Validation of a Vitamin D Specific Questionnaire to Determine Vitamin D Status in Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study 1: Biomarker Validation
2.1.1. Study Population
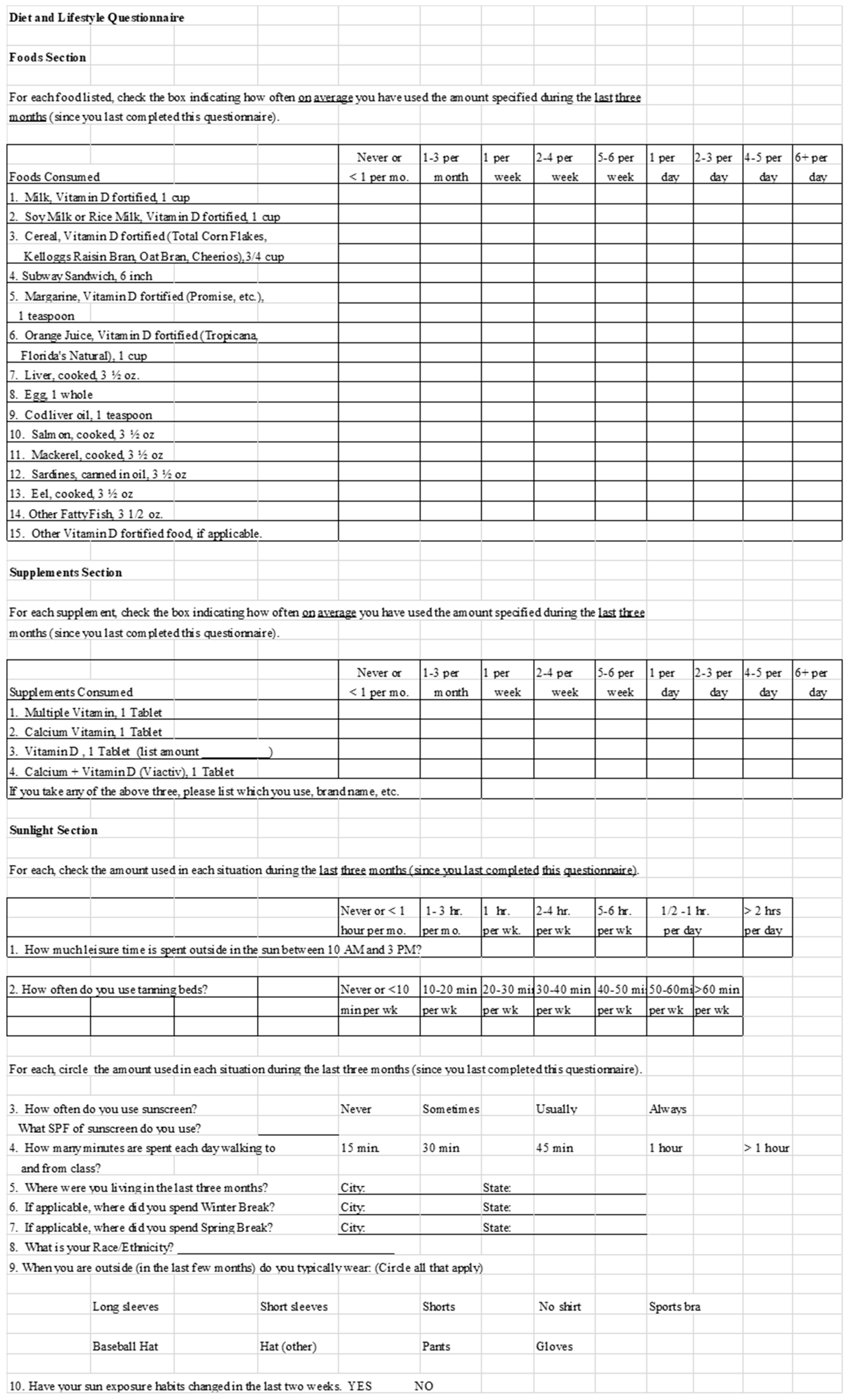
2.1.2. Food Frequency and Lifestyle Questionnaire
2.1.3. Serum 25(OH)D Concentration/Vitamin D Status
2.1.4. Statistical Analysis
2.2. Study 2: Food Diary Cross-Validation
2.2.1. Study Population
2.2.2. Food Frequency and Lifestyle Questionnaire
2.2.3. Food Diaries
2.2.4. Statistical Analysis
3. Results
3.1. Study 1: Biomarker Validation
3.1.1. Physical Characteristics and Vitamin D Status
3.1.2. Vitamin D Intake and Association between Intake and Status
3.1.3. UV Exposure and Association between Vitamin D Status and UV Exposure
3.1.4. Predictors of Serum 25(OH)D
3.2. Study 2: Food Diary Cross-Validation
3.2.1. Physical Characteristics and Vitamin D Intake
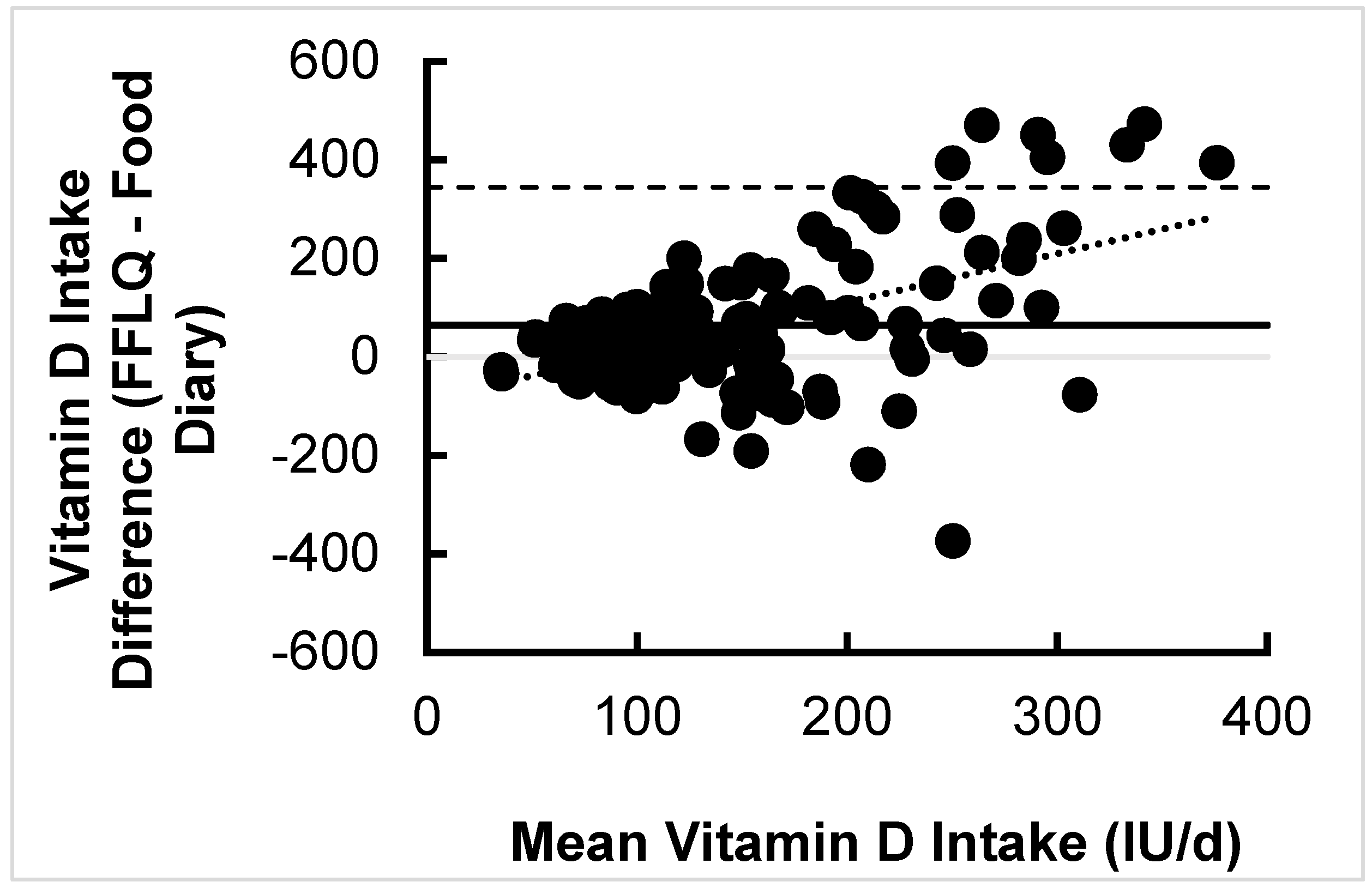
3.2.2. Association and Difference between Vitamin D Intake Estimates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
References
- Larson-Meyer, D.E.; Willis, K.S. Vitamin D and athletes. Curr. Sports Med. Rep. 2010, 9, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E. The Importance of Vitamin D for Athletes. Sports Sci. Exch. 2015, 28, 1–8. [Google Scholar]
- Halliday, T.; Peterson, N.; Thomas, J.; Kleppinger, K.; Hollis, B.; Larson-Meyer, D. Vitamin D Status Relative to Diet, Lifestyle, Injury and Illness in College Athletes. Med. Sci. Sports Exerc. 2011, 42, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen-Veromaa, M.; Mottonen, T.; Irjala, K.; Karkkainen, M.; Lamberg-Allardt, C.; Hakola, P.; Viikari, J. Vitamin D intake is low and hypovitaminosis D common in healthy 9- to 15-year-old Finnish girls. Eur. J. Clin. Nutr. 1999, 53, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Constantini, N.W.; Arieli, R.; Chodick, G.; Dubnov-Raz, G. High prevalence of vitamin D insufficiency in athletes and dancers. Clin. J. Sport Med. 2010, 20, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.; Grantham, J.; Racinais, S.; Chalabi, H. Vitamin D deficiency is endemic in Middle Eastern sportsmen. Public Health Nutr. 2010, 13, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.E.; Thomas, J.J.; Hollis, B.W.; Larson-Meyer, D.E. Relation Between Vitamin D Status and Body Composition in Collegiate Athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C.; Lu, Z.; Sauter, E. Vitamin D and skin physiology: A D-lightful story. J. Bone Min. Res. 2007, 22 (Suppl. 2), V28–V33. [Google Scholar] [CrossRef]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, L.Y.; Ide, L.; Wortsman, J.; MacLaughlin, J.A.; Holick, M.F. Sunscreens suppress cutaneous vitamin D3 synthesis. J. Clin. Endocrinol. Metab. 1987, 64, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, L.Y.; Wortsman, J.; Dannenberg, M.J.; Hollis, B.W.; Lu, Z.; Holick, M.F. Clothing prevents ultraviolet-B radiation-dependent photosynthesis of vitamin D3. J. Clin. Endocrinol. Metab. 1992, 75, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Oh, I.H.; Baek, H.J.; Lee, C.H.; Lee, S.S. Effects of sun exposure and dietary vitamin D intake on serum 25-hydroxyvitamin D status in hemodialysis patients. Nutr. Res. Pract. 2015, 9, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Woolf, K.; Burke, L. Assessment of Nutrient Status in Athletes and the Need for Supplementation. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Barcal, J.N.; Thomas, J.T.; Hollis, B.W.; Austin, K.J.; Alexander, B.M.; Larson-Meyer, D.E. Vitamin D and Weight Cycling: Impact on Injury, Illness, and Inflammation in Collegiate Wrestlers. Nutrients 2016, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.S.; Peterson, B.J.; Wilson, P.B.; Rhodes, G.S.; Ingraham, S.J. Vitamin D status is associated with adiposity in male ice hockey players. Med. Sci. Sports Exerc. 2015, 47, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.M.; Redzic, M.; Thomas, D.T. The effects of season-long vitamin d supplementation on collegiate swimmers and divers. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, K.; Pritchett, R.C.; Stark, L.; Broad, E.; LaCroix, M. Effect of Vitamin D Supplementation on 25(OH)D Status in Elite Athletes With Spinal Cord Injury. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.M.; Seechurn, T.; Atkinson, S.A. A food frequency questionnaire for the assessment of calcium, vitamin D and vitamin K: A pilot validation study. Nutrients 2010, 2, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Hacker-Thompson, A.; Sellmeyer, D.E.; Schloetter, M. Validation of a Dietary Vitamin D Questionnaire Using Multiple Diet Records and the Block 98 Health Habits and History Questionnaire in Healthy Postmenopausal Women in Northern California. J. Acad. Nutr. Diet. 2012, 112, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Glabska, D.; Guzek, D.; Sidor, P.; Wlodarek, D. Vitamin D Dietary Intake Questionnaire Validation Conducted among Young Polish Women. Nutrients 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, L.; Lind, T.; Åkeson, P.K.; Sandström, A.K. A validation study of an interviewer-administered short food frequency questionnaire in assessing dietary vitamin D and calcium intake in swedish children. Nutrients 2017, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gozdzik, A.; Barta, J.L.; Wagner, D. The development and evaluation of a food frequency questionnaire used in assessing vitamin D intake in a sample of healthy young Canadian adults of diverse ancestry. Nutr. Res. 2009, 29, 255–261. [Google Scholar] [CrossRef] [PubMed]
- USDA. USDA Food Composition Databases. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 23 July 2019).
- Chen, T.C.; Chimeh, F.; Lu, Z.; Mathieu, J.; Person, K.S.; Zhang, A.; Kohn, N.; Martinello, S.; Berkowitz, R.; Holick, M.F. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch. Biochem. Biophys. 2007, 460, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Cannell, J.J.; Hollis, B.W.; Zasloff, M.; Heaney, R.P. Diagnosis and treatment of vitamin D deficiency. Expert Opin. Pharmacother. 2008, 9, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.C.; Huffman, A.E.; Yoder, H.A.; Dolci, A.; Perrier, E.T.; Larson-Meyer, D.E.; Armstrong, L.E. Urinary markers of hydration during 3-day water restriction and graded rehydration. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Kiely, M.; Collins, A.; Lucey, A.J.; Andersen, R. Development, validation and implementation of a quantitative food frequency questionnaire to assess habitual vitamin D intake. J. Hum. Nutr. Diet. 2016, 29, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Weir, R.R.; Weir, R.R.; Carson, E.L.; Mulhern, M.S.; Laird, E. Validation of a food frequency questionnaire to determine vitamin D intakes using the method of triads. J. Hum. Nutr. Diet. 2016, 29, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.D.; Nieman, D.C. Nutritional Assessment, 6th ed.; McGraw Hill: New York, NY, USA, 2013. [Google Scholar]
- Jones, K.S.; Assar, S.; Harnpanich, D.; Bouillon, R.; Lambrechts, D.; Prentice, A.; Schoenmakers, I. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J. Clin. Endocrinol. Metab. 2014, 99, 3373–3381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cade, J.E.; Burley, V.J.; Warm, D.L.; Thompson, R.L.; Margetts, B.M. Food-frequency questionnaires: A review of their design, validation and utilisation. Nutr. Res. Rev. 2004, 17, 5–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burild, A.; Frandsen, H.L.; Poulsen, M.; Jakobsen, J. Tissue content of vitamin D3 and 25-hydroxy vitamin D3 in minipigs after cutaneous synthesis, supplementation and deprivation of vitamin D3. Steroids 2015, 98, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Martinaityte, I.; Kamycheva, E.; Didriksen, A.; Jakobsen, J.; Jorde, R. Vitamin D Stored in Fat Tissue During a 5-Year Intervention Affects Serum 25-Hydroxyvitamin D Levels the Following Year. J. Clin. Endocrinol. Metab. 2017, 102, 3731–3738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassity, E.P.; Redzic, M.; Teager, C.R.; Thomas, D.T. The effect of body composition and BMI on 25(OH)D response in vitamin D-supplemented athletes. Eur. J. Sport Sci. 2016, 16, 773–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A. Serum 25-hydroxy vitamin D levels in relation to body mass index: A systematic review and meta-analysis: 25(OH) vitamin D and BMI. Obes. Rev. 2013, 14, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Tosunbayraktar, G.; Bas, M.; Kut, A.; Buyukkaragoz, A.H. Low serum 25(OH)D levels are assocıated to hıgher BMI and metabolic syndrome parameters in adult subjects in Turkey. Afr. Health Sci. 2015, 15, 1161–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsen, J.; Jakobsen, J.; Maribo, H.; Bysted, A.; Sommer, H.M. 25-Hydroxyvitamin D-3 affects vitamin D status similar to vitamin D-3 in pigs—But the meat produced has a lower content of vitamin D. Br. J. Nutr. 2007, 98, 908–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Køster, B.; Søndergaard, J.; Nielsen, J.B.; Allen, M.; Olsen, A.; Bentzen, J. The validated sun exposure questionnaire: Association of objective and subjective measures of sun exposure in a Danish population-based sample. Br. J. Dermatol. 2017, 176, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Koutkia, P.; Lu, Z.; Chen, T.C.; Holick, M.F. Treatment of vitamin d deficiency due to crohn’s disease with tanning bed ultraviolet b radiation. Gastroenterology 2001, 121, 1485–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holden, J.M.; Lemar, L.E. Assessing vitamin D contents in foods and supplements: Challenges and needs. Am. J. Clin. Nutr. 2008, 88, 551S–553S. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Ingold, B.C.; Fensterseifer, S.R.; Austin, K.J.; Wechsler, P.J.; Hollis, B.W.; Makowski, A.J.; Alexander, B.M. Sun exposure in pigs increases the vitamin D nutritional quality of pork. PLoS ONE 2017, 12, e0187877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, C.L.; Patterson, K.Y.; Roseland, J.M.; Wise, S.A.; Merkel, J.M.; Pehrsson, P.R.; Yetley, E.A. Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. J. Nutr. 2014, 144, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Fall (n = 86) | Winter (n = 49) | Spring (n = 67) | |
|---|---|---|---|
| Male:Female | 54:32 | 30:19 | 44:23 |
| Race/Ethnicity (C, A, H, B) | 78/3/2/3 | 45/2/1/1 | 60/2/1/4 |
| Age (years) | 20.2 ± 1.5 | 20.4 ± 1.7 | 20.6 ± 1.6 |
| BMI (kg/m2) | 23.8 ± 3.8 | 24.1 ± 4.2 | 23.9 ± 3.7 |
| Sport (Indoor/Outdoor) | 58/28 | 26/23 | 49/18 |
| 25(OH)D (ng/mL) | 42.4 ± 15.5 | 27.9 ± 9.0 | 35.0 ± 15.5 |
| Total Vitamin D Intake (IU/day) | 800 ± 820 | 1236 ± 1131 | 727 ± 977 |
| Vitamin D from Food Only (IU/day) | 341 ± 228 | 584 ± 593 | 393 ± 616 |
| Vitamin D from Supplements Only (IU/day) | 459 ± 777 | 653 ± 987 | 334 ± 764 |
| Leisure Time Outside in the Sun (h/day) | 0.8 ± 0.7 | 0.7 ± 0.5 | 0.6 ± 0.6 |
| Tanning Bed Use (h/day) | 0.0008 ± 0.004 | 0.0002 ± 0.001 | 0.001 ± 0.004 |
| Total UV Exposure (h/day) | 0.83 ± 0.69 | 0.65 ± 0.52 | 0.61 ± 0.63 |
| Correlation Coefficients | BMI | Vitamin D from Food Per Day | Vitamin D from Supplements Per Day | Total Vitamin D Per Day | Leisure Time Outside Per Day | Tanning Bed Use Per Day | Total UV Exposure Per Day | Frequency Sunscreen Use |
|---|---|---|---|---|---|---|---|---|
| Fall 25(OH)D (n = 86) | 0.330** | −0.126 | 0.280** | 0.133 | 0.130 | 0.176 | 0.133 | 0.226* |
| Winter 25(OH)D (n = 49) | −0.324* | −0.332* | 0.152 | −0.080 | 0.079 | 0.189 | 0.130 | 0.08 |
| Spring 25(OH)D (n = 67) | 0.472** | 0.008 | 0.155 | 0.126 | 0.074 | 0.391** | 0.096 | 0.479** |
| Fall Model (R = 0.424, p < 0.001) | Winter Model (R = 0.315, p = 0.029) | Spring Model (R = 0.516, p<0.001) | |||
|---|---|---|---|---|---|
| Predictor | Beta | Predictor | Beta | Predictor | Beta |
| BMI | −0.313 | BMI | −0.315 | BMI | −0.376 |
| Tanning Bed Use | 0.210 | Tanning Bed Use | 0.337 | ||
| Outside Exposure | 0.213 |
| Characteristic | Mean (n = 123) |
|---|---|
| Male:Female | 60:63 |
| Race/Ethnicity (C, A, H, B, AI, O) | 112/5/3/1/1/1 |
| Age (years) | 31.3 ± 8.5 |
| BMI (kg/m2) | 24.8 ± 4.8 |
| FFLQ Vitamin D Intake (IU/day) | 186.4 ± 125.7 |
| FFLQ Vitamin D Intake from Food Plus Supplements (IU/day) | 341.5 ± 533.4 |
| Food Diary Vitamin D Intake (IU/day) | 148.5 ± 228.2 |
| Food Diary Caloric Intake (kcal/day) | 1871.0 ± 474.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larson-Meyer, D.E.; Douglas, C.S.; Thomas, J.J.; Johnson, E.C.; Barcal, J.N.; Heller, J.E.; Hollis, B.W.; Halliday, T.M. Validation of a Vitamin D Specific Questionnaire to Determine Vitamin D Status in Athletes. Nutrients 2019, 11, 2732. https://doi.org/10.3390/nu11112732
Larson-Meyer DE, Douglas CS, Thomas JJ, Johnson EC, Barcal JN, Heller JE, Hollis BW, Halliday TM. Validation of a Vitamin D Specific Questionnaire to Determine Vitamin D Status in Athletes. Nutrients. 2019; 11(11):2732. https://doi.org/10.3390/nu11112732
Chicago/Turabian StyleLarson-Meyer, D. Enette, Corey S. Douglas, Joi J. Thomas, Evan C. Johnson, Jacqueline N. Barcal, Jenna E. Heller, Bruce W. Hollis, and Tanya M. Halliday. 2019. "Validation of a Vitamin D Specific Questionnaire to Determine Vitamin D Status in Athletes" Nutrients 11, no. 11: 2732. https://doi.org/10.3390/nu11112732
APA StyleLarson-Meyer, D. E., Douglas, C. S., Thomas, J. J., Johnson, E. C., Barcal, J. N., Heller, J. E., Hollis, B. W., & Halliday, T. M. (2019). Validation of a Vitamin D Specific Questionnaire to Determine Vitamin D Status in Athletes. Nutrients, 11(11), 2732. https://doi.org/10.3390/nu11112732







