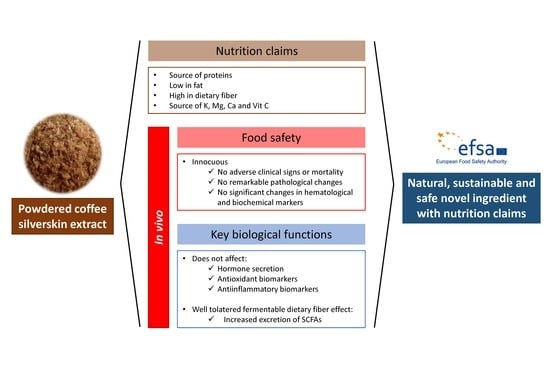
Coffee Silverskin Extract: Nutritional Value, Safety and Effect on Key Biological Functions
Abstract
1. Introduction
2. Materials and Methods
2.1. Coffee Silverskin Extract (CSE)
2.2. Nutritional Characterization of CSE
2.2.1. Protein Content and Amino acid Composition
2.2.2. Fat and Fatty Acid Profile
2.2.3. Soluble Simple Sugars
2.2.4. Dietary Fiber
2.2.5. Ions and Ascorbic Acid
2.3. Repeated-Dose Study
2.3.1. Safety
Necropsy, Macroscopic Analysis and Organ Weight
Histopathological Examination
Hematological and Biochemical Parameters
2.3.2. Effect on key Biological Functions
Hormone Levels
Oxidative Stress Biomarkers
Inflammation Biomarkers
Dietary Fiber Effect
2.4. Statistical Analysis
3. Results
3.1. Nutritional Characterization
3.2. Repeated-Dose Study
3.2.1. Safety
Food and Water Intake
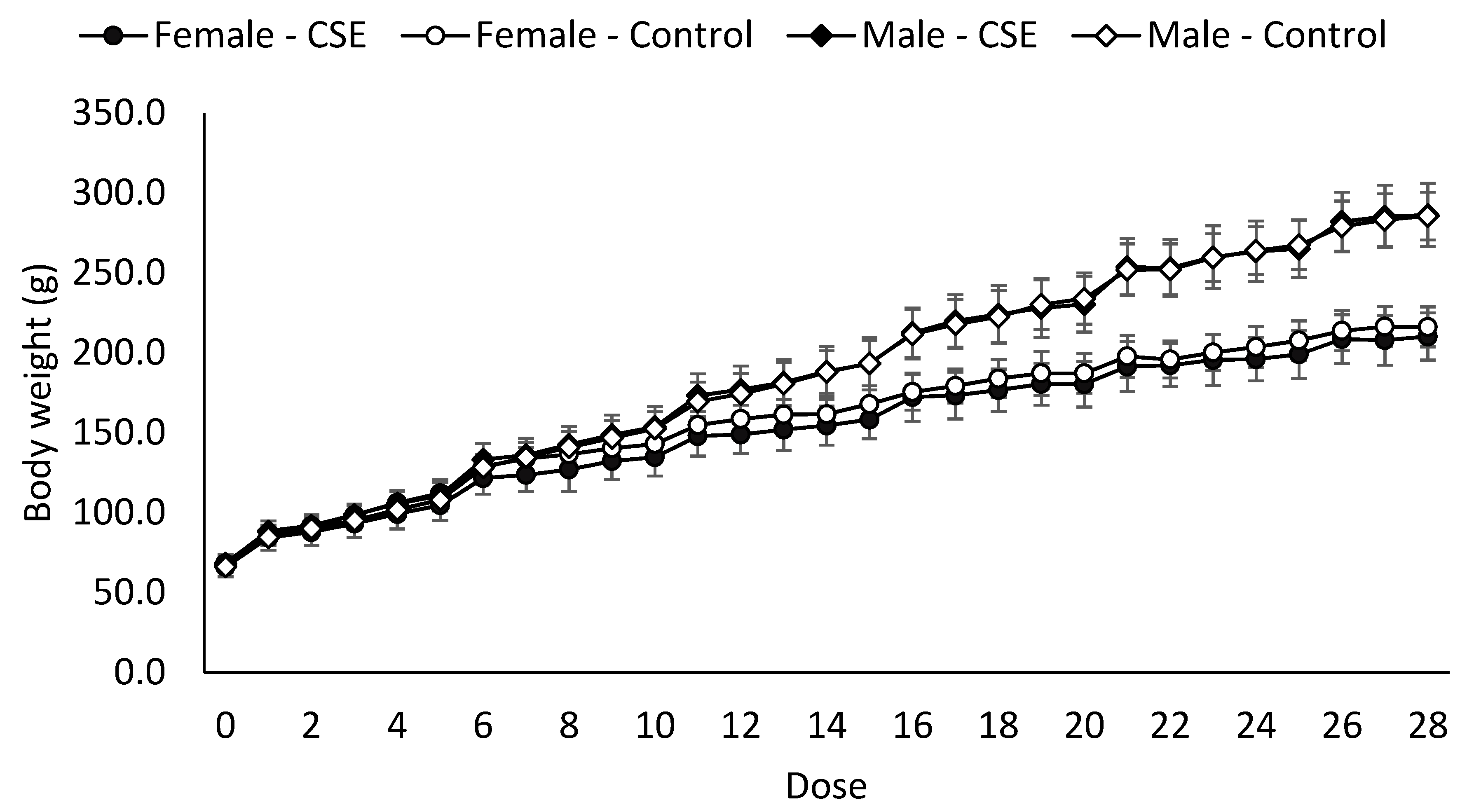
Body and Organ Weight
Histopathology
Hematological and Biochemical Parameters
3.2.2. Effect on Key Biological Functions
Hormone Levels
Oxidative Stress and Inflammation Biomarkers
Dietary Fiber Effect
4. Discussion
4.1. Nutritional Quality
4.2. Safety
4.3. Effect on Key Biological Functions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EFSA Panel on Dietetic Products. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2016, 14, e04594. [Google Scholar] [CrossRef]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.G.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Zamora, A.; Pastoriza, S.; Rufián-Henares, J.A. Revalorization of coffee by-products. Prebiotic, antimicrobial and antioxidant properties. LWT Food Sci. Technol. 2015, 61, 12–18. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary fiber and health outcomes: An umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- World Health Organization. e-Library of Evidence for Nutrition Actions (eLENA). Available online: https://www.who.int/elena/nutrient/en/ (accessed on 22 August 2019).
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a new potential functional ingredient: Coffee silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef]
- Mesías, M.; Navarro, M.; Martínez-Saez, N.; Ullate, M.; del Castillo, M.D.; Morales, F.J. Antiglycative and carbonyl trapping properties of the water soluble fraction of coffee silverskin. Food Res. Int. 2014, 62, 1120–1126. [Google Scholar] [CrossRef]
- Ateş, G.; Elmacı, Y. Physical, chemical and sensory characteristics of fiber-enriched cakes prepared with coffee silverskin as wheat flour substitution. J. Food Meas. Charact. 2019, 13, 755–763. [Google Scholar] [CrossRef]
- The European Parliament; The Council of the European Union. REGULATION (EC) No 1924/2006 of the european parliament and of the council on nutrition and health claims made on foods. Off. J. Eur. Union 2006, 49, 9–25. [Google Scholar]
- Picó, C.; Serra, F.; Rodríguez, A.M.; Keijer, J.; Palou, A. Biomarkers of nutrition and health: New tools for new approaches. Nutrients 2019, 11, 1092. [Google Scholar] [CrossRef]
- Fernandez-Gomez, B.; Lezama, A.; Amigo-Benavent, M.; Ullate, M.; Herrero, M.; Martín, M.Á.; Mesa, M.D.; del Castillo, M.D. Insights on the health benefits of the bioactive compounds of coffee silverskin extract. J. Funct. Foods 2016, 25, 197–207. [Google Scholar] [CrossRef]
- Parasuraman, S. Toxicological screening. J. Pharmacol. Pharmacother. 2011, 2, 74–79. [Google Scholar] [CrossRef] [PubMed]
- del Castillo, M.D.; Fernandez-Gomez, B.; Ullate, M.; Mesa, M.D. Use of Products of the Husk of Coffee for the Prevention and Treatment of the Diseases that Form the Metabolic Syndrome and the Risk Factors Thereof. Patent WO/2016/097450, 23 June 2016. [Google Scholar]
- Shilo, L.; Sabbah, H.; Hadari, R.; Kovatz, S.; Weinberg, U.; Dolev, S.; Dagan, Y.; Shenkman, L. The effects of coffee consumption on sleep and melatonin secretion. Sleep Med. 2002, 3, 271–273. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Aparicio García, N.; Velazquez Escobar, F.; San Andres, M.I.; Sanchez-Fortun, S.; Blanch, G.P.; Fernandez-Gomez, B.; Guisantes Batan, E.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- del Castillo, M.D.; Ibañez, M.E.; Amigo, M.; Herrero, M.; Plaza del Moral, M.; Ullate, M. Application of Products of Coffee Silverskin in Anti-Ageing cosmetics and Functional Food. Patent WO 2013/004873, 10 January 2013. [Google Scholar]
- Martinez-Saez, N.; Tamargo García, A.; Domínguez Pérez, I.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; Martín-Cabrejas, M.A.; del Castillo, M.D. Use of spent coffee grounds as food ingredient in bakery products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Cornejo, F.S.; Fernandez-Gomez, B.; Vera, G.; Guisantes-Batan, E.; Alonso, S.G.; Andres, M.I.S.; Sanchez-Fortun, S.; Lopez-Gomez, L.; Uranga, J.A.; et al. Bioaccesibility, Metabolism, and Excretion of Lipids Composing Spent Coffee Grounds. Nutrients 2019, 11, 1411. [Google Scholar] [CrossRef]
- OECD/OCDE. OECD guideline for the testing of chemicals. Guidel. Test. Chem. 1995, 8, 1–8. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oki, T.; Nagai, S.; Yoshinaga, M.; Nishiba, Y.; Suda, I. Contribution of b-Carotene to Radical Scavenging Capacity Varies among Orange-fleshed Sweet Potato Cultivars. Food Sci. Technol. Res. 2006, 12, 156–160. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Carlos Espín, J.; Larrosa, M. Alternative method for gas chromatography-mass spectrometry analysis of short-chain fatty acids in faecal samples. J. Sep. Sci. 2012, 35, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Saez, N.; Ullate, M.; Martin-Cabrejas, M.A.; Martorell, P.; Genovés, S.; Ramon, D.; Del Castillo, M.D. A novel antioxidant beverage for body weight control based on coffee silverskin. Food Chem. 2014, 150, 227–234. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT Food Sci. Technol. 2013, 50, 599–606. [Google Scholar] [CrossRef]
- The European Commission. Commission Regulation (EU) No 432/2012. Off. J. Eur. Union 2012, 13, L136. [Google Scholar]
- Wolford, S.T.; Schroer, R.A.; Gohs, F.X.; Gallo, P.P.; Brodeck, M.; Falk, H.B.; Ruhren, R. Reference range data base for serum chemistry and hematology values in laboratory animals. J. Toxicol. Environ. Health 1986, 18, 161–188. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Guidance on the scientific requirements for health claims related to muscle function and physical performance: (Revision 1). EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Napolitano, A.; Fogliano, V.; Tafuri, A.; Ritieni, A. Natural occurrence of ochratoxin A and antioxidant activities of green and roasted coffees and corresponding byproducts. J. Agric. Food Chem. 2007, 55, 10499–10504. [Google Scholar] [CrossRef] [PubMed]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-estrada, M.T. Coffee Silverskin: Characterization, Possible Uses, and Safety Aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Potassium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- European Council Council. Directive of 24 September 1990 on nutrition labelling for foodstuffs (90/496/EEC). Off. J. Eur. Union 1990, 33, 18–63. [Google Scholar]
- Malla, S.; Hobbs, J.E.; Kofi Sogah, E. Functional Foods, Health Benefits and Health Claims. Athens J. Health 2018, 1, 37–46. [Google Scholar] [CrossRef]
- Guglielmetti, A.; Fernandez-Gomez, B.; Zeppa, G.; del Castillo, M.D. Nutritional quality, potential health promoting properties and sensory perception of an improved gluten free bread formulation containing inulin, rice protein and bioactive compounds extracted from coffee byproducts. Pol. J. Food Nutr. Sci. 2019, 69, 157–166. [Google Scholar] [CrossRef]
- Garcia-Serna, E.; Martinez-Saez, N.; Mesias, M.; Morales, F.J.; Castillo, M.D. Del Use of Coffee Silverskin and Stevia to Improve the Formulation of Biscuits. Pol. J. Food Nutr. Sci. 2014, 64, 243–251. [Google Scholar] [CrossRef]
- Ateş, G.; Elmacı, Y. Coffee silverskin as fat replacer in cake formulations and its effect on physical, chemical and sensory attributes of cakes. LWT 2018, 90, 519–525. [Google Scholar] [CrossRef]
- Bertolino, M.; Barbosa-Pereira, L.; Ghirardello, D.; Botta, C.; Rolle, L.; Guglielmetti, A.; Borotto Dalla Vecchia, S.; Zeppa, G. Coffee silverskin as nutraceutical ingredient in yogurt: Its effect on functional properties and its bioaccessibility. J. Sci. Food Agric. 2019, 99, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Haza, A.I.; Ávalos, A.; del Castillo, M.D.; Morales, P. Validation of coffee silverskin extract as a food ingredient by the analysis of cytotoxicity and genotoxicity. Food Res. Int. 2017, 100, 791–797. [Google Scholar] [CrossRef]
- Hayes, A.W.; Kruger, C.L. Hayes’ Principles and Methods of Toxicology; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781842145371. [Google Scholar]
- Delaney, B.; Carlson, T.; Frazer, S.; Zheng, T.; Hess, R.; Ostergren, K.; Kierzek, K.; Haworth, J.; Knutson, N.; Junker, K.; et al. Evaluation of the toxicity of concentrated barley b-glucan in a 28-day feeding study in Wistar rats. Food Chem. Toxicol. 2003, 41, 477–487. [Google Scholar] [CrossRef]
- El Kabbaoui, M.; Chda, A.; El-Akhal, J.; Azdad, O.; Mejrhit, N.; Aarab, L.; Bencheikh, R.; Tazi, A. Acute and sub-chronic toxicity studies of the aqueous extract from leaves of Cistus ladaniferus L. in mice and rats. J. Ethnopharmacol. 2017, 209, 147–156. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.P.d.S.; da Silva Oliveira, G.L.; Medeiros, S.C.; Sousa, A.M.L.; Lopes, L.D.S.; David, J.M.; Junior, J.S.D.C.; De Freitas, R.M. Pre-clinical toxicology of garcinielliptone FC, a tautomeric pair of polyprenylated benzophenone, isolated from Platonia insignis Mart seeds. Phytomedicine 2016, 23, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Al-Afifi, N.A.; Alabsi, A.M.; Bakri, M.M.; Ramanathan, A. Acute and sub-acute oral toxicity of Dracaena cinnabari resin methanol extract in rats. BMC Complement. Altern. Med. 2018, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Afzan, A.; Abdullah, N.R.; Halim, S.Z.; Rashid, B.A.; Semail, R.H.R.; Abdullah, N.; Jantan, I.; Muhammad, H.; Ismail, Z. Repeated dose 28-days oral toxicity study of Carica papaya L. Leaf extract in Sprague Dawley rats. Molecules 2012, 17, 4326–4342. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Denny Joseph, K.M.; Muralidhara; Ramesh, H.P.; Giridhar, P.; Ravishankar, G.A. Acute, subacute and subchronic safety assessment of betalains rich Rivina humilis L. berry juice in rats. Food Chem. Toxicol. 2011, 49, 3154–3157. [Google Scholar] [CrossRef]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef]
- Charles River Laboratories. Baseline Hematology and Clinical Chemistry Values for Charles River Wistar Rats—(CRL:(WI)BR) as a Function of Sex and Age; Charles River Laboratories: Wilmington, MA, USA, 1998. [Google Scholar]
- Boehm, O.; Zur, B.; Koch, A.; Tran, N.; Freyenhagen, R.; Hartmann, M.; Zacharowski, K. Clinical chemistry reference database for Wistar rats and C57/BL6 mice. Biol. Chem. 2007, 388, 547–554. [Google Scholar] [CrossRef]
- Imafidon, K.E.; Okunrobo, L.O. Study on Biochemical Indices of Liver Function Tests of Albino Rats Supplemented with Three Sources of Vegetable Oils. Niger. J. Basic Appl. Sci. 2012, 19, 105–110. [Google Scholar] [CrossRef]
- Fernandez-Gomez, B.; Ramos, S.; Goya, L.; Mesa, M.D.; del Castillo, M.D.; Martín, M.Á. Coffee silverskin extract improves glucose-stimulated insulin secretion and protects against streptozotocin-induced damage in pancreatic INS-1E beta cells. Food Res. Int. 2016. [Google Scholar] [CrossRef]
- Paredes, S.D.; Barriga, C.; Reiter, R.J.; Rodríguez, A.B. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia risoria as a model. Int. J. Tryptophan Res. 2009, 2, 23–36. [Google Scholar] [CrossRef]
- Thor, P.J.; Krolczyk, G.; Gil, K.; Zurowski, D.; Nowak, L. Melatonin and serotonin effects on gastrointestinal motility. J. Physiol. Pharmacol. 2007, 58, 97–103. [Google Scholar] [PubMed]
- del Castillo, M.D.; Fernandez-Gomez, B.; Martinez-Saez, N.; Iriondo-Dehond, A.; Martirosyan, D.M.; Mesa, M.D. Coffee Silverskin Extract for Aging and Chronic Diseases. In Functional Foods for Chronic Diseases; Martirosyan, D.M., Ed.; CreateSpace: Scotts Valley, CA, USA, 2016; pp. 386–409. ISBN 978-1536919431. [Google Scholar]
- Weibel, J.; Lin, Y.-S.; Landolt, H.-P.; Garbazza, C.; Kolodyazhniy, V.; Kistler, J.; Rehm, S.; Rentsch, K.; Borgwardt, S.; Cajochen, C.; et al. Caffeine-dependent changes of sleep-wake regulation: Evidence for adaptation after repeated intake. bioRxiv 2019, 641480. [Google Scholar] [CrossRef]
- Burke, T.M.; Markwald, R.R.; McHill, A.W.; Chinoy, E.D.; Snider, J.A.; Bessman, S.C.; Jung, C.M.; O’Neill, J.S.; Wright, K.P. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci. Transl. Med. 2015, 7, 305ra146. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef]
- Tores de la Cruz, S.; Iriondo-DeHond, A.; Herrera, T.; Lopez-Tofiño, Y.; Galvez-Robleño, C.; Prodanov, M.; Velazquez-Escobar, F.; Abalo, R.; Castillo, M.D. An Assessment of the Bioactivity of Coffee Silverskin Melanoidins. Foods 2019, 8, 68. [Google Scholar] [CrossRef]
- Kleessen, B.; Stoof, G.; Schmiedl, D.; Noack, J.; Blaut, M. Feeding Resistant Starch Affects Fecal and Cecal Microflora and Short-Chain Fatty Acids in Rats 1. Blood 1994, 75, 2453–2462. [Google Scholar] [CrossRef]
- Shastri, P.; McCarville, J.; Kalmokoff, M.; Brooks, S.P.J.; Green-Johnson, J.M. Sex differences in gut fermentation and immune parameters in rats fed an oligofructose-supplemented diet. Biol. Sex Differ. 2015, 6, 13. [Google Scholar] [CrossRef]
- Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 1994, 35, 35–38. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Reijngoud, D.-J.; Venema, K.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of coffee consumption on the gut microbiota: A human volunteer study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef] [PubMed]
- López-Barrera, D.M.; Vázquez-Sánchez, K.; Loarca-Piña, M.G.F.; Campos-Vega, R. Spent coffee grounds, an innovative source of colonic fermentable compounds, inhibit inflammatory mediators in vitro. Food Chem. 2016, 212, 282–290. [Google Scholar] [CrossRef] [PubMed]

| CSE | ||
|---|---|---|
| Total Protein (%) | 16.17 ± 0.06 | |
| Amino acids (mg/g) | Free | Total |
| Alanine (Ala) | 0.66 ± 0.01 | 2.83 ± 0.13 |
| Arginine (Arg) | 0.79 ± 0.01 | 1.14 ± 0.06 |
| Asparagine (Asp) | 0.92 ± 0.02 | 6.41 ± 0.34 |
| Cysteine (Cys) | 0.18 ± 0.02 | 0.35 ± 0.01 |
| γ-Amino butyric acid (GABA) | 0.31 ± 0.00 | N.D. |
| Glutamic acid (Glu) | 0.77 ± 0.00 | 5.61 ± 0.31 |
| Glycine (Gly) | 0.23 ± 0.00 | 3.11 ± 0.14 |
| Histidine (His) | 0.19 ± 0.00 | 0.92 ± 0.04 |
| Isoleucine (Ile) | 0.21 ± 0.02 | 1.04 ± 0.06 |
| Leucine (Leu) | 0.26 ± 0.01 | 1.54 ± 0.09 |
| Lysine (Lys) | 0.59 ± 0.01 | 1.77 ± 0.10 |
| Methionine (Met) | 0.04 ± 0.03 | 0.57 ± 0.02 |
| Phenylalanine (Phe) | 0.29 ± 0.15 | 2.05 ± 0.09 |
| Proline (Pro) | 0.41 ± 0.02 | 2.32 ± 0.10 |
| Serine (Ser) | 1.32 ± 0.00 | 2.72 ± 0.14 |
| Threonine (Thr) | 0.19 ± 0.00 | 1.78 ± 0.09 |
| Tryptophan (Trp) | 0.25 ± 0.02 | N.D. |
| Tyrosine (Tyr) | 0.65 ± 0.22 | 1.48 ± 0.08 |
| Valine (Val) | 0.42 ± 0.01 | 1.79 ± 0.07 |
| EAA (% total) | 37.12 ± 0.46 | 33.70 ± 0.11 |
| BCAA (Val + Leu + Ile) (% total) | 10.18 ± 0.06 | 11.68 ± 0.05 |
| AAA (Phe + Tyr + Trp) (% total) | 13.60 ± 3.30 | 9.44 ± 0.39 |
| Male | Female | |||
|---|---|---|---|---|
| Control | CSE | Control | CSE | |
| Food (g/rat/day) | ||||
| Week 1 | 14.1 ± 2.8 a | 13.3 ± 1.4 a | 15.5 ± 3.4 a | 11.9 ± 0.9 a |
| Week 2 | 17.5 ± 3.0 bc | 18.3 ± 1.2 c | 15.2 ± 1.1 ab | 14.2 ± 1.0 a |
| Week 3 | 21.3 ± 1.2 b | 21.0 ± 2.7 b | 17.7 ± 4.4 ab | 16.2 ± 2.1 a |
| Week 4 | 22.3 ± 0.9 b | 22.0 ± 1.0 b | 16.5 ± 1.7 a | 17.2 ± 1.7 a |
| Fiber intake (g/rat/day) | ||||
| From diet | 0.9 ± 0.0 a | 0.9 ± 0.0 a | 0.7 ± 0.1 a | 0.7 ± 0.1 a |
| From CSE | - | 0.1 ± 0.0 | - | 0.1 ± 0.0 |
| Total | 0.9 ± 0.0 a | 1.0 ± 0.0 b | 0.7 ± 0.1 a | 0.8 ± 0.1 a |
| Water (ml/rat/day) | ||||
| Week 1 | 21.6 ± 5.3 a | 27.4 ± 9.2 a | 24.1 ± 4.2 a | 25.3 ± 9.0 a |
| Week 2 | 33.1 ± 9.8 a | 30.1 ± 5.8 a | 26.6 ± 8.6 a | 31.9 ± 7.3 a |
| Week 3 | 43.0 ± 7.5 a | 39.3 ± 9.9 a | 32.9 ± 4.7 a | 38.6 ± 5.7 a |
| Week 4 | 37.9 ± 6.3 b | 41.0 ± 4.2 b | 28.0 ± 4.2 a | 35.5 ± 3.8 b |
| Male | Female | |||
|---|---|---|---|---|
| Parameter | Control | CSE | Control | CSE |
| Body weight (g) | 263.8 ± 15.0 b | 261.7 ± 18.5 b | 197.2 ± 10.4 a | 191.4 ± 12.8 a |
| Absolute organ weights (g) | ||||
| Thymus | 0.4 ± 0.1 a | 0.6 ± 0.2 a | 0.6 ± 0.2 a | 0.5 ± 0.1 a |
| Lungs | 1.7 ± 0.4 ab | 2.1 ± 0.4 b | 1.2 ± 0.6 a | 1.5 ± 0.3 ab |
| Liver | 10.0 ± 1.5 b | 9.2 ± 1.3 b | 6.6 ± 0.6 a | 7.2 ± 1.0 a |
| Kidneys | 1.0 ± 0.1 ab | 1.4 ± 0.6 b | 0.8 ± 0.1 a | 0.8 ± 0.1 a |
| Adrenal glands | 0.2 ± 0.0 a | 0.2 ± 0.1 a | 0.3 ± 0.1 a | 0.2 ± 0.1 a |
| Sex organs | 2.5 ± 0.2 b | 2.7 ± 0.4 b | 1.4 ± 0.3 a | 1.1 ± 0.3 a |
| Brain | 1.6 ± 0.1 a | 1.6 ± 0.2 a | 1.6 ± 0.2 a | 1.5 ± 0.3 a |
| Heart | 1.0 ± 0.1 b | 0.9 ± 0.1 ab | 0.8 ± 0.2 ab | 0.7 ± 0.1 a |
| Spleen | 0.5 ± 0.1 a | 0.4 ± 0.1 a | 0.5 ± 0.2 a | 0.5 ± 0.1 a |
| Relative organ weights (g%) | ||||
| Thymus | 0.2 ± 0.0 a | 0.2 ± 0.1 ab | 0.3 ± 0.1 b | 0.2 ± 0.0 ab |
| Lungs | 0.6 ± 0.2 a | 0.8 ± 0.1 a | 0.6 ± 0.3 a | 0.8 ± 0.1 a |
| Liver | 3.8 ± 0.4 a | 3.5 ± 0.4 a | 3.3 ± 0.3 a | 3.7 ± 0.4 a |
| Kidneys | 0.4 ± 0.0 a | 0.5 ± 0.2 a | 0.4 ± 0.0 a | 0.4 ± 0.1 a |
| Adrenal glands | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.1 a | 0.1 ± 0.1 a |
| Sex organs | 0.9 ± 0.1 b | 1.0 ± 0.2 b | 0.7 ± 0.2 a | 0.6 ± 0.2 a |
| Brain | 0.6 ± 0.0 a | 0.6 ± 0.1 a | 0.8 ± 0.1 b | 0.8 ± 0.1 b |
| Heart | 0.4 ± 0.0 a | 0.3 ± 0.0 a | 0.4 ± 0.1 a | 0.4 ± 0.1 a |
| Spleen | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.3 ± 0.1 a | 0.2 ± 0.1 a |
| Male | Female | |||
| Control | CSE | Control | CSE | |
| BIOCHEMICAL ANALYSES | ||||
| Glucose (mg/dL) | 109.7 ± 42.2 a | 101.0 ± 12.3 a | 117.7 ± 7.9 a | 106.0 ± 16.9 a |
| Urea (mg/dL) | 41.2 ± 3.5 ab | 38.5 ± 4.0 a | 46.1 ± 6.4 b | 39.2 ± 5.2 ab |
| Creatinine (mg/dL) | <0.5 a | <0.5 a | <0.5 a | <0.5 a |
| Proteins (g/dL) | 6.0 ± 0.7 a | 5.9 ± 0.4 a | 6.1 ± 0.3 a | 5.5 ± 0.2 a |
| ALT (U/L) | 32.4 ± 6.6 b | 26.6 ± 4.3 ab | 23.1 ± 5.4 a | 23.5 ± 3.5 a |
| GGT (U/L) | <5.0 a | <5.0 a | <5.0 a | <5.0 a |
| Cholesterol (mg/dL) | 104.8 ± 4.5 a | 103.3 ± 5.9 a | 102.3 ± 3.6 a | 105.6 ± 4.8 a |
| Potassium (mEq/L) | 3.5 ± 0.2 bc | 3.7 ± 0.5 c | 2.8 ± 0.1 a | 3.2 ± 0.3 ab |
| Sodium /mEq/L) | 144.0 ± 4.6 a | 145.2 ± 1.0 a | 144.0 ± 1.1 a | 142.2 ± 1.4 a |
| Albumin (g/dL) | 3.4 ± 0.1 ab | 3.3 ± 0.1 a | 3.5 ± 0.2 b | 3.2 ± 0.1 a |
| Bile acid (μmol/mL) | 20.7 ± 10.9 a | 15.0 ± 11.6 a | 11.7 ± 4.5 a | 14.0 ± 9.9 a |
| ERYTHROCYTE PARAMETERS | ||||
| RBCs (×106/µL) | 8.5 ± 0.5 b | 8.1 ± 0.3 ab | 7.9 ± 0.4 ab | 7.8 ± 0.4 a |
| Hemoglobin (g/dL) | 16.2 ± 0.4 b | 16.1 ± 0.5 b | 15.2 ± 0.6 a | 14.8 ± 0.6 a |
| HCT (%) | 48.4 ± 1.5 b | 47.4 ± 1.2 b | 44.5 ± 2.5 a | 43.3 ± 2.3 a |
| MCV (fl) | 56.6 ± 2.4 ab | 58.3 ± 2.4 b | 56.2 ± 0.9 ab | 55.3 ± 1.4 a |
| MCH (pg) | 19.0 ± 0.8 a | 19.8 ± 0.8 a | 19.2 ± 0.3 a | 19.0 ± 0.8 a |
| MCHC (g/dL) | 33.6 ± 0.3 a | 34.1 ± 0.7 a | 34.2 ± 0.6 a | 34.3 ± 0.8 a |
| RDW (%) | 11.8 ± 0.6 b | 11.5 ± 0.4 ab | 11.1 ± 0.3 a | 11.6 ± 0.4 ab |
| LEUKOCYTE PARAMETERS | ||||
| WBCs (×103/µL) | 8.1 ± 1.4 b | 7.1 ± 3.0 ab | 4.7 ± 1.4 a | 4.6 ± 1.4 a |
| Segmented Neutrophils (μL) | 1155.0 ± 303.5 b | 586.3 ± 304.4 a | 864.4 ± 396.2 ab | 499.0 ± 226.2 a |
| Band Neutrophils (μL) | N.D. | N.D. | N.D. | N.D. |
| Lymphocytes (µL) | 6919.0 ± 1323.6 c | 6395.7 ± 2738.7 bc | 3683.0 ± 1132.9 a | 3996.8 ± 1550.2 ab |
| Monocytes (µL) | 37.7 ± 47.5 a | 71.0 ± 67.0 a | 82.8 ± 82.3 a | 57.1 ± 42.2 a |
| Eosinophils (μL) | 59.7 ± 66.5 a | 84.3 ± 98.7 a | 69.2 ± 94.9 a | 75.5 ± 18.4 a |
| Basophils (μL) | N.D. | N.D. | 43.2 ± 93.9 | N.D. |
| PLATELET PARAMETERS | ||||
| Platelets (×103 /µL) | 801.8 ± 213.9 a | 773.5 ± 309.3 a | 763.0 ± 272.9 a | 755.4 ± 368.4 a |
| MPV (fl) | 9.0 ± 0.5 a | 9.0 ± 0.8 a | 8.7 ± 0.6 a | 9.1 ± 0.4 a |
| Plateletcrit (%) | 0.7 ± 0.1 a | 0.6 ± 0.2 a | 0.6 ± 0.2 a | 0.6 ± 0.3 a |
| Male | Female | |||
|---|---|---|---|---|
| Control | CSE | Control | CSE | |
| Oxidative stress | ||||
| Total antioxidant capacity (Eq. Trolox mM) | 2.0 ± 0.1 a | 2.0 ± 0.1 a | 2.0 ± 0.0 a | 2.0 ± 0.1 a |
| Glutathione Peroxidase (GPx) (U/mL) | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.0 a |
| Glutathione Reductase (GR) (U/mL) | 3.5 ± 0.8 a | 3.9 ± 1.2 a | 4.1 ± 0.9 a | 3.7 ± 0.9 a |
| Superoxide Dismutase (SOD) (U/mL) | 2.9 ± 0.8 a | 3.1 ± 1.4 a | 3.0 ± 1.3 a | 3.2 ± 1.3 a |
| Catalase (CAT) (U/mL) | 7.0 ± 4.2 a | 7.4 ± 5.1 a | 5.3 ± 1.5 a | 7.1 ± 4.3 a |
| Inflammation | ||||
| C Reactive Protein (μL U/mL) | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a |
| Hormones | ||||
| Insulin (ng/mL) | 34.9 ± 19.3 a | 29.4 ± 28.8 a | 21.9 ± 12.6 a | 26.9 ± 8.9 a |
| Serotonin (ng/mL) | 240.8 ± 43.8 a | 239.1 ± 46.0 a | 208.4 ± 26.4 a | 210.7 ± 20.2 a |
| Melatonin (pg/mL) | 671.9 ± 258.1 a | 853.4 ± 161.5 a | 784.5 ± 196.3 a | 900.6 ± 45.4 a |
| Male | Female | |||
|---|---|---|---|---|
| Control | CSE | Control | CSE | |
| Number pellet/rat | 53.0 ± 0.5 b | 44.0 ± 1.6 a | 44.0 ± 0.1 a | 41.0 ± 2.0 a |
| Feces weight (g)/pellet | 0.04 ± 0.0 a | 0.03 ± 0.0 a | 0.03 ± 0.0 a | 0.03 ± 0.0 a |
| Feces weight (g)/rat | 5.5 ± 0.2 b | 5.2 ± 0.0 ab | 4.2 ± 0.4 a | 4.4 ± 0.2 a |
| pH | 6.3 ± 0.1 a | 6.3 ± 0.1 a | 6.3 ± 0.1 a | 6.3 ± 0.1 a |
| Total antioxidant capacity (Eq. Trolox mM) | 8.2 ± 0.4 a | 7.8 ± 0.3 a | 8.1 ± 0.4 a | 7.8 ± 0.7 a |
| SCFAs (µmol/g) | ||||
| Acetic | 46.42 ± 1.75 a | 68.49 ± 8.23 c | 59.64 ± 1.79 b | 62.18 ± 4.22 bc |
| Propionic | 5.14 ± 0.24 a | 5.94 ± 0.90 a | 7.09 ± 0.34 b | 5.29 ± 0.38 a |
| Isobutyric | 0.24 ± 0.01 a | 0.28 ± 0.04 ab | 0.40 ± 0.02 c | 0.31 ± 0.03 b |
| Butyric | 6.71 ± 1.08 c | 2.33 ± 0.20 a | 10.63 ± 0.73 d | 4.51 ± 0.34 b |
| Isovaleric | 0.17 ± 0.02 a | 0.16 ± 0.03 a | 0.28 ± 0.01 c | 0.21 ± 0.02 b |
| Valeric | 0.49 ± 0.05 b | 0.40 ± 0.06 a | 0.78 ± 0.03 c | 0.48 ± 0.04 b |
| Caproic | 0.40 ± 0.04 b | 0.30 ± 0.04 a | 0.92 ± 0.03 d | 0.55 ± 0.06 c |
| Heptanoic | N.D. | N.D. | N.D. | N.D. |
| Total SCFAs | 59.57 ± 3.14 a | 77.90 ± 9.42 b | 79.75 ± 2.81 b | 73.52 ± 5.05 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iriondo-DeHond, A.; Rios, M.B.; Herrera, T.; Rodriguez-Bertos, A.; Nuñez, F.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Coffee Silverskin Extract: Nutritional Value, Safety and Effect on Key Biological Functions. Nutrients 2019, 11, 2693. https://doi.org/10.3390/nu11112693
Iriondo-DeHond A, Rios MB, Herrera T, Rodriguez-Bertos A, Nuñez F, San Andres MI, Sanchez-Fortun S, del Castillo MD. Coffee Silverskin Extract: Nutritional Value, Safety and Effect on Key Biological Functions. Nutrients. 2019; 11(11):2693. https://doi.org/10.3390/nu11112693
Chicago/Turabian StyleIriondo-DeHond, Amaia, Maria Belen Rios, Teresa Herrera, Antonio Rodriguez-Bertos, Fernando Nuñez, Manuel Ignacio San Andres, Sebastian Sanchez-Fortun, and Maria Dolores del Castillo. 2019. "Coffee Silverskin Extract: Nutritional Value, Safety and Effect on Key Biological Functions" Nutrients 11, no. 11: 2693. https://doi.org/10.3390/nu11112693
APA StyleIriondo-DeHond, A., Rios, M. B., Herrera, T., Rodriguez-Bertos, A., Nuñez, F., San Andres, M. I., Sanchez-Fortun, S., & del Castillo, M. D. (2019). Coffee Silverskin Extract: Nutritional Value, Safety and Effect on Key Biological Functions. Nutrients, 11(11), 2693. https://doi.org/10.3390/nu11112693