Dysregulated Iron Metabolism-Associated Dietary Pattern Predicts an Altered Body Composition and Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Definitions
2.3. Questionnaires
2.4. Anthropometric Measurements
2.5. Laboratory Measurements
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population According to Dysregulated Iron Metabolism (DIM)
3.2. DIM and Risk of Altered Body Composition and Metabolic Syndrome (MetS)
3.3. DIM-Associated Dietary Pattern Scores by the Reduced-Rank Regression (RRR)
3.4. Relationships among DIM-Associated Dietary Pattern Scores, MetS, Central Obesity, and Body Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, H.C.; Yang, H.C.; Chang, H.Y.; Yeh, C.J.; Chen, H.H.; Huang, K.C.; Pan, W.H. Morbid obesity in Taiwan: Prevalence, trends, associated social demographics, and lifestyle factors. PLoS ONE 2017, 12, e0169577. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Shen, Y.; Fang, X.; Wang, Y.; Wang, F. Obesity and iron deficiency: a quantitative meta-analysis. Obes. Rev. 2015, 16, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Lin, S.M.; Huang, T.C.; Chao, J.C.; Chen, Y.C.; Pan, W.H.; Bai, C.H. Serum ferritin and risk of the metabolic syndrome: a population-based study. Asia. Pac. J. Clin. Nutr. 2013, 22, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S.; Gammella, E.; Cairo, G. Dysregulation of iron metabolism in cancer stem cells. Free Radic. Biol. Med. 2019, 133, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, Y.Z.; Feola, M.; Zimran, E.; Varkonyi, J.; Ganz, T.; Hoffman, R. Dysregulated iron metabolism in polycythemia vera: etiology and consequences. Leukemia 2018, 32, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Halon-Golabek, M.; Borkowska, A.; Herman-Antosiewicz, A.; Antosiewicz, J. Iron Metabolism of the Skeletal Muscle and Neurodegeneration. Front. Neurosci. 2019, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Aigner, E.; Feldman, A.; Datz, C. Obesity as an emerging risk factor for iron deficiency. Nutrients 2014, 6, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Deugnier, Y.; Bardou-Jacquet, E.; Laine, F. Dysmetabolic iron overload syndrome (DIOS). Presse Med. 2017, 46, e306–e311. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.S.; Kang, M.J.; Oh, Y.J.; Baek, J.W.; Yang, S.; Hwang, I.T. Association of serum ferritin with insulin resistance, abdominal obesity, and metabolic syndrome in Korean adolescent and adults: The Korean National Health and Nutrition Examination Survey, 2008 to 2011. Medicine 2017, 96, e6179. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Ortegon, M.F.; Ensaldo-Carrasco, E.; Shi, T.; McLachlan, S.; Fernandez-Real, J.M.; Wild, S.H. Ferritin, metabolic syndrome and its components: A systematic review and meta-analysis. Atheroscler. 2018, 275, 97–106. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.; Wilman, H.R.; Dennis, A.; Kelly, M.; Gyngell, M.L.; Neubauer, S.; Bell, J.D.; Banerjee, R.; Thomas, E.L. Measurement of liver iron by magnetic resonance imaging in the UK Biobank population. PLoS ONE 2018, 13, e0209340. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Pham, V.T.; Mori, H.; MacDougald, O.A.; Shah, Y.M.; Bodary, P.F. Iron elevation and adipose tissue remodeling in the epididymal depot of a mouse model of polygenic obesity. PLoS ONE 2017, 12, e0179889. [Google Scholar] [CrossRef] [PubMed]
- Sabrina, N.; Bai, C.H.; Chang, C.C.; Chien, Y.W.; Chen, J.R.; Chang, J.S. Serum Iron:Ferritin Ratio Predicts Healthy Body Composition and Reduced Risk of Severe Fatty Liver in Young Adult Women. Nutrients 2017, 9, 833. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Girelli, D.; Nemeth, E.; Swinkels, D.W. Hepcidin in the diagnosis of iron disorders. Blood 2016, 127, 2809–2813. [Google Scholar] [CrossRef] [PubMed]
- Rishi, G.; Wallace, D.F.; Subramaniam, V.N. Hepcidin: regulation of the master iron regulator. Biosci. Rep. 2015, 35. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.J. Regulation of Iron Metabolism by Hepcidin under Conditions of Inflammation. J. Biol. Chem. 2015, 290, 18975–18983. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.; Conlon, C.; Kruger, R.; Coad, J. Dietary Determinants of and Possible Solutions to Iron Deficiency for Young Women Living in Industrialized Countries: A Review. Nutrients 2014, 6, 3747–3776. [Google Scholar] [CrossRef] [PubMed]
- Quintana Pacheco, D.A.; Sookthai, D.; Wittenbecher, C.; Graf, M.E.; Schubel, R.; Johnson, T.; Katzke, V.; Jakszyn, P.; Kaaks, R.; Kuhn, T. Red meat consumption and risk of cardiovascular diseases-is increased iron load a possible link? Am. J. Clin. Nutr. 2018, 107, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nothlings, U.; Boeing, H. Application of a New Statistical Method to Derive Dietary Patterns in Nutritional Epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Fracanzani, A.L.; Fargion, S.; Valenti, L. Iron in fatty liver and in the metabolic syndrome: a promising therapeutic target. J. Hepatol. 2011, 55, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gotzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Initiative, S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare. 2017 Annual Report of Health Promotion Administration. Available online: https://www.hpa.gov.tw/EngPages/List.aspx?nodeid=1070 (accessed on 7 June 2018).
- Niu, J.; Seo, D.-C. Central obesity and hypertension in Chinese adults: a 12-year longitudinal examination. Prev. Med. 2014, 62, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Datz, C.; Felder, T.K.; Niederseer, D.; Aigner, E. Iron Homeostasis in the Metabolic Syndrome. Eur. J. Clin. Invest. 2013, 43, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Utzschneider, K.M.; Largajolli, A.; Bertoldo, A.; Marcovina, S.; Nelson, J.E.; Yeh, M.M.; Kowdley, K.V.; Kahn, S.E. Serum ferritin is associated with non-alcoholic fatty liver disease and decreased Β-cell function in non-diabetic men and women. J. Diabetes Complicat. 2014, 28, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Hwang, H.J.; Kim, S.H. Relationship between serum ferritin levels and sarcopenia in Korean females aged 60 years and older using the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV-2, 3), 2008-2009. PLoS ONE 2014, 9, e90105. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Troutt, J.S.; Konrad, R.J.; Ghabril, M.; Saxena, R.; Bell, L.N.; Kowdley, K.V.; Chalasani, N. Serum hepcidin levels are associated with obesity but not liver disease. Obesity (Silver Spring) 2014, 22, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Chen, Y.C.; Owaga, E.; Palupi, K.C.; Pan, W.H.; Bai, C.H. Interactive effects of dietary fat/carbohydrate ratio and body mass index on iron deficiency anemia among Taiwanese women. Nutrients 2014, 6, 3929–3941. [Google Scholar] [CrossRef] [PubMed]
- Avila, F.; Echeverria, G.; Perez, D.; Martinez, C.; Strobel, P.; Castillo, O.; Villaroel, L.; Mezzano, D.; Rozowski, J.; Urquiaga, I.; et al. Serum Ferritin Is Associated with Metabolic Syndrome and Red Meat Consumption. Oxid. Med. Cell. Longev. 2015, 2015, 769739. [Google Scholar] [CrossRef] [PubMed]
- Meli, R.; Mattace Raso, G.; Irace, C.; Simeoli, R.; Di Pascale, A.; Paciello, O.; Pagano, T.B.; Calignano, A.; Colonna, A.; Santamaria, R. High Fat Diet Induces Liver Steatosis and Early Dysregulation of Iron Metabolism in Rats. PLoS ONE 2013, 8, e66570. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.A.; Bhatia, R. Nutrition standards for away-from-home foods in the USA. Obes. Rev. 2012, 13, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Bozoglu, M.; Bilgic, A.; Yen, S.T.; Huang, C.L. Household food expenditures at home and away from home in Turkey. In Proceedings of the Agricultural & Applied Economics Association′s 2013 AAEA & CAES Joint Annual Meeting, Washington, DC, USA, 4–6 August 2013. [Google Scholar]
- WHO. Serum Ferritin Concentrations for The Assessment of IronStatus and Iron Deficiency in Populations. In Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Branisso, P.P.F.; Oliveira, C.P.; Leão Filho, H.M.; Santos, A.; Lima, F.R.; Mancini, M.; Carrilho, F.J.; Rocha, M.; Cercato, C. Iron-Overload Evaluation by Noninvasive Methods in Patients with Nonalcoholic Fatty Liver Disease, Overweight, and Hyperferritinemia. Diabetes 2018, 67. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Steffen, L.M.; Schulze, M.B.; Jenny, N.S.; Barr, R.G.; Bertoni, A.G.; Jacobs, D.R., Jr. Associations between markers of subclinical atherosclerosis and dietary patterns derived by principal component analysis and reduce rank regression in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2007, 85, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Weikert, C.; Schulze, M.B. Evaluating dietary patterns: the role of reduced rank regression. Curr. Opin. Clin. Nutr. Metab. Care 2016. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Kourlaba, G.; Grammatikaki, E.; Androutsos, O.; Ioannou, E.; Roma-Giannikou, E. Comparison of two methods for identifying dietary patterns associated with obesity in preschool children: the GENESIS study. Eur. J. Clin. Nutr. 2010, 64, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.; Tucker, K.L. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr. Rev. 2004, 62, 177–203. [Google Scholar] [CrossRef] [PubMed]
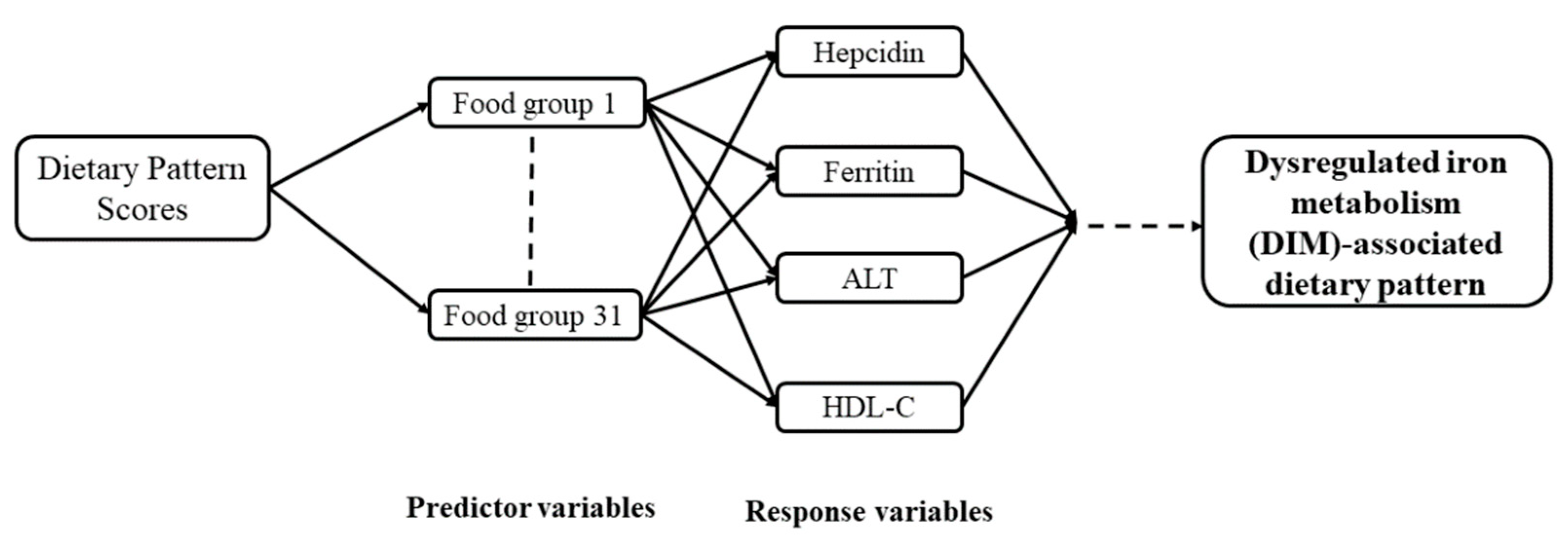
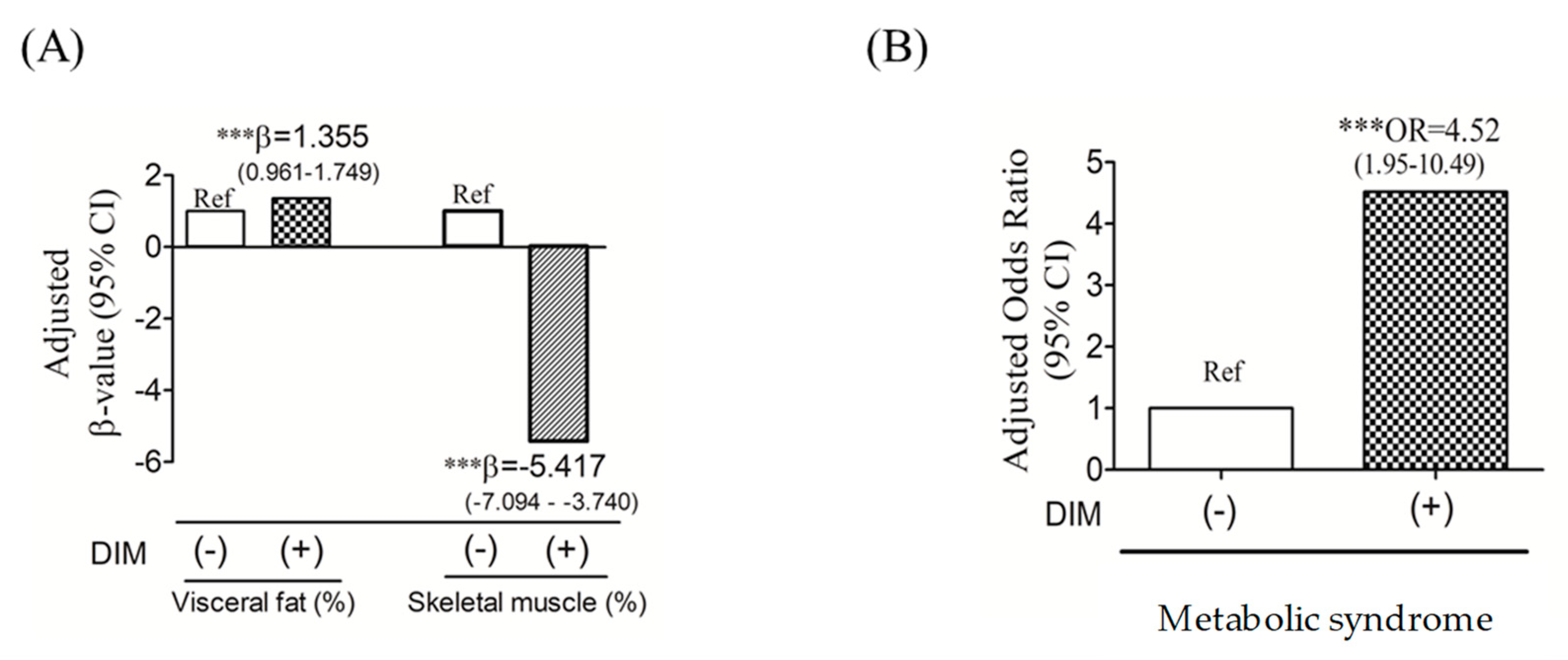
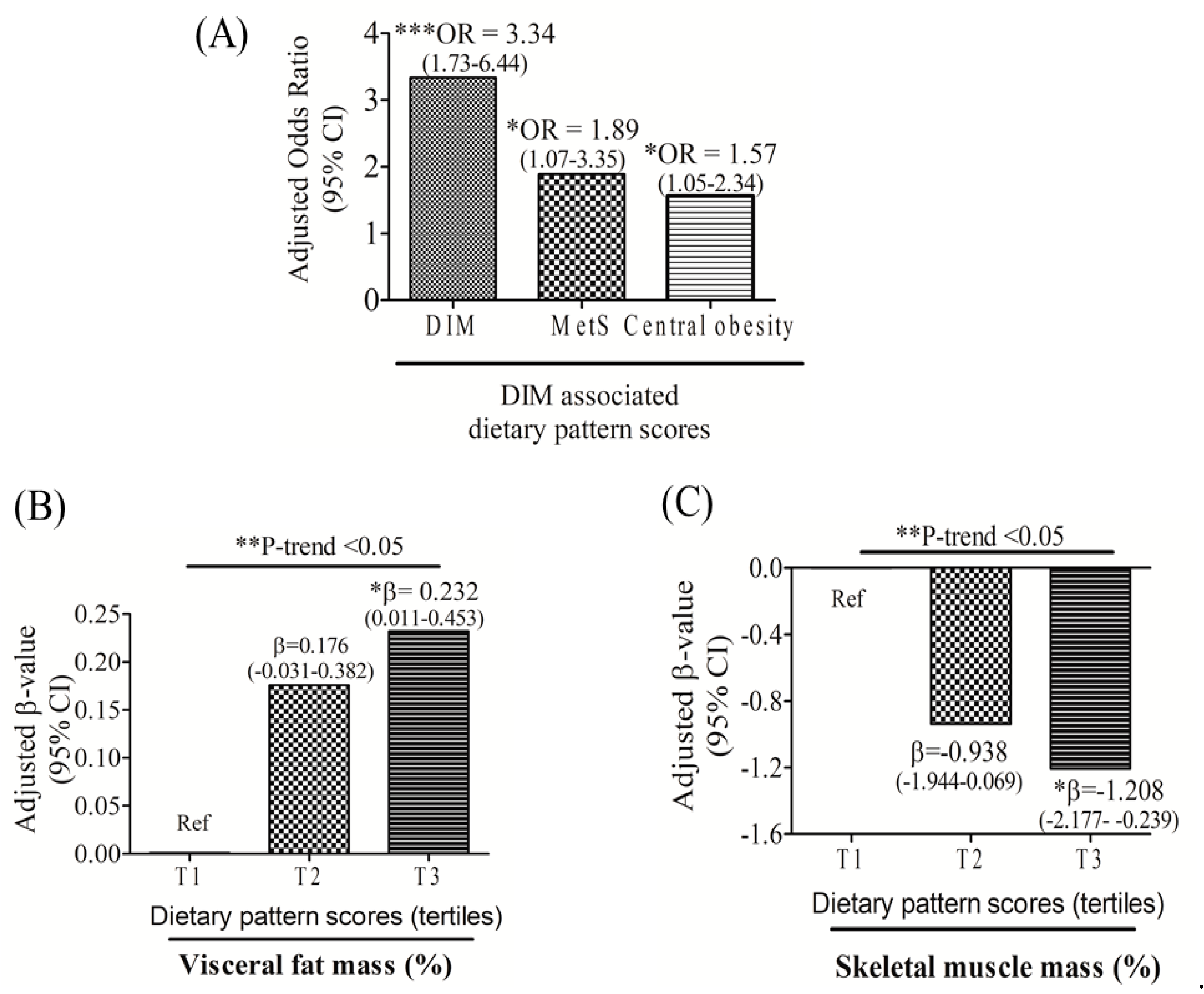
| Characteristic | Study Population (N = 208) | p Value * | |
|---|---|---|---|
| DIM (−) (n = 161) | DIM (+) (n = 47) | ||
| Age (years) | 40.55 ± 12.76 | 47.15 ± 10.82 | 0.001 |
| Gender (n, %) | |||
| Male | 81 (50.3) | 24 (51.1) | 0.928 |
| Female | 80 (49.7) | 23 (48.9) | |
| NAFLD (n, %) | 124 (77.0) | 46 (97.9) | 0.001 |
| MetS (n, %) | 22 (13.7) | 29 (61.7) | <0.001 |
| Central obesity (n, %) | 54 (33.5) | 45 (95.7) | <0.001 |
| Diabetes mellitus (n, %) | 15 (9.3) | 10 (21.3) | 0.027 |
| Dyslipidemia (n, %) | 32 (19.9) | 28 (59.6) | <0.001 |
| Hypertension (n, %) | 69 (42.9) | 35 (74.5) | <0.001 |
| Abnormal ALT (n, %) | 19 (11.8) | 23 (48.9) | <0.001 |
| Anthropometry | |||
| BMI (kg/m2) | 23.41 ± 4.37 | 28.59 ± 5.77 | <0.001 |
| Waist circumference (cm) | 83.04 ± 11.80 | 96.86 ± 12.88 | <0.001 |
| Male | 87.98 ± 9.32 | 101.63 ± 10.26 | <0.001 |
| Female | 78.04 ± 11.98 | 91.88 ± 13.65 | <0.001 |
| Body fat mass (%) | 26.47 ± 5.90 | 32.38 ± 5.53 | <0.001 |
| Skeletal muscle mass (%) | 67.79 ± 5.83 | 61.93 ± 5.48 | <0.001 |
| Visceral fat mass (%) | 3.37 ± 1.18 | 4.85 ± 1.23 | <0.001 |
| Glucose biomarkers | |||
| Fasting blood glucose (mg/dL) | 89.83 ± 16.35 | 97.36 ± 24.81 | 0.045 |
| Insulin (mIU/mL) | 9.62 ± 5.43 | 9.11 ± 6.08 | 0.327 |
| HbA1C (%) | 5.72 ± 0.83 | 6.16 ± 1.31 | 0.001 |
| Lipid biomarkers | |||
| Total cholesterol (mg/dL) | 197.45 ± 37.59 | 206.49 ± 38.13 | 0.052 |
| TGs (mg/dL) | 102.05 ± 67.34 | 174.23 ± 67.47 | <0.001 |
| HDL-C (mg/dL) | 59.00 ± 16.25 | 48.80 ± 11.81 | <0.001 |
| LDL-C (mg/dL) | 115.99 ± 32.15 | 127.85 ± 31.96 | 0.012 |
| Iron-related biomarkers | |||
| Hb (gm/dL) | 14.55 ± 2.41 | 15.66 ± 3.12 | 0.003 |
| Iron (µg/dL) | 102.97 ± 38.63 | 107.13 ± 34.14 | 0.632 |
| Hepcidin (ng/mL) | 118.61 ± 93.54 | 259.77 ± 67.59 | <0.001 |
| Ferritin (ng/mL) | 117.16 ± 121.84 | 263.64 ± 169.26 | <0.001 |
| TS (%) | 29.69 ± 12.62 | 30.33 ± 10.31 | 0.765 |
| Elevated hepcidin (n, %) | 38 (23.6) | 46 (97.9) | <0.001 |
| Anemia (n, %) | 13 (8.1) | 2 (4.3) | 0.373 |
| Iron-deficiency anemia (n, %) | 14 (8.7) | 0 (0.0) | 0.036 |
| Hyperferritinemia (n, %) | 8 (5.0) | 26 (55.3) | <0.001 |
| Liver injury and oxidative stress biomarkers | |||
| Nitrite oxide (μM) | 44.44 ± 28.01 | 61.67 ± 24.33 | <0.001 |
| ALT (U/L) | 26.14 ± 17.88 | 54.96 ± 37.98 | <0.001 |
| MDA (μM) | 40.13 ± 27.54 | 47.89 ± 21.23 | 0.005 |
| Variable | Univariate | Multivariate * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | ||||
| Age (years) | 1.044 | 1.02 | 1.07 | 0.002 | 1.036 | 0.977 | 1.099 | 0.235 | |
| Sex (n, %) | |||||||||
| Female | Ref | ||||||||
| Male | 1.031 | 0.54 | 1.97 | 0.928 | |||||
| BMI (kg/m2) | 1.224 | 1.13 | 1.32 | <0.001 | |||||
| Hemoglobin (g/dL) | 1.159 | 1.03 | 1.31 | 0.014 | |||||
| Iron (μg/dL) | 1.003 | 0.99 | 1.01 | 0.504 | |||||
| Hepcidin (ng/mL) | 1.02 | 1.01 | 1.02 | <0.001 | 1.018 | 1.010 | 1.025 | <0.001 | |
| Ferritin (ng/mL) | 1.01 | 1.00 | 1.01 | <0.001 | 1.007 | 1.002 | 1.012 | 0.005 | |
| Transferrin saturation (%) | 1.01 | 0.98 | 1.03 | 0.753 | |||||
| ALT (U/L) | 1.04 | 1.03 | 1.06 | <0.001 | 1.029 | 1.004 | 1.055 | 0.021 | |
| MDA (μM) | 1.01 | 0.998 | 1.02 | 0.115 | |||||
| Total cholesterol (mg/dL) | 1.01 | 0.998 | 1.02 | 0.151 | |||||
| Triglycerides (mg/dL) | 1.01 | 1.01 | 1.02 | <0.001 | 1.003 | 0.993 | 1.012 | 0.582 | |
| LDL-C (mg/dL) | 1.0 | 1.00 | 1.02 | 0.029 | |||||
| HDL-C (mg/dL) | 0.944 | 0.916 | 0.97 | <0.001 | 0.929 | 0.865 | 0.999 | 0.046 | |
| Fasting blood glucose (mg/dL) | 1.02 | 1.00 | 1.03 | 0.023 | |||||
| Insulin (mIU/mL) | 0.983 | 0.906 | 1.07 | 0.679 | |||||
| HbA1C (%) | 1.48 | 1.07 | 2.05 | 0.018 | 0.689 | 0.335 | 1.41 | 0.309 | |
| Visceral Fat Mass (%) | ||||||
|---|---|---|---|---|---|---|
| # Model 1 | * Model 2 | & Model 3 | ||||
| β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | |
| Hepcidin (ng/mL) | 0.003 (0.002–0.005) | <0.001 | 0.003 (0.002–0.005) | <0.001 | 0.001 (0.0001–0.002) | 0.025 |
| Ferritin (ng/mL) | 0.002 (0.0001–0.003) | 0.008 | 0.002 (0.0001–0.003) | 0.030 | 1.187 (−0.001–0.001) | 0.972 |
| ALT (U/L) | 0.020 (0.014–0.026) | <0.001 | 0.020 (0.014–0.026) | <0.001 | 0.004 (0.001–0.007) | 0.018 |
| HDL (mg/dL) | −0.030 (−0.041–−0.020) | <0.001 | −0.032 (−0.044–−0.021) | <0.001 | −0.005 (−0.011–0.001) | 0.109 |
| Skeletal muscle mass (%) | ||||||
| Hepcidin (ng/mL) | −0.003 (−0.011–0.004) | 0.388 | −0.014 (−0.021–−0.006) | <0.001 | −0.004 (−0.008–−0.0001) | 0.033 |
| Ferritin (ng/mL) | 0.005 (−0.001–0.010) | 0.102 | −0.006 (−0.012–0.0001) | 0.039 | −6.395 (−0.003–0.003) | 0.968 |
| ALT (U/L) | −0.067 (−0.097–−0.038) | <0.001 | −0.084 (−0.110–−0.059) | <0.001 | −0.020 (−0.035–−0.004) | 0.012 |
| HDL (mg/dL) | 0.045 (−0.007–0.097) | 0.087 | 0.142 (0.094–0.191) | <0.001 | 0.032 (0.004–0.060) | 0.027 |
| Food Groups | Explained Variation (%) | Factor Loading * |
|---|---|---|
| Deep-fried foods | 15.87 | 0.41 |
| Processed meats | 11.08 | 0.34 |
| Chicken and pork | 7.88 | 0.29 |
| Eating out | 7.33 | 0.28 |
| Coffee | 4.85 | 0.23 |
| Animal fat/skin | 4.80 | 0.22 |
| Steamed/boiled/raw food | 7.57 | −0.28 |
| Dairy products | 5.36 | −0.24 |
| Total explained variation (%): | 64.73 |
| Variable | Tertile of Dietary Pattern Scores $ | * p for Trend | #p Value | ||
|---|---|---|---|---|---|
| T1 (N = 69) | T2 (N = 69) | T3 (N = 69) | |||
| Age (years) | 41.32 ± 13.80 | 41.04 ± 13.13 | 43.81 ± 10.86 | 0.730 | 0.752 |
| Sex (n, %) | |||||
| Male | 24 (34.8) | 37 (53.6) | 44 (63.8) | 0.003 | 0.002 |
| Female | 45 (65.2) | 32 (46.4) | 25 (36.2) | ||
| NAFLD (n, %) | 56 (81.2) | 55 (79.7) | 58 (84.1) | 0.798 | 1.000 |
| Metabolic syndrome (n, %) | 10 (14.5) | 18 (26.1) | 22 (31.9) | 0.052 | 0.051 |
| Central obesity (n, %) | 26 (37.7) | 32 (46.4) | 40 (58.0) | 0.057 | 0.051 |
| Diabetes mellitus (n, %) | 7 (10.1) | 11 (15.9) | 7 (10.1) | 0.483 | 1.000 |
| Dyslipidemia (n, %) | 14 (20.3) | 23 (33.3) | 23 (33.3) | 0.149 | 0.277 |
| Hypertension (n, %) | 32 (46.4) | 34 (49.3) | 37 (53.6) | 0.693 | 1.000 |
| DIM (n, %) | 9 (13.0) | 13 (18.8) | 25 (36.2) | 0.003 | 0.003 |
| Abnormal ALT (n, %) | 7 (10.1) | 15 (21.7) | 20 (29.0) | 0.021 | 0.018 |
| Anthropometry | |||||
| BMI (kg/m2) | 23.13 ± 5.13 | 24.83 ± 5.35 | 25.60 ± 4.62 | 0.005 | 0.014 |
| Waist circumference (cm) | 81.69 ± 13.13 | 86.98 ± 13.92 | 89.41 ± 11.52 | 0.001 | 0.002 |
| Male | 89.26 ± 12.84 | 91.72 ± 11.54 | 91.58 ± 9.80 | 0.415 | 1.000 |
| Female | 77.65 ± 11.50 | 81.50 ± 14.59 | 85.59 ± 13.43 | 0.016 | 0.048 |
| Body fat mass (%) | 26.86 ± 6.10 | 27.97 ± 6.12 | 28.43 ± 6.59 | 0.142 | 0.427 |
| Skeletal muscle mass (%) | 67.40 ± 6.02 | 66.30 ± 6.06 | 65.85 ± 6.53 | 0.144 | 0.431 |
| Visceral fat mass (%) | 3.27 ± 1.28 | 3.75 ± 1.33 | 4.05 ± 1.30 | 0.001 | 0.002 |
| Glucose biomarkers | |||||
| Fasting blood glucose (mg/dL) | 88.88 ± 13.27 | 92.96 ± 17.93 | 92.70 ± 23.82 | 0.236 | 0.708 |
| Insulin (mIU/mL) | 10.02 ± 6.24 | 8.42 ± 4.41 | 9.84 ± 5.92 | 0.894 | 1.000 |
| HbA1C (%) | 5.67 ± 0.69 | 5.91 ± 1.06 | 5.88 ± 1.12 | 0.198 | 0.595 |
| Lipid biomarkers | |||||
| Total cholesterol (mg/dL) | 198.84 ± 30.93 | 199.25 ± 40.46 | 201.35 ± 41.11 | 0.697 | 1.000 |
| Triglyceride (mg/dL) | 100.93 ± 71.69 | 121.74 ± 76.02 | 132.46 ± 71.40 | 0.012 | 0.036 |
| HDL-C (mg/dL) | 61.73 ± 16.02 | 54.10 ± 15.39 | 54.50 ± 15.34 | 0.007 | 0.779 |
| LDL-C (mg/dL) | 115.30 ± 28.60 | 119.81 ± 34.02 | 121.54 ± 34.24 | 0.260 | 0.021 |
| Iron-related biomarkers | |||||
| Hemoglobin (g/dL) | 14.31 ± 2.57 | 14.91 ± 2.62 | 15.24 ± 2.60 | 0.036 | 0.109 |
| Iron (μg/dL) | 101.75 ± 38.06 | 107.06 ± 39.20 | 104.10 ± 34.79 | 0.713 | 1.000 |
| Hepcidin (ng/mL) | 123.97 ± 93.68 | 150.94 ± 115.39 | 178.80 ± 101.92 | 0.002 | 0.007 |
| Ferritin (ng/mL) | 103.59 ± 130.04 | 144.32 ± 129.73 | 204.96 ± 162.65 | <0.001 | <0.001 |
| Transferrin saturation (%) | 28.76 ± 12.17 | 31.07 ± 12.88 | 30.03 ± 11.02 | 0.536 | 1.000 |
| Elevated serum hepcidin (n, %) | 12 (17.4) | 21 (30.4) | 31 (44.9) | 0.010 | 0.007 |
| Anemia (n, %) | 7 (10.1) | 4 (5.8) | 3 (4.3) | 0.369 | 0.533 |
| Iron-deficiency anemia (n, %) | 7 (10.1) | 5 (7.2) | 1 (1.4) | 0.100 | 0.107 |
| Hyperferritinemia (n, %) | 5 (7.2) | 11 (15.9) | 19 (27.5) | 0.006 | 0.004 |
| Liver injury and oxidative stress biomarkers | |||||
| Nitrite oxide (μM) | 41.97 ± 21.76 | 54.79 ± 33.36 | 48.90 ± 26.90 | 0.193 | 0.579 |
| ALT (U/L) | 25.77 ± 18.71 | 32.30 ± 24.51 | 40.14 ± 33.35 | 0.001 | 0.004 |
| MDA (μM) | 43.41 ± 30.40 | 38.21 ± 19.32 | 44.69 ± 28.12 | 0.798 | 1.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cempaka, A.R.; Tseng, S.-H.; Yuan, K.-C.; Bai, C.-H.; Tinkov, A.A.; Skalny, A.V.; Chang, J.-S. Dysregulated Iron Metabolism-Associated Dietary Pattern Predicts an Altered Body Composition and Metabolic Syndrome. Nutrients 2019, 11, 2733. https://doi.org/10.3390/nu11112733
Cempaka AR, Tseng S-H, Yuan K-C, Bai C-H, Tinkov AA, Skalny AV, Chang J-S. Dysregulated Iron Metabolism-Associated Dietary Pattern Predicts an Altered Body Composition and Metabolic Syndrome. Nutrients. 2019; 11(11):2733. https://doi.org/10.3390/nu11112733
Chicago/Turabian StyleCempaka, Anggun Rindang, Sung-Hui Tseng, Kuo-Ching Yuan, Chyi-Huey Bai, Alexey A. Tinkov, Anatoly V. Skalny, and Jung-Su Chang. 2019. "Dysregulated Iron Metabolism-Associated Dietary Pattern Predicts an Altered Body Composition and Metabolic Syndrome" Nutrients 11, no. 11: 2733. https://doi.org/10.3390/nu11112733
APA StyleCempaka, A. R., Tseng, S.-H., Yuan, K.-C., Bai, C.-H., Tinkov, A. A., Skalny, A. V., & Chang, J.-S. (2019). Dysregulated Iron Metabolism-Associated Dietary Pattern Predicts an Altered Body Composition and Metabolic Syndrome. Nutrients, 11(11), 2733. https://doi.org/10.3390/nu11112733





