Plant-Based Diets in the Reduction of Body Fat: Physiological Effects and Biochemical Insights
Abstract
:1. Introduction
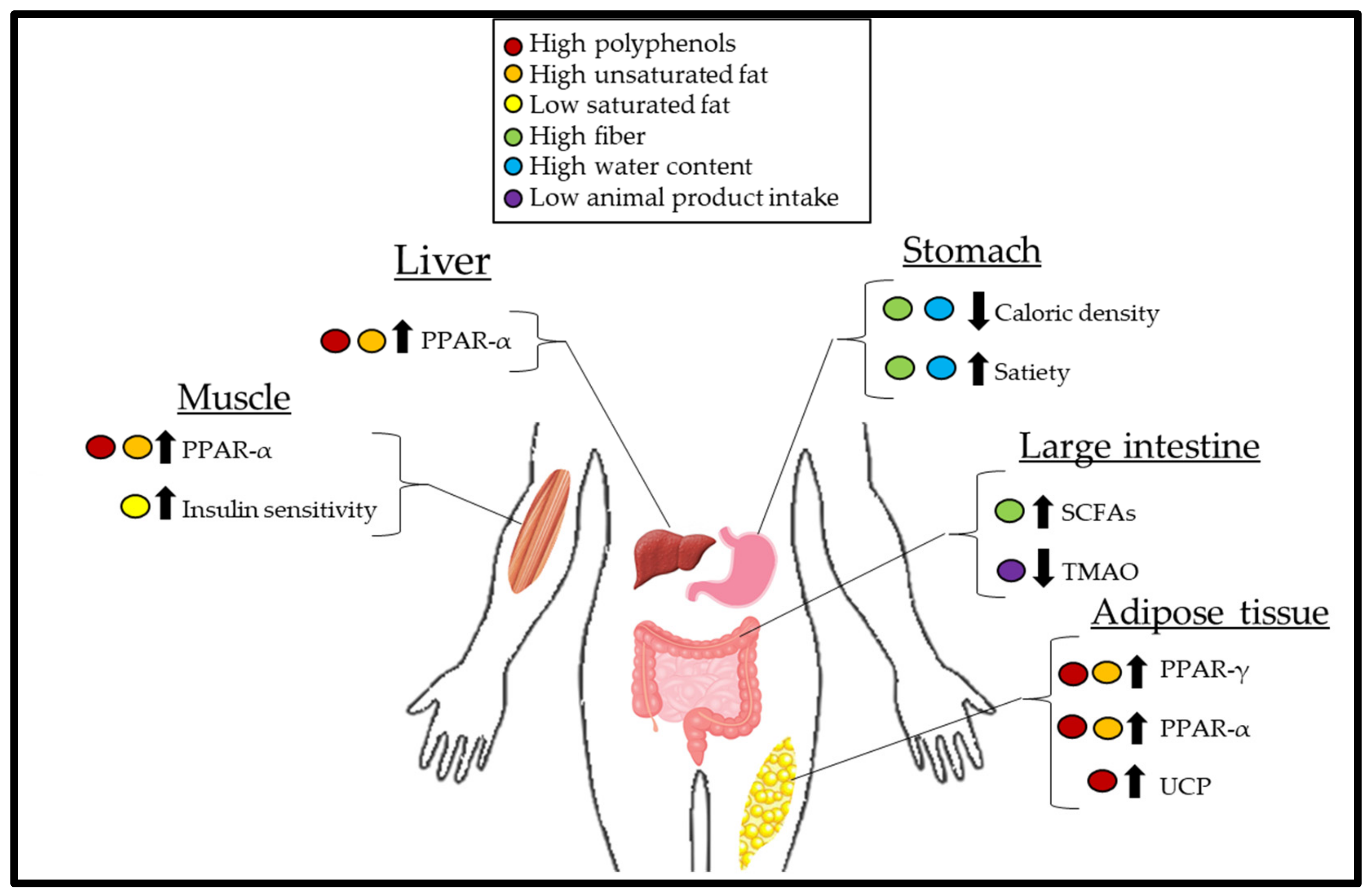
2. Mechanisms of Weight Loss
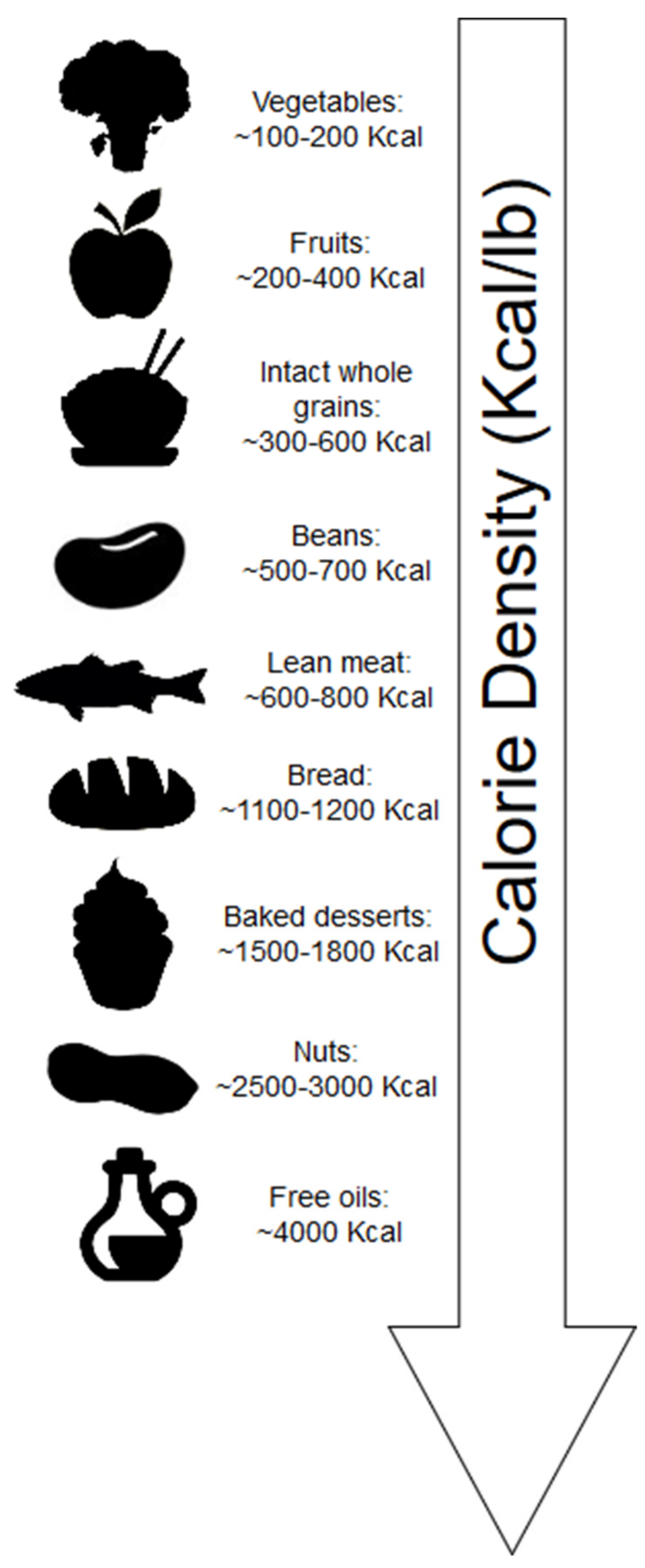
2.1. Calorie Density
2.2. Role of the Gut Microbiota
2.3. Insulin Sensitivity, Carbohydrates, and Diet-Induced Thermogenesis
2.4. Obesogenic Effects of Trimethylamine-N-Oxide
2.5. Unsaturated Fatty Acids and the Role of PPAR
2.6. The Role of Polyphenols on Uncoupling Proteins (UCP) and PPAR
3. Considerations for Health beyond Weight Loss
3.1. Plant-Based Versus Animal-Based Diets for Weight Loss
3.2. Health Effects of Plant-Based Diets
4. Conclusions
Funding
Conflicts of Interest
References
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Peeters, A.; Barendregt, J.J.; Willekens, F.; Mackenbach, J.P.; Al Mamun, A.; Bonneux, L. NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group. Obesity in adulthood and its consequences for life expectancy: A life-table analysis. Ann. Intern. Med. 2003, 138, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Prospective Studies Collaboration; Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef]
- Adams, K.F.; Leitzmann, M.F.; Ballard-Barbash, R.; Albanes, D.; Harris, T.B.; Hollenbeck, A.; Kipnis, V. Body mass and weight change in adults in relation to mortality risk. Am. J. Epidemiol. 2014, 179, 135–144. [Google Scholar] [CrossRef]
- Kaila, B.; Raman, M. Obesity: A review of pathogenesis and management strategies. Can. J. Gastroenterol. 2008, 22, 61–68. [Google Scholar] [CrossRef]
- Barnard, N.D.; Levin, S.M.; Yokoyama, Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J. Acad. Nutr. Diet. 2015, 115, 954–969. [Google Scholar] [CrossRef]
- Huang, R.Y.; Huang, C.C.; Hu, F.B.; Chavarro, J.E. Vegetarian diets and weight reduction: A meta-analysis of randomized controlled trials. J. Gen. Intern. Med. 2016, 31, 109–116. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.M.; Davidson, C.R.; Wingard, E.E.; Wilcox, S.; Frongillo, E.A. Comparative effectiveness of plant-based diets for weight loss: A randomized controlled trial of five different diets. Nutrition 2015, 31, 350–358. [Google Scholar] [CrossRef]
- Ferdowsian, H.R.; Barnard, N.D.; Hoover, V.J.; Katcher, H.I.; Levin, S.M.; Green, A.A.; Cohen, J.L. A multicomponent intervention reduces body weight and cardiovascular risk at a GEICO corporate site. Am. J. Health Promot. 2010, 24, 384–387. [Google Scholar] [CrossRef]
- Wright, N.; Wilson, L.; Smith, M.; Duncan, B.; McHugh, P. The BROAD study: A randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr. Diabetes 2017, 7, e256. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Butler, T.; Yan, R.; Fraser, G.E. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 2009, 32, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.; Carlson, J. Carbohydrates. Adv. Nutr. 2014, 5, 760–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolls, B.J. The relationship between dietary energy density and energy intake. Physiol. Behav. 2009, 97, 609–615. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, M.C.; Sichieri, R.; Mozzer, R.A.V. A low-energy-dense diet adding fruit reduces weight and energy intake in women. Appetite 2008, 51, 291–295. [Google Scholar] [CrossRef]
- Flood-Obbagy, J.E.; Rolls, B.J. The effect of fruit in different forms on energy intake and satiety at a meal. Appetite 2009, 52, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Rolls, B.J.; Roe, L.S.; Meengs, J.S. Reductions in portion size and energy density of foods are additive and lead to sustained decreases in energy intake. Am. J. Clin. Nutr. 2006, 83, 11–17. [Google Scholar] [CrossRef]
- Pérez-Escamilla, R.; Obbagy, J.E.; Altman, J.M.; Essery, E.V.; McGrane, M.M.; Wong, Y.P.; Spahn, J.M.; Williams, C.L. Dietary energy density and body weight in adults and children: A systematic review. J. Acad. Nutr. Diet. 2012, 112, 671–684. [Google Scholar] [CrossRef]
- Shintani, T.T.; Hughes, C.K.; Beckham, S.; O’Connor, H.K. Obesity and cardiovascular risk intervention through the ad libitum feeding of traditional Hawaiian diet. Am. J. Clin. Nutr. 1991, 53, 1647S–1651S. [Google Scholar] [CrossRef]
- Najjar, R.S.; Moore, C.E.; Montgomery, B.D. A defined, plant-based diet utilized in an outpatient cardiovascular clinic effectively treats hypercholesterolemia and hypertension and reduces medications. Clin. Cardiol. 2018, 41, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Najjar, R.S.; Moore, C.E.; Montgomery, B.D. Consumption of a defined, plant-based diet reduces lipoprotein(a), inflammation, and other atherogenic lipoproteins and particles within 4 weeks. Clin. Cardiol. 2018, 41, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, K.M.; Conterno, L.; Gasperotti, M.; Viola, R. Up-regulating the human intestinal microbiome using whole plant foods, polyphenols, and/or fiber. J. Agric. Food Chem. 2012, 60, 8776–8782. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Castaner, O.; Goday, A.; Park, Y.M.; Lee, S.H.; Magkos, F.; Shiow, S.A.T.E.; Schröder, H. The gut microbiome profile in obesity: A systematic review. Int. J. Endocrinol. 2018, 2018, 4095789. [Google Scholar] [CrossRef]
- Lin, H.; An, Y.; Tang, H.; Wang, Y. Alterations of bile acids and gut microbiota in obesity induced by high fat diet in rat model. J. Agric. Food Chem. 2019, 67, 3624–3632. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef] [Green Version]
- Fiorucci, S.; Mencarelli, A.; Palladino, G.; Cipriani, S. Bile-acid-activated receptors: Targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol. Sci. 2009, 30, 570–580. [Google Scholar] [CrossRef]
- Islam, K.B.M.S.; Fukiya, S.; Hagio, M.; Fujii, N.; Ishizuka, S.; Ooka, T.; Ogura, Y.; Hayashi, T.; Yokota, A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011, 141, 1773–1781. [Google Scholar] [CrossRef]
- Wu, C.C.; Weng, W.L.; Lai, W.L.; Tsai, H.P.; Liu, W.H.; Lee, M.H.; Tsai, Y.C. Effect of lactobacillus plantarum strain K21 on high-fat diet-fed obese mice. Evid. Based Complement. Alternat. Med. 2015, 2015, 391767. [Google Scholar] [CrossRef]
- Ibrugger, S.; Vigsnaes, L.K.; Blennow, A.; Skuflic, D.; Raben, A.; Lauritzen, L.; Kristensen, M. Second meal effect on appetite and fermentation of wholegrain rye foods. Appetite 2014, 80, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Mollard, R.C.; Wong, C.L.; Luhovyy, B.L.; Anderson, G.H. First and second meal effects of pulses on blood glucose, appetite, and food intake at a later meal. Appl. Physiol. Nutr. Metab. 2011, 36, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Jenkins, D.J.; Ocana, A.M.; Rao, V.A.; Collier, G.R. Second-meal effect: Low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am. J. Clin. Nutr. 1988, 48, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Wolever, T.; Taylor, R.H.; Griffiths, C.; Krzeminska, K.; Lawrie, J.A.; Bennett, C.M.; Goff, D.V.; Sarson, D.L.; Bloom, S.R. Slow release dietary carbohydrate improves second meal tolerance. Am. J. Clin. Nutr. 1982, 35, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Mollard, R.C.; Wong, C.L.; Luhovyy, B.L.; Cho, F.; Anderson, G.H. Second-meal effects of pulses on blood glucose and subjective appetite following a standardized meal 2 h later. Appl. Physiol. Nutr. Metab. 2014, 39, 849–851. [Google Scholar] [CrossRef]
- Kristensen, M.D.; Bendsen, N.T.; Christensen, S.M.; Astrup, A.; Raben, A. Meals based on vegetable protein sources (beans and peas) are more satiating than meals based on animal protein sources (veal and pork)—A randomized cross-over meal test study. Food Nutr. Res. 2016, 60, 32634. [Google Scholar] [CrossRef]
- Brighenti, F.; Benini, L.; Del Rio, D.; Casiraghi, C.; Pellegrini, N.; Scazzina, F.; Jenkins, D.J.; Vantini, I. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am. J. Clin. Nutr. 2006, 83, 817–822. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.-H.; Wu, C.-Y. The gut microbiome in obesity. J. Formos. Med. Assoc. 2019, 118, S3–S9. [Google Scholar] [CrossRef]
- Nilsson, A.; Johansson, E.; Ekström, L.; Björck, I. Effects of a brown beans evening meal on metabolic risk markers and appetite regulating hormones at a subsequent standardized breakfast: A randomized cross-over study. PLoS ONE 2013, 8, e59985. [Google Scholar] [CrossRef]
- Freeland, K.R.; Wilson, C.; Wolever, T.M.S. Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br. J. Nutr. 2010, 103, 82–90. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Hwang, S.S.; Park, E.J.; Bae, J.W. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ. Microbiol. Rep. 2013, 5, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.W.; Yi, C.H.; Liu, T.T.; Lei, W.Y.; Hung, J.S.; Lin, C.L.; Lin, S.Z.; Chen, C.L. Impact of vegan diets on gut microbiota: An update on the clinical implications. Tzu Chi Med. J. 2018, 30, 200–203. [Google Scholar]
- Barazzoni, R.; Gortan Cappellari, G.; Ragni, M.; Nisoli, E. Insulin resistance in obesity: An overview of fundamental alterations. Eat. Weight Disord. 2018, 23, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Boucher, J.L.; Cypress, M.; Dunbar, S.A.; Franz, M.J.; Mayer-Davis, E.J.; Neumiller, J.J.; Nwankwo, R.; Verdi, C.; Urbanski, P.; et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2013, 36, 3821–3842. [Google Scholar] [CrossRef]
- Bremer, A.A.; Auinger, P.; Byrd, R.S. Sugar-sweetened beverage intake trends in US adolescents and their association with insulin resistance-related parameters. J. Nutr. Metab. 2010, 2010, 196476. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef]
- Khan, T.A.; Sievenpiper, J.L. Controversies about sugars: Results from systematic reviews and meta-analyses on obesity, cardiometabolic disease and diabetes. Eur. J. Nutr. 2016, 55 (Suppl. 2), 25–43. [Google Scholar] [CrossRef]
- Della Pepa, G.; Vetrani, C.; Vitale, M.; Riccardi, G. Wholegrain intake and risk of type 2 diabetes: Evidence from epidemiological and intervention studies. Nutrients 2018, 10, 1288. [Google Scholar] [CrossRef]
- Anderson, J.W.; Ward, K. High-carbohydrate, high-fiber diets for insulin-treated men with diabetes mellitus. Am. J. Clin. Nutr. 1979, 32, 2312–2321. [Google Scholar] [CrossRef]
- Rachek, L.I. Free fatty acids and skeletal muscle insulin resistance. Prog. Mol. Biol. Transl. Sci. 2014, 121, 267–292. [Google Scholar] [PubMed]
- Nolan, C.J.; Larter, C.Z. Lipotoxicity: Why do saturated fatty acids cause and monounsaturates protect against it? J. Gastroenterol. Hepatol. 2009, 24, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Jheng, H.F.; Tsai, P.J.; Guo, S.M.; Kuo, L.H.; Chang, C.S.; Su, I.J.; Chang, C.R.; Tsai, Y.S. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell. Biol. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zuurmond, M.G.; van der Schaft, N.; Nano, J.; Wijnhoven, H.A.H.; Ikram, M.A.; Franco, O.H.; Voortman, T. Plant versus animal based diets and insulin resistance, prediabetes and type 2 diabetes: The Rotterdam Study. Eur. J. Epidemiol. 2018, 33, 883–893. [Google Scholar] [CrossRef]
- US Department of Agriculture. Nutrient Database. Available online: http://www.nal.usda.gov/fnic/foodcomp/search (accessed on 16 October 2019).
- Bachmann, O.P.; Dahl, D.B.; Brechtel, K.; Machann, J.; Haap, M.; Maier, T.; Loviscach, M.; Stumvoll, M.; Claussen, C.D.; Schick, F.; et al. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes 2001, 50, 2579–258484. [Google Scholar] [CrossRef]
- Lee, S.; Boesch, C.; Kuk, J.L.; Arslanian, S. Effects of an overnight intravenous lipid infusion on intramyocellular lipid content and insulin sensitivity in African-American versus Caucasian adolescents. Metabolism 2013, 62, 417–423. [Google Scholar] [CrossRef]
- Roden, M.; Krssak, M.; Stingl, H.; Gruber, S.; Hofer, A.; Furnsinn, C.; Moser, E.; Waldhäusl, W. Rapid impairment of skeletal muscle glucose transport/phosphorylation by free fatty acids in humans. Diabetes 1999, 48, 358–364. [Google Scholar] [CrossRef]
- Santomauro, A.T.; Boden, G.; Silva, M.E.; Rocha, D.M.; Santos, R.F.; Ursich, M.J.; Strassmann, P.G.; Wajchenberg, B.L. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 1999, 48, 1836–1841. [Google Scholar] [CrossRef]
- Hocking, S.; Samocha-Bonet, D.; Milner, K.L.; Greenfield, J.R.; Chisholm, D.J. Adiposity and insulin resistance in humans: The role of the different tissue and cellular lipid depots. Endocr. Rev. 2013, 34, 463–500. [Google Scholar] [CrossRef]
- Pankow, J.S.; Duncan, B.B.; Schmidt, M.I.; Ballantyne, C.M.; Couper, D.J.; Hoogeveen, R.C.; Golden, S.H. Fasting plasma free fatty acids and risk of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes Care 2004, 27, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Guth, E.; Islam, A.; Livingston, E. Rapid improvement in diabetes after gastric bypass surgery: Is it the diet or surgery? Diabetes Care 2013, 36, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Calorie restriction and reversal of type 2 diabetes. Expert Rev. Endocrinol. Metab. 2016, 11, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Varela, J.E. Bariatric surgery: A cure for diabetes? Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 396–401. [Google Scholar] [CrossRef]
- Ravussin, E.; Acheson, K.J.; Vernet, O.; Danforth, E.; Jéquier, E. Evidence that insulin resistance is responsible for the decreased thermic effect of glucose in human obesity. J. Clin. Investig. 1985, 76, 1268–1273. [Google Scholar] [CrossRef]
- Calcagno, M.; Kahleova, H.; Alwarith, J.; Burgess, N.N.; Flores, R.A.; Busta, M.L.; Barnard, N.D. The thermic effect of food: A review. J. Am. Coll. Nutr. 2019, 38, 547–551. [Google Scholar] [CrossRef]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Green, A.; Ferdowsian, H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 2009, 89, 1588S–1596S. [Google Scholar] [CrossRef]
- Kahleova, H.; Klementova, M.; Herynek, V.; Skoch, A.; Herynek, S.; Hill, M.; Mari, A.; Pelikanova, T. The effect of a vegetarian vs. conventional hypocaloric diabetic diet on thigh adipose tissue distribution in subjects with type 2 diabetes: A randomized study. J. Am. Coll. Nutr. 2017, 36, 364–369. [Google Scholar] [CrossRef]
- Kahleova, H.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. A plant-based dietary intervention improves beta-cell function and insulin resistance in overweight adults: A 16-week randomized clinical trial. Nutrients 2018, 10, 189. [Google Scholar] [CrossRef]
- Kahleova, H.; Dort, S.; Holubkov, R.; Barnard, N.D. A plant-based high-carbohydrate, low-fat diet in overweight individuals in a 16-week randomized clinical trial: The role of carbohydrates. Nutrients 2018, 10, 1302. [Google Scholar] [CrossRef]
- Barnard, N.D.; Scialli, A.R.; Turner-McGrievy, G.; Lanou, A.J.; Glass, J. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am. J. Med. 2005, 118, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Shepherd, J.A.; Looker, A.C.; Graubard, B.I.; Borrud, L.G.; Ogden, C.L.; Harris, T.B.; Everhart, J.E.; Schenker, N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am. J. Clin. Nutr. 2009, 89, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J. Carnitine. In Modern Nutrition in Health and Disease, 9th ed.; Shils, M.E., Olson, J.A., Shike, M., Ross, A.C., Eds.; Lippincott Williams and Wilkins: New York, NY, USA, 1999; pp. 505–512. [Google Scholar]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab. Dispos. 2016, 44, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as novel potential biomarker of early predictors of metabolic syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Rep. 2017, 19, 2451–2461. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Wu, W.K.; Chen, C.C.; Liu, P.Y.; Panyod, S.; Liao, B.Y.; Chen, P.C.; Kao, H.L.; Kuo, H.C.; Kuo, C.H.; Chiu, T.H.T.; et al. Identification of TMAO-producer phenotype and host-diet-gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut 2019, 68, 1439–1449. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef]
- O’Neil, C.E.; Fulgoni, V.L., 3rd; Nicklas, T.A. Tree Nut consumption is associated with better adiposity measures and cardiovascular and metabolic syndrome health risk factors in U.S. Adults: NHANES 2005–2010. Nutr. J. 2015, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Dhillon, J.; Mattes, R.D. A review of the effects of nuts on appetite, food intake, metabolism, and body weight. Am. J. Clin. Nutr. 2014, 100, 412S–422S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, S.; Cooper, J.A. Effect of dietary fatty acid composition on substrate utilization and body weight maintenance in humans. Eur. J. Nutr. 2014, 53, 691–710. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.E.; Allred, J.B.; Kien, C.L. Fractional oxidation of chylomicron-derived oleate is greater than that of palmitate in healthy adults fed frequent small meals. J. Lipid Res. 1999, 40, 2322–2332. [Google Scholar] [PubMed]
- Piers, L.S.; Walker, K.Z.; Stoney, R.M.; Soares, M.J.; O’Dea, K. Substitution of saturated with monounsaturated fat in a 4-week diet affects body weight and composition of overweight and obese men. Br. J. Nutr. 2003, 90, 717–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiadi, A.; Kersten, S. Mechanisms of gene regulation by fatty acids. Adv. Nutr. 2012, 3, 127–134. [Google Scholar] [CrossRef]
- Hihi, A.K.; Michalik, L.; Wahli, W. PPARs: Transcriptional effectors of fatty acids and their derivatives. Cell. Mol. Life Sci. 2002, 59, 790–798. [Google Scholar] [CrossRef]
- Rangwala, S.M.; Lazar, M.A. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol. Sci. 2004, 25, 331–336. [Google Scholar] [CrossRef]
- Nolan, J.J.; Ludvik, B.; Beerdsen, P.; Joyce, M.; Olefsky, J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N. Engl. J. Med. 1994, 331, 1188–1193. [Google Scholar] [CrossRef]
- He, W.; Barak, Y.; Hevener, A.; Olson, P.; Liao, D.; Le, J.; Nelson, M.; Ong, E.; Olefsky, J.M.; Evans, R.M. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. USA 2003, 100, 15712–15717. [Google Scholar] [CrossRef]
- Tindall, A.M.; Petersen, K.S.; Lamendella, R.; Shearer, G.C.; Murray-Kolb, L.E.; Proctor, D.N.; Kris-Etherton, P.M. Tree Nut consumption and adipose tissue mass: Mechanisms of action. Curr. Dev. Nutr. 2018, 2, nzy069. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Rienks, J.; Barbaresko, J.; Nöthlings, U. Association of polyphenol biomarkers with cardiovascular disease and mortality risk: A systematic review and meta-analysis of observational studies. Nutrients 2017, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Nachvak, S.M.; Moradi, S.; Anjom-Shoae, J.; Rahmani, J.; Nasiri, M.; Maleki, V.; Sadeghi, O. Soy, soy isoflavones, and protein intake in relation to mortality from all causes, cancers, and cardiovascular diseases: A systematic review and dose-response meta-analysis of prospective cohort studies. J. Acad. Nutr. Diet. 2019, 119, 1483–1500. [Google Scholar] [CrossRef]
- Kimble, R.; Keane, K.; Lodge, J.K.; Howatson, G. Dietary intake of anthocyanins and risk of cardiovascular disease: A systematic review and metaanalysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 3032–3043. [Google Scholar] [CrossRef]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ 2016, 352, i17. [Google Scholar] [CrossRef]
- Vernarelli, J.A.; Lambert, J.D. Flavonoid intake is inversely associated with obesity and C-reactive protein, a marker for inflammation, in US adults. Nutr. Diabetes 2017, 7, e276. [Google Scholar] [CrossRef]
- Cases, J.; Romain, C.; Dallas, C.; Gerbi, A.; Cloarec, M. Regular consumption of Fiit-ns, a polyphenol extract from fruit and vegetables frequently consumed within the Mediterranean diet, improves metabolic ageing of obese volunteers: A. randomized, double-blind, parallel trial. Int. J. Food. Sci. Nutr. 2015, 66, 120–125. [Google Scholar] [CrossRef]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Brand, M.D. The regulation and physiology of mitochondrial proton-leak. Physiology 2011, 26, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Esteves, T.C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005, 2, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabalina, I.G.; Backlund, E.C.; Bar-Tana, J.; Cannon, B.; Nedergaard, J. Within brown-fat cells, UCP1-mediated fatty acid-induced uncoupling is independent of fatty acid metabolism. Biochim. Biophys. Acta 2008, 1777, 642–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lameloise, N.; Muzzin, P.; Prentki, M.; Assimacopoulos-Jeannet, F. Uncoupling protein 2: A possible link between fatty acid excess and impaired glucose-induced insulin secretion? Diabetes 2001, 50, 803–809. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, Y. (−)-Epigallocatechin-3-gallate enhances uncoupling protein 2 gene expression in 3T3–L1 adipocytes. Biosci. Biotechnol. Biochem. 2009, 73, 434–436. [Google Scholar] [CrossRef]
- Nomura, S.; Ichinose, T.; Jinde, M.; Kawashima, Y.; Tachiyashiki, K.; Imaizumi, K. Tea catechins enhance the mrna expression of uncoupling protein 1 in rat brown adipose tissue. J. Nutr. Biochem. 2008, 19, 840–847. [Google Scholar] [CrossRef]
- Andrade, J.M.; Frade, A.C.; Guimaraes, J.B.; Freitas, K.M.; Lopes, M.T.; Guimaraes, A.L. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur. J. Nutr. 2014, 53, 1503–1510. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; de la Rosa, L.A. Modulation of PPAR expression and activity in response to polyphenolic compounds in high fat diets. Int. J. Mol. Sci. 2016, 17, 1002. [Google Scholar] [CrossRef]
- Arcari, D.P.; Bartchewsky, W.; dos Santos, T.W.; Oliveira, K.A.; Funck, A.; Pedrazzoli, J.; de Souza, M.F.; Saad, M.J.; Bastos, D.H.; Gambero, A.; et al. Antiobesity effects of yerba mate extract (Ilex paraguariensis) in high-fat diet-induced obese mice. Obesity (Silver Spring). 2009, 17, 2127–2133. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.H.; Chung, I.M.; Park, Y. Sorghum extract exerts an anti-diabetic effect by improving insulin sensitivity via PPAR-γ in mice fed a high-fat diet. Nutr. Res. Pract. 2012, 6, 322–327. [Google Scholar] [CrossRef]
- Yang, D.J.; Chang, Y.Y.; Hsu, C.L.; Liu, C.W.; Wang, Y.; Chen, Y.C. Protective effect of a litchi (Litchi chinensis Sonn.)-flower-water-extract on cardiovascular health in a high-fat/cholesterol-dietary hamsters. Food Chem. 2010, 119, 1457–1464. [Google Scholar] [CrossRef]
- Jang, H.H.; Park, M.Y.; Kim, H.W.; Lee, Y.M.; Hwang, K.A.; Park, J.H.; Park, D.S.; Kwon, O. Black rice (Oryza sativa L.) extract attenuates hepatic steatosis in C57BL/6 J mice fed a high-fat diet via fatty acid oxidation. Nutr. Metab. 2012, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Serisier, S.; Leray, V.; Poudroux, W.; Magot, T.; Ouguerram, K.; Nguyen, P. Effects of green tea on insulin sensitivity, lipid profile and expression of pparalpha and ppargamma and their target genes in obese dogs. Br. J. Nutr. 2008, 99, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Herranz-Lopez, M.; Barrajon-Catalan, E.; Segura-Carretero, A.; Menendez, J.A.; Joven, J.; Micol, V. Lemon verbena (Lippia citriodora) polyphenols alleviate obesity-related disturbances in hypertrophic adipocytes through ampk-dependent mechanisms. Phytomedicine 2015, 22, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Kuo, D.H.; Yeh, C.H.; Shieh, P.C.; Cheng, K.C.; Chen, F.A.; Cheng, J.T. Effect of shanzha, a chinese herbal product, on obesity and dyslipidemia in hamsters receiving high-fat diet. J. Ethnopharmacol. 2009, 124, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Stefani, M. Nutraceutical properties of olive oil polyphenols. An itinerary from cultured cells through animal models to humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef]
- Razquin, C.; Sanchez-Tainta, A.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Fito, M.; Ros, E.; Estruch, R.; Aros, F.; Gomez-Gracia, E.; et al. Dietary energy density and body weight changes after 3 years in the predimed study. Int. J. Food Sci. Nutr. 2017, 68, 865–872. [Google Scholar] [CrossRef]
- Cândido, F.G.; Valente, F.X.; Silva, L.E.; Coelho, O.G.L.; Peluzio, M.C.G.; Alfenas, R.C.G. Consumption of extra virgin olive oil improves body composition and blood pressure in women with excess body fat: A randomized, double-blinded, placebo-controlled clinical trial. Eur. J. Nutr. 2017, 57, 2445–2455. [Google Scholar] [CrossRef]
- Rodriguez-Villar, C.; Manzanares, J.M.; Casals, E.; Perez-Heras, A.; Zambon, D.; Gomis, R.; Ros, E. High-monounsaturated fat, olive oil-rich diet has effects similar to a high-carbohydrate diet on fasting and postprandial state and metabolic profiles of patients with type 2 diabetes. Metabolism 2000, 49, 1511–1517. [Google Scholar] [CrossRef]
- Maki, K.C.; Lawless, A.L.; Kelley, K.M.; Kaden, V.N.; Geiger, C.J.; Dicklin, M.R. Corn oil improves the plasma lipoprotein lipid profile compared with extra-virgin olive oil consumption in men and women with elevated cholesterol: Results from a randomized controlled feeding trial. J. Clin. Lipidol. 2015, 9, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Keita, H.; Ramírez-San Juan, E.; Paniagua-Castro, N.; Garduño-Siciliano, L.; Quevedo, L. The long-term ingestion of a diet high in extra virgin olive oil produces obesity and insulin resistance but protects endothelial function in rats: A preliminary study. Diabetol. Metab. Syndr. 2013, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Oi-Kano, Y.; Kawada, T.; Watanabe, T.; Koyama, F.; Watanabe, K.; Senbongi, R.; Iwai, K. Extra virgin olive oil increases uncoupling protein 1 content in brown adipose tissue and enhances noradrenaline and adrenaline secretions in rats. J. Nutr. Biochem. 2007, 18, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, V.M.; Portillo, M.P.; Picó, C.; Macarulla, M.T.; Palou, A. Olive oil feeding up-regulates uncoupling protein genes in rat brown adipose tissue and skeletal muscle. Am. J. Clin. Nutr. 2002, 75, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, N.S.; Jaceldo-Siegl, K.; Sabate, J.; Fraser, G.E. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J. Acad. Nutr. Diet. 2013, 113, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Churuangsuk, C.; Kherouf, M.; Combet, E.; Lean, M. Low-carbohydrate diets for overweight and obesity: A systematic review of the systematic reviews. Obes. Rev. 2018, 19, 1700–1718. [Google Scholar] [CrossRef]
- Hall, K.D.; Guo, J. Obesity energetics: Body weight regulation and the effects of diet composition. Gastroenterology 2017, 152, 1718–1727. [Google Scholar] [CrossRef]
- Hall, K.D.; Bemis, T.; Brychta, R.; Chen, K.Y.; Courville, A.; Crayner, E.J.; Goodwin, S.; Guo, J.; Howard, L.; Knuth, N.D.; et al. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab. 2015, 22, 427–436. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, Q.; Guo, W.; Bao, W.; Wang, X. Association of whole grain intake with all-cause, cardiovascular, and cancer mortality: A systematic review and dose–response meta-analysis from prospective cohort studies. Eur. J. Clin. Nutr. 2017, 72, 57–65. [Google Scholar] [CrossRef]
- Marventano, S.; Izquierdo Pulido, M.; Sanchez-Gonzalez, C.; Godos, J.; Speciani, A.; Galvano, F.; Grosso, G. Legume consumption and CVD risk: A systematic review and meta-analysis. Public Health Nutr. 2017, 20, 245–254. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Shen, Y.; Wang, J.; Zhou, D. Legume consumption and all-cause and cardiovascular disease mortality. Biomed. Res. Int. 2017, 2017, 8450618. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Chen, K.Y.; Guo, J.; Lam, Y.Y.; Leibel, R.L.; Mayer, L.E.; Reitman, M.L.; Rosenbaum, M.; Smith, S.R.; Walsh, B.T.; et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am. J. Clin. Nutr. 2016, 104, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roden, M. How free fatty acids inhibit glucose utilization in human skeletal muscle. News Physiol. Sci. 2004, 19, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Hall, K.D.; Guo, J.; Ravussin, E.; Mayer, L.S.; Reitman, M.L.; Smith, S.R.; Walsh, B.T.; Leibel, R.L. Glucose and lipid homeostasis and inflammation in humans following an isocaloric ketogenic diet. Obesity 2019, 27, 971–981. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Low-carbohydrate diets impair flow-mediated dilatation: Evidence from a systematic review and meta-analysis. Br. J. Nutr. 2013, 110, 969–970. [Google Scholar] [CrossRef]
- Merino, J.; Kones, R.; Ferré, R.; Plana, N.; Girona, J.; Aragonés, G.; Ibarretxe, D.; Heras, M.; Masana, L. Negative effect of a low-carbohydrate, high-protein, high-fat diet on small peripheral artery reactivity in patients with increased cardiovascular risk. Br. J. Nutr. 2013, 109, 1241–1247. [Google Scholar] [CrossRef]
- Higashi, Y.; Noma, K.; Yoshizumi, M.; Kihara, Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ. J. 2009, 73, 411–418. [Google Scholar] [CrossRef]
- Fleming, R.M. The effect of high-protein diets on coronary blood flow. Angiology 2000, 51, 817–826. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D.; et al. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Ornish, D.; Brown, S.E.; Scherwitz, L.W.; Billings, J.H.; Armstrong, W.T.; Ports, T.A.; McLanahan, S.M.; Kirkeeide, R.L.; Brand, R.J.; Gould, K.L. Can lifestyle changes reverse coronary heart disease? The lifestyle heart trial. Lancet Lond. Engl. 1990, 336, 129–133. [Google Scholar] [CrossRef]
- Esselstyn, C.B., Jr.; Gendy, G.; Doyle, J.; Golubic, M.; Roizen, M.F. A way to reverse CAD? J. Fam. Pract. 2014, 63, 356–364. [Google Scholar] [PubMed]
- Romeu, M.; Aranda, N.; Giralt, M.; Ribot, B.; Nogues, M.R.; Arija, V. Diet, iron biomarkers and oxidative stress in a representative sample of Mediterranean population. Nutr. J. 2013, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Ornish, D.; Weidner, G.; Fair, W.R.; Marlin, R.; Pettengill, E.B.; Raisin, C.J.; Dunn-Emke, S.; Crutchfield, L.; Jacobs, F.N.; Barnard, R.J.; et al. Intensive lifestyle changes may affect the progression of prostate cancer. J. Urol. 2005, 174, 1065–1069. [Google Scholar] [CrossRef]
- Barnard, R.J.; Gonzalez, J.H.; Liva, M.E.; Ngo, T.H. Effects of a low-fat, high-fiber diet and exercise program on breast cancer risk factors in vivo and tumor cell growth and apoptosis in vitro. Nutr. Cancer 2006, 55, 28–34. [Google Scholar] [CrossRef]
- Ornish, D.; Lin, J.; Chan, J.M.; Epel, E.; Kemp, C.; Weidner, G.; Marlin, R.; Frenda, S.J.; Magbanua, M.J.; Daubenmier, J.; et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013, 14, 1112–1120. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: Results from three prospective cohort studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Kim, H.; Caulfield, L.E.; Rebholz, C.M. Healthy plant-based diets are associated with lower risk of all-cause mortality in US adults. J. Nutr. 2018, 148, 624–631. [Google Scholar] [CrossRef]
- Bolori, P.; Setaysh, L.; Rasaei, N.; Jarrahi, F.; Saeid Yekaninejad, M. Adherence to a healthy plant diet may reduce inflammatory factors in obese and overweight women-a cross-sectional study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2795–2802. [Google Scholar] [CrossRef]
| Food Type | Total Saturated Fat g/100 g | Total Unsaturated Fat g/100g 1 |
|---|---|---|
| Animal-derived foods | ||
| Butter, unsalted | 50.5 | 26.4 |
| Cheese, cheddar | 19.4 | 9.8 |
| Pork, cured, bacon, baked | 14.2 | 23.8 |
| Cream, fluid, light (coffee cream) | 10.2 | 5.3 |
| Beef, ground, 80% lean, baked | 6.2 | 9.3 |
| Eggs, hard-boiled | 3.3 | 5.4 |
| Fish, salmon, Atlantic, farmed, cooked, dry heat | 2.4 | 8.6 |
| Milk, whole | 1.9 | 1.0 |
| Yogurt, Greek, plain, low-fat | 1.2 | 0.6 |
| Chicken breast, skin removed, baked | 1.0 | 2.0 |
| High-fat plant-derived foods | ||
| Oil, Coconut | 82.5 | 8 |
| Oil, palm | 49.3 | 46.3 |
| Oil, Olive | 13.8 | 83.5 |
| Nuts, almonds | 3.8 | 43.8 |
| Avocados, raw | 2.1 | 11.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najjar, R.S.; Feresin, R.G. Plant-Based Diets in the Reduction of Body Fat: Physiological Effects and Biochemical Insights. Nutrients 2019, 11, 2712. https://doi.org/10.3390/nu11112712
Najjar RS, Feresin RG. Plant-Based Diets in the Reduction of Body Fat: Physiological Effects and Biochemical Insights. Nutrients. 2019; 11(11):2712. https://doi.org/10.3390/nu11112712
Chicago/Turabian StyleNajjar, Rami S., and Rafaela G. Feresin. 2019. "Plant-Based Diets in the Reduction of Body Fat: Physiological Effects and Biochemical Insights" Nutrients 11, no. 11: 2712. https://doi.org/10.3390/nu11112712
APA StyleNajjar, R. S., & Feresin, R. G. (2019). Plant-Based Diets in the Reduction of Body Fat: Physiological Effects and Biochemical Insights. Nutrients, 11(11), 2712. https://doi.org/10.3390/nu11112712






