H.-C., & Calbet, J. A. L. (2019). Exercise Mitigates the Loss of Muscle Mass by Attenuating the Activation of Autophagy during Severe Energy Deficit. Nutrients, 11(11), 2824. https://doi.org/10.3390/nu11112824