Discovery of Nobiletin from Citrus Peel as a Potent Inhibitor of β-Amyloid Peptide Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Aβ25-35 Stock Solution
2.2. Cell Viability Analysis
2.3. Intracellular ROS Analysis
2.4. Apoptosis Assay by Hoechst 33342 Staining
2.5. Fluorescence-Activated Cell Sorting (FACS) Analysis
2.6. Assessment of Levels of Nitric Oxide (NO) and Prostaglandin E2 (PGE2)
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results and Discussion
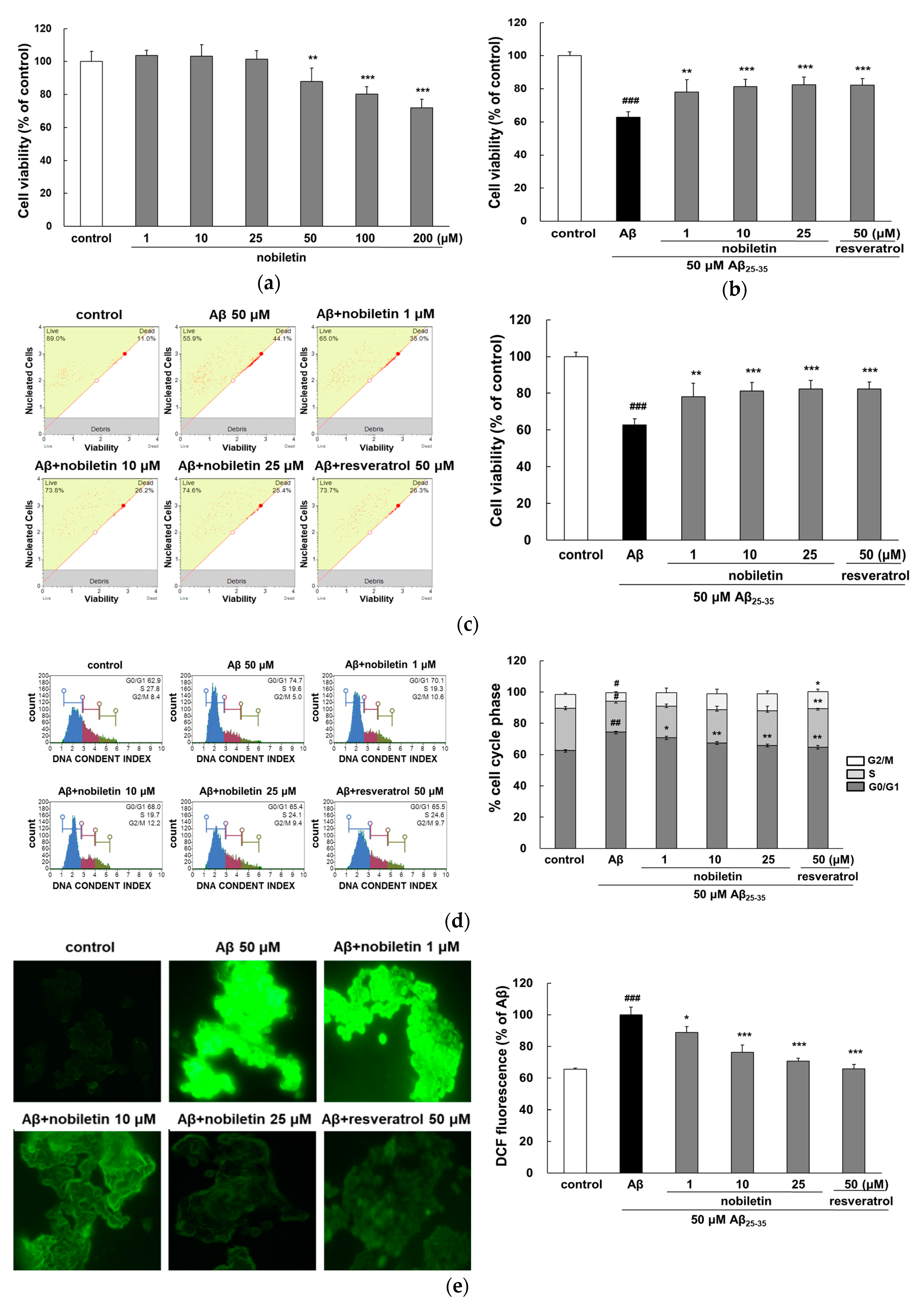
3.1. Nobiletin Inhibits Cytotoxicity Evoked by Aβ25-35
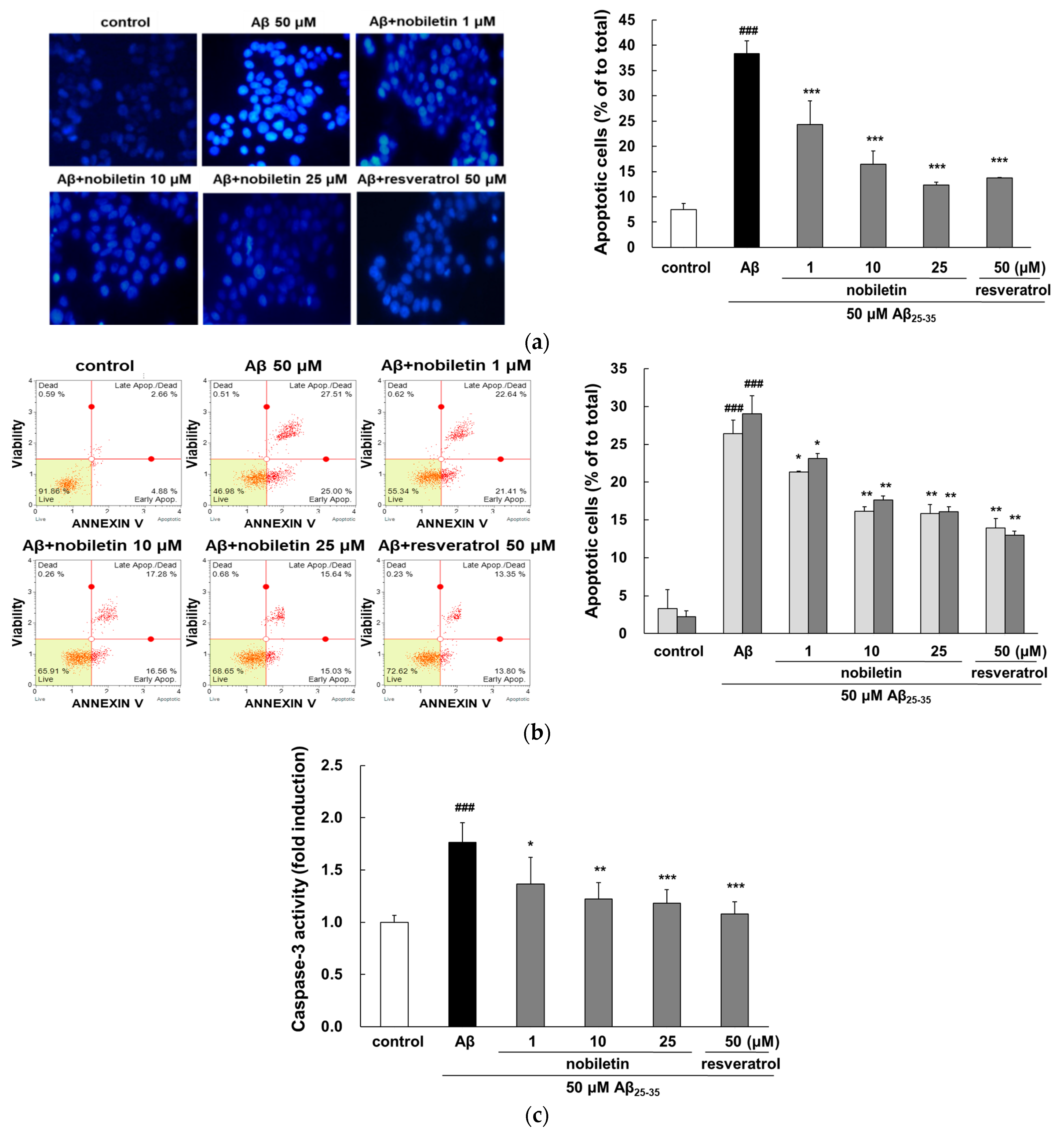
3.2. Nobiletin Reduces Aβ25–35-Mediated Apoptosis and Caspase-3 Activation
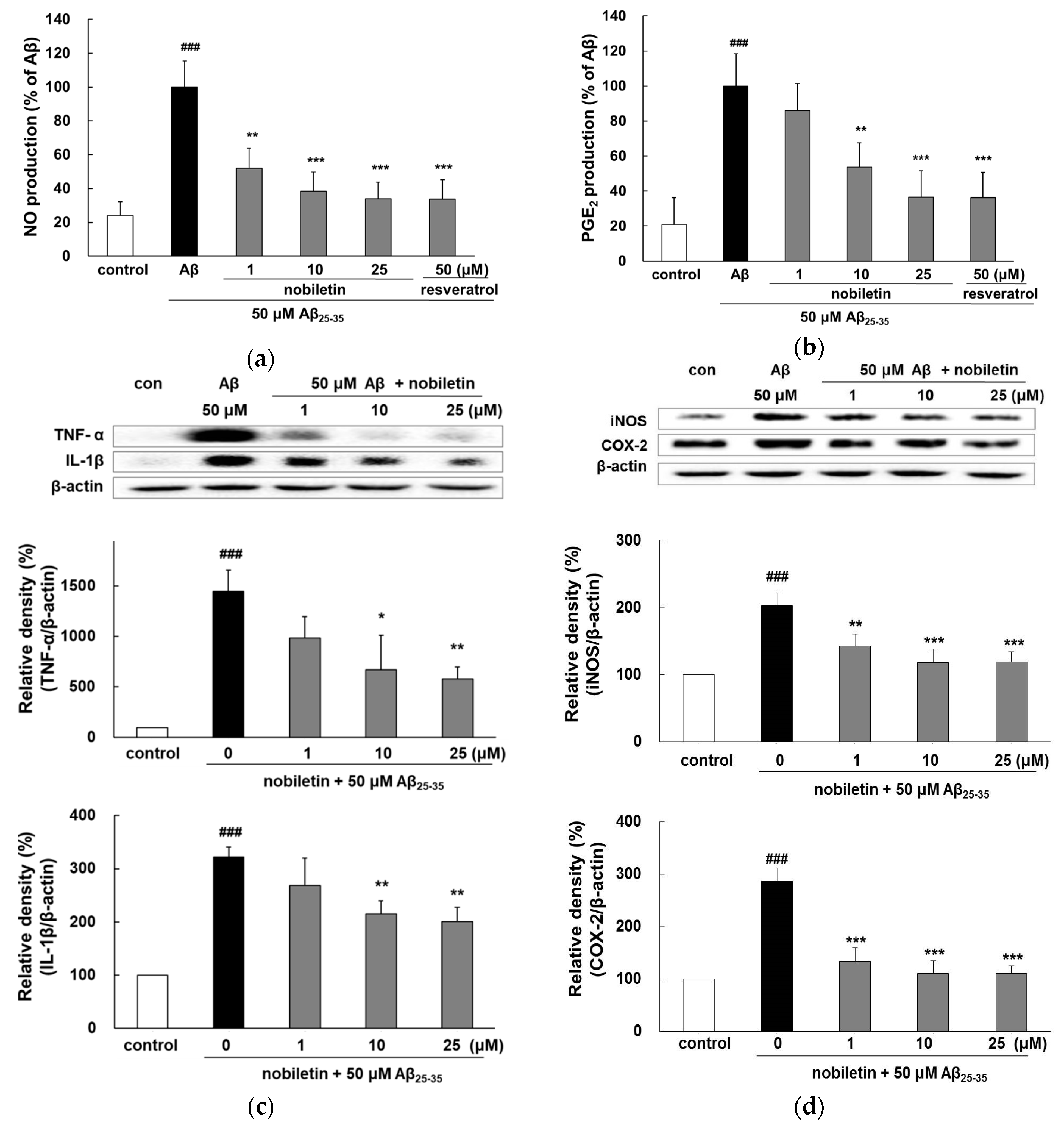
3.3. Nobiletin Suppresses Aβ25-35-Induced Release of Inflammatory Markers
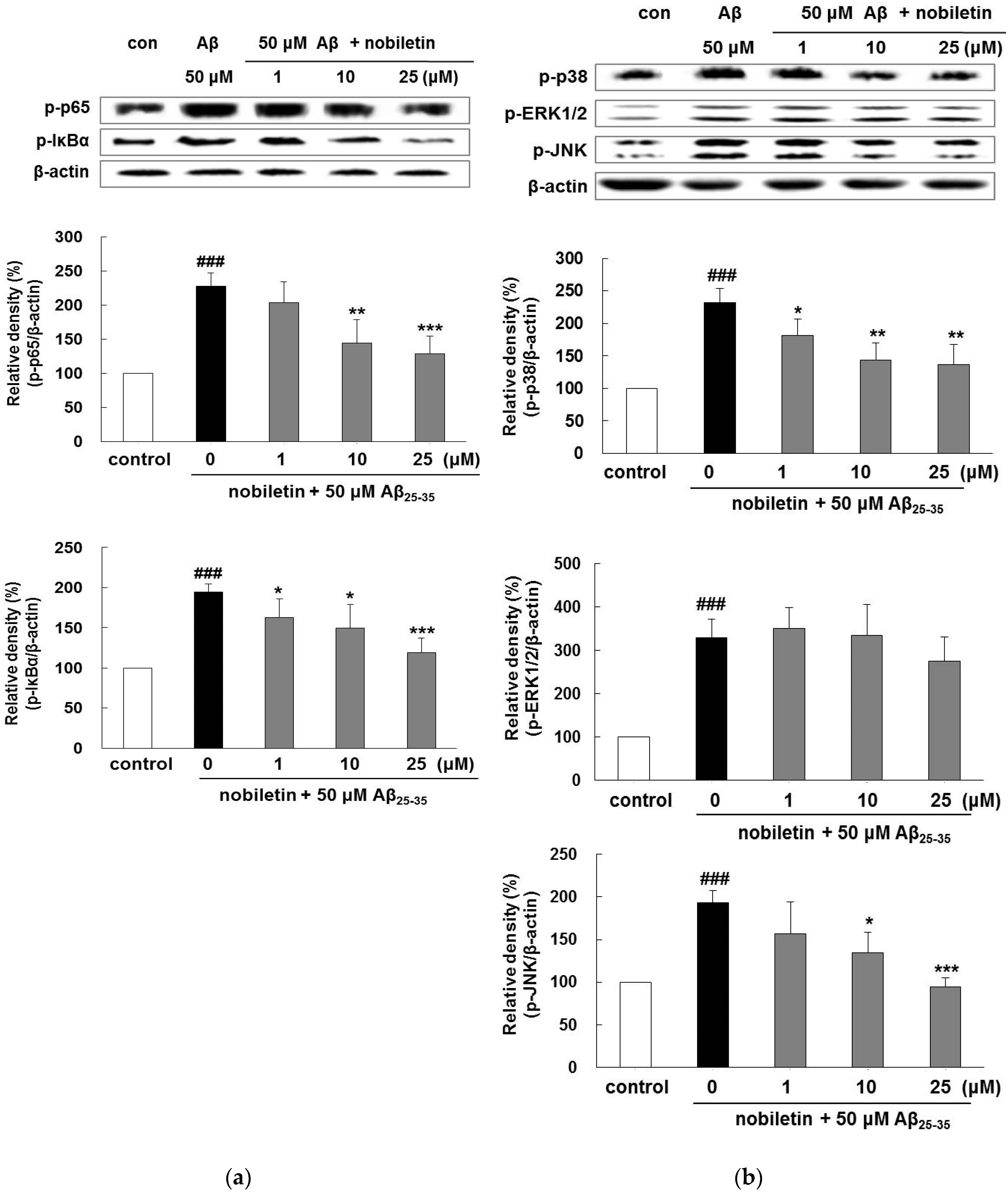
3.4. Nobiletin Regulates Aβ25-35-Induced NF-κB and MAPK Signaling Pathways
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Selkoe, D.J. Translating cell biology into therapeutic advances in Alzheimer′s disease. Nature 1999, 399, A23–A31. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; He, P.; Zhong, Z.; McAllister, C.; Lindholm, K. Distinct destructive signal pathways of neuronal death in Alzheimer′s disease. Trends Mol. Med. 2006, 12, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid beta-peptide. Trends Mol. Med. 2001, 12, 548–554. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Dodwell, S.A.; Meyer-Luehmann, M.; Hyman, B.T.; Bacskai, B.J. Plaque-derived oxidative stress mediates distorted neurite trajectories in the Alzheimer mouse model. J. Neuropathol. Exp. Neurol. 2006, 65, 1082–1089. [Google Scholar] [CrossRef]
- Robinson, R.A.; Lange, M.B.; Sultana, R.; Galvan, V.; Fombonne, J.; Gorostiza, O.; Zhang, J.; Warrier, G.; Cai, J.; Pierce, W.M.; et al. Differential expression and redox proteomics analyses of an Alzheimer disease transgenic mouse model: Effects of the amyloid-β peptide of amyloid precursor protein. Neuroscience 2011, 177, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Sultana, R. Methionine-35 of Aβ (1–42): Importance for oxidative stress in Alzheimer disease. J. Amino Acid. 2011, 2011, 198430. [Google Scholar] [CrossRef] [PubMed]
- Wojsiat, J.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. Oxidant/Antioxidant imbalance in alzheimer’s disease: Therapeutic and diagnostic prospects. Oxid. Med. Cell Longev. 2018, 2018, 6435861. [Google Scholar] [CrossRef]
- Chen, C.H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar] [CrossRef]
- Chami, L.; Buggia-Prévot, V.; Duplan, E.; Del Prete, D.; Chami, M.; Peyron, J.F.; Checler, F. Nuclear factor-κB regulates βAPP and β- and γ-secretases differently at physiological and supraphysiological Aβ concentrations. J. Biol. Chem. 2012, 287, 24573–24584. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechonol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef]
- Wang, M.; Meng, D.; Zhang, P.; Wang, X.; Du, G.; Brennan, C.; Li, S.; Ho, C.T.; Zhao, H. Antioxidant protection of nobiletin, 5-dimethylnobiletin, tangeretin, and 5-demethyltangeretin from citrus peel in Sacch. Cerevisiae. J. Agric. Food Chem. 2018, 66, 3155–3160. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Hwang, J.H.; Ko, H.C.; Park, J.G.; Kim, S.J. Nobiletin from Citrus fruit peel inhibits the DNA-binding activity of NF-kappab and ROS production in LPS-activated RAW 264.7 cells. J. Ethnopharmacol. 2007, 113, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Cha, B.Y.; Saito, K.; Yamakawa, H.; Choi, S.S.; Yamaguchi, K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem. Pharmacol. 2010, 79, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Assini, J.M.; Lee, J.K.; Allister, E.M.; Sutherland, B.G.; Koppes, J.B.; Sawyez, C.G.; Edwards, J.Y.; Telford, D.E.; Charbonneau, A.; et al. Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes 2011, 60, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Yasui, Y.; Tanaka, T.; Ohigashi, H.; Murakami, A. Suppressive effects of nobiletin on hyperleptinemia and colitis-related colon carcinogenesis in male ICR mice. Carcinogenesis 2008, 29, 1057–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, A.; Yamakuni, T.; Haraguchi, M.; Omae, N.; Song, S.Y.; Kato, C.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid that improves memory impairment, rescues bulbectomy induced cholinergic neurodegeneration in mice. J. Pharmacol. Sci. 2007, 105, 122–126. [Google Scholar] [CrossRef]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shin, R.W.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer’s disease. J. Pharmacol. Exper. Thera. 2008, 326, 739–744. [Google Scholar] [CrossRef]
- Nakajima, A.; Aoyama, Y.; Shin, E.J.; Nam, Y.; Kim, H.C.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; Yokoi, T.; Ohizumi, Y.; et al. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Abeta levels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD). Behav. Brain Res. 2015, 289, 69–77. [Google Scholar] [CrossRef]
- Youn, K.; Yu, Y.; Lee, J.; Jeong, W.S.; Ho, C.T.; Jun, M. Polymethoxyflavones: Novel β-secretase (BACE1) inhibitors from Citrus peels. Nutrients 2017, 9, 973. [Google Scholar] [CrossRef]
- Giovanni, A.; Wirtz-Brugger, F.; Keramaris, E.; Slack, R.; Park, D.S. Involvement of cell cycle elements, cycline-dependent kinases, pRb, and E2F x DP, in B-amyloid-induced neuronal death. J. Biol. Chem. 1999, 274, 19011–19016. [Google Scholar] [CrossRef]
- Lu, Y.H.; Su, M.Y.; Huang, H.Y.; Lin-Li; Yuan, C.G. Protective effects of the citrus flavanones to PC12 cells against cytotoxicity induced by hydrogen peroxide. Neurosci. Lett. 2010, 484, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Amarsanaa, K.; Wu, J.; Jeon, S.C.; Cui, Y.; Jung, S.C.; Park, D.B.; Kim, S.J.; Han, S.H.; Kim, H.W.; et al. Nobiletin attenuates neurotoxic mitochondrial calcium overload through K+ influx and ΔΨm across mitochondrial inner membrane. Korean J. Physiol. Pharmacol. 2018, 3, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Aoyama, Y.; Nguyen, T.T.; Shin, E.J.; Kim, H.C.; Yamada, S.; Nakai, T.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, ameliorates cognitive impairment, oxidative burden, and hyperphosphorylation of tau in senescence-accelerated mouse. Behav. Brain Res. 2013, 250, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.F.; Lin, Z.X.; Mao, Q.Q.; Ip, S.P.; Su, Z.R.; Lai, X.P. Protective effect of isorhynchophylline against beta-amyloid-induced neurotoxicity in PC12 cells. Cell Mol. Neurobiol. 2012, 32, 353–360. [Google Scholar] [CrossRef]
- Li, Z.R.; Yang, L.; Zhen, J.; Zhao, Y.; Lu, Z.N. Nobiletin protects PC12 cells from ERS-induced apoptosis in OGD/R injury via activation of the PI3K/AKT pathway. Exp. Ther. Med. 2018, 16, 1470–1476. [Google Scholar] [CrossRef]
- Cho, H.W.; Jung, S.Y.; Lee, G.H.; Cho, J.H.; Choi, I.Y. Neuroprotective effect of Citrus unshiu immature peel and nobiletin inhibiting hydrogen peroxide-induced oxidative stress in HT22 murine hippocampal neuronal cells. Pharmacogn. Mag. 2015, 11, S284–S289. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, J.; Jung, S.C.; Park, D.B.; Maeng, Y.H.; Hong, J.Y.; Kim, S.J.; Lee, S.R.; Kim, S.J.; Kim, S.J.; et al. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol. Pharm. Bull. 2010, 33, 1814–1821. [Google Scholar] [CrossRef]
- Ho, S.C.; Kuo, C.T. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium). Food Chem. Toxicol. 2014, 71, 176–182. [Google Scholar] [CrossRef]
- Wang, Y.; Zang, W.; Ji, S.; Cao, J.; Sun, C. Three Polymethoxyflavones purified from Ougan (Citrus reticulata Cv. Suavissima) inhibited LPS-Induced NO elevation in the neuroglia BV-2 cell line via the JAK2/STAT3 pathway. Nutrients 2019, 11, 791. [Google Scholar] [CrossRef]
- Qi, G.; Mi, Y.; Fan, R.; Li, R.; Liu, Z.; Liu, X. Nobiletin Protects against systemic inflammation-stimulated memory impairment via MAPK and NF-κB signaling pathways. J. Agric. Food Chem. 2019, 67, 5122–5134. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Yamakuni, T.; Hashimoto, M.; Haque, A.M.; Shido, O.; Mimaki, Y.; Sashida, Y.; Ohizumi, Y. Nobiletin restoring beta-amyloid-impaired CREB phosphorylation rescues memory deterioration in Alzheimer’s disease model rats. Neurosci. Lett. 2006, 400, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Wahajuddin; Tewari, D.; Patel, K.; Jain, G.K. Permeability determination and pharmacokinetic study of nobiletin in rat plasma and brain by validated high-performance liquid chromatography method. Fitoterapia 2011, 82, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Cha, B.Y.; Choi, S.S.; Choi, B.K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Nobiletin improves obesity and insulin resistance in high-fat diet-induced obese mice. J. Nutr. Biochem. 2013, 24, 156–162. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youn, K.; Lee, S.; Jun, M. Discovery of Nobiletin from Citrus Peel as a Potent Inhibitor of β-Amyloid Peptide Toxicity. Nutrients 2019, 11, 2648. https://doi.org/10.3390/nu11112648
Youn K, Lee S, Jun M. Discovery of Nobiletin from Citrus Peel as a Potent Inhibitor of β-Amyloid Peptide Toxicity. Nutrients. 2019; 11(11):2648. https://doi.org/10.3390/nu11112648
Chicago/Turabian StyleYoun, Kumju, Seonah Lee, and Mira Jun. 2019. "Discovery of Nobiletin from Citrus Peel as a Potent Inhibitor of β-Amyloid Peptide Toxicity" Nutrients 11, no. 11: 2648. https://doi.org/10.3390/nu11112648
APA StyleYoun, K., Lee, S., & Jun, M. (2019). Discovery of Nobiletin from Citrus Peel as a Potent Inhibitor of β-Amyloid Peptide Toxicity. Nutrients, 11(11), 2648. https://doi.org/10.3390/nu11112648






