The Impact of a Large Bolus Dose of l-leucine and l-isoleucine on Enteroendocrine and Pancreatic Hormones, and Glycemia in Healthy, Inactive Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Ethics Approval
2.2. Anthropometric, Body Composition, and Dietary Control
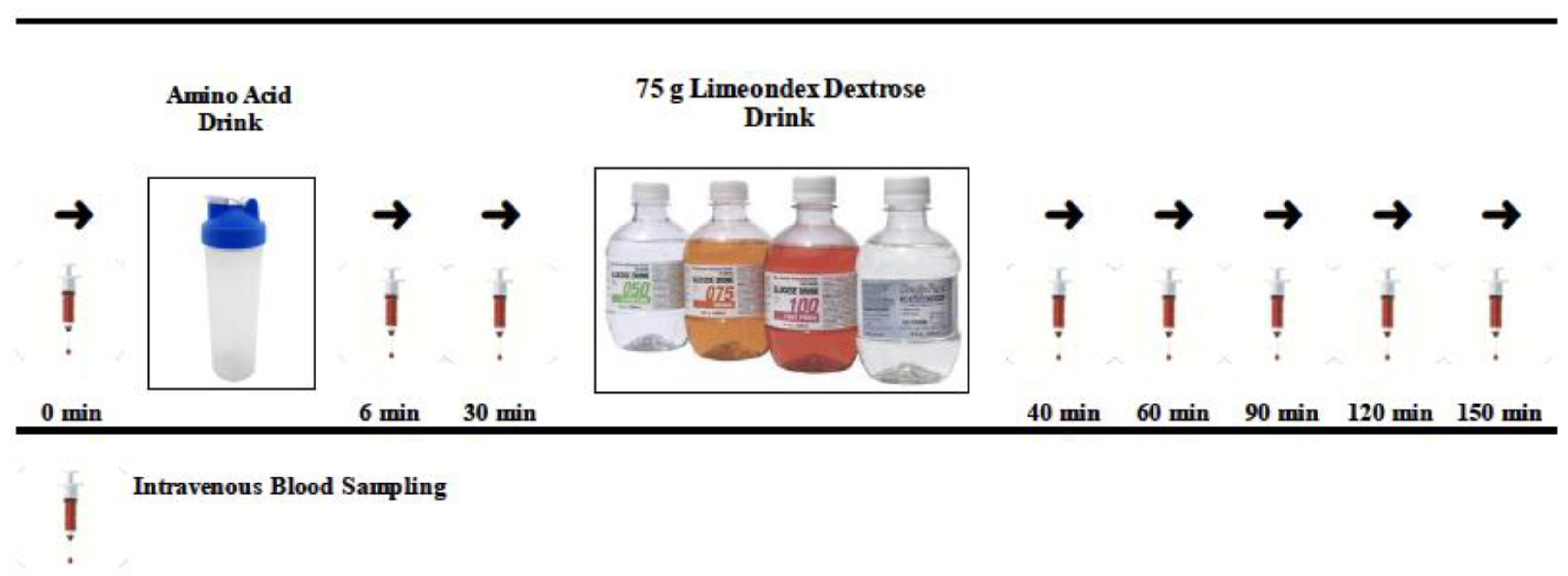
2.3. Experimental Study Design
2.4. Amino Acids and Placebo Control Treatments
2.5. Sampling Protocol, Storage, and Biochemistry Analysis
2.6. Statistical Analysis
3. Results
3.1. Treatment Dose
3.2. MyFitnessPal 3-day Dietary Recall
3.3. Participant Compliance and Data Integrity
3.4. Plasma Analysis
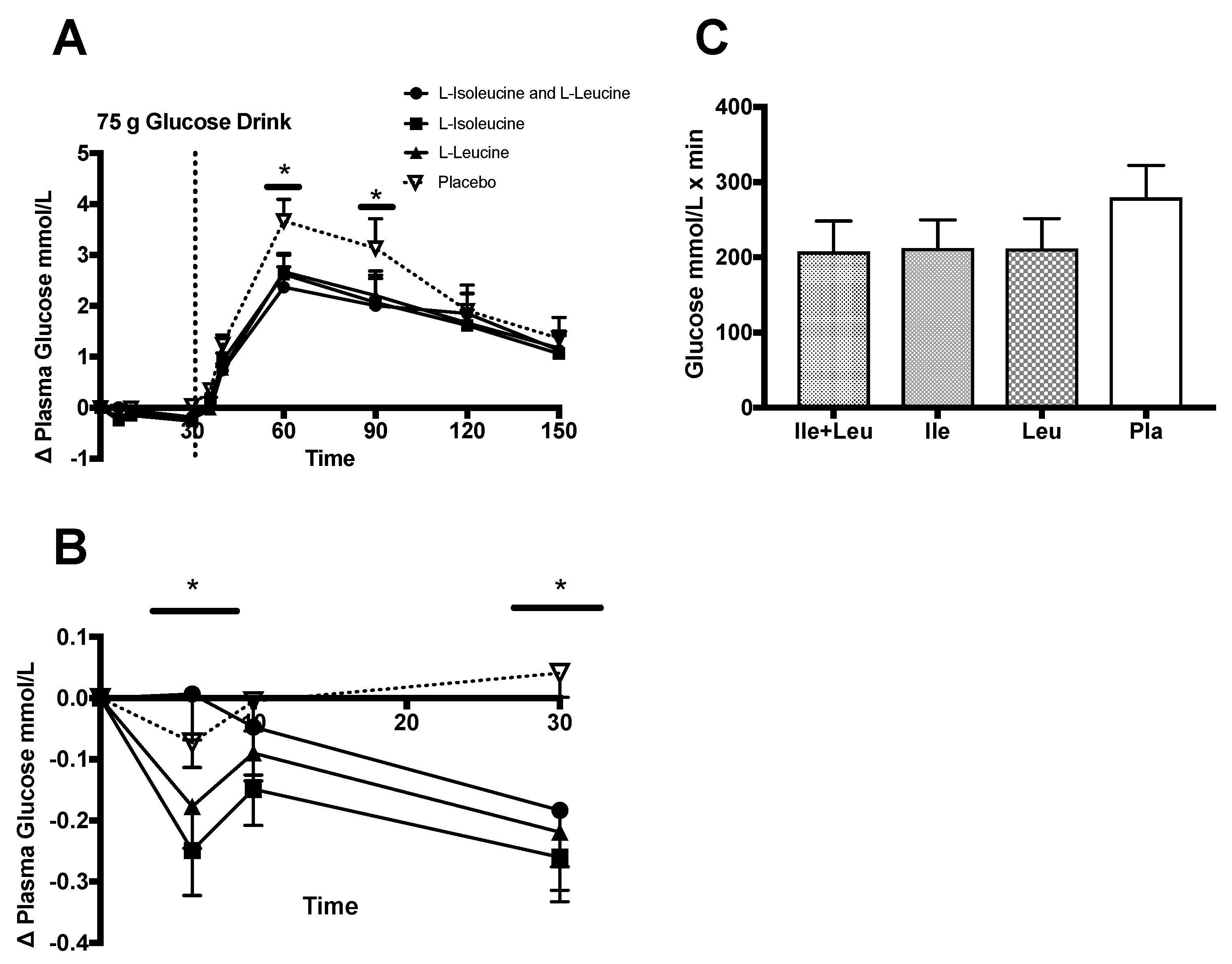
3.4.1. Glucose
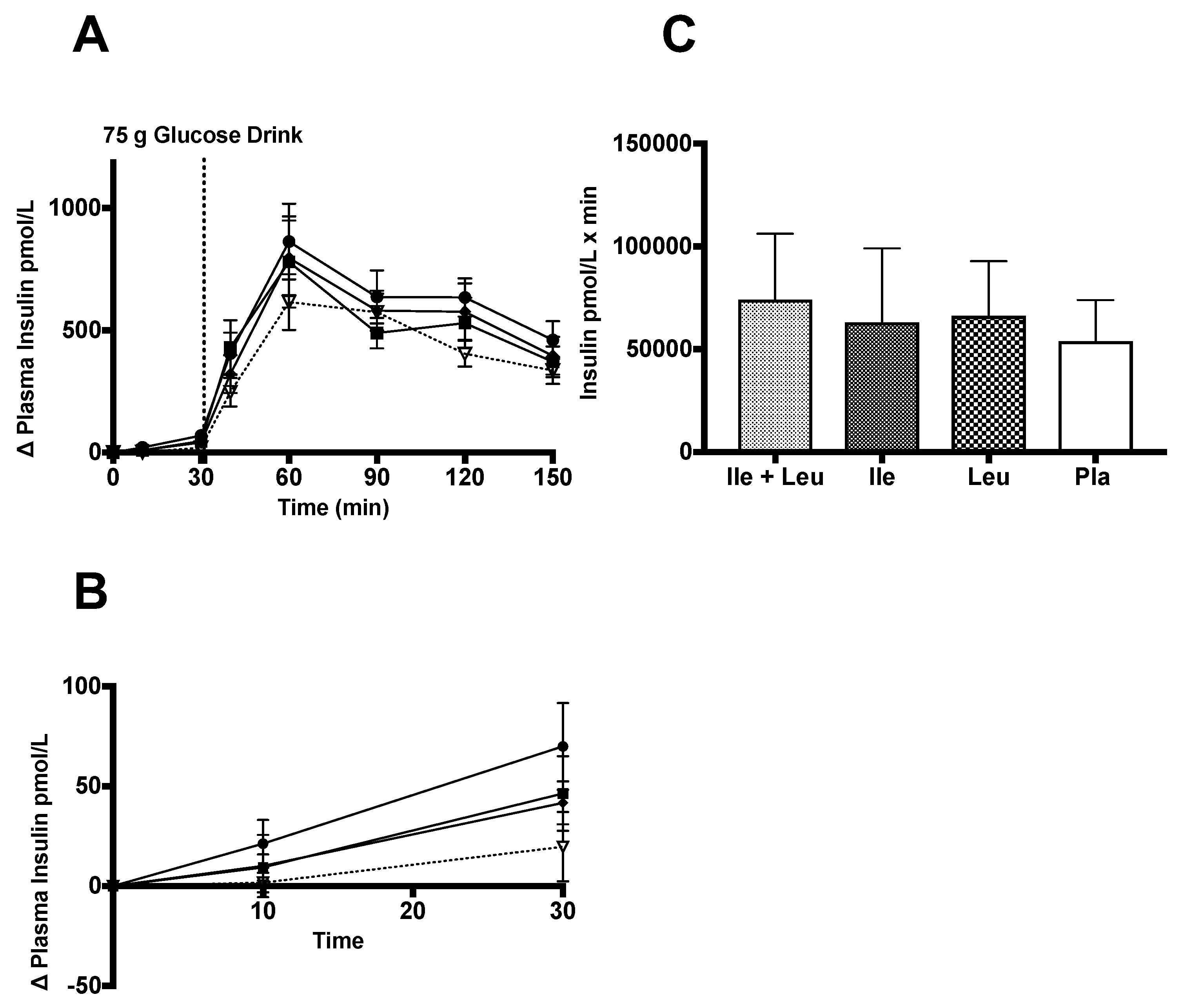
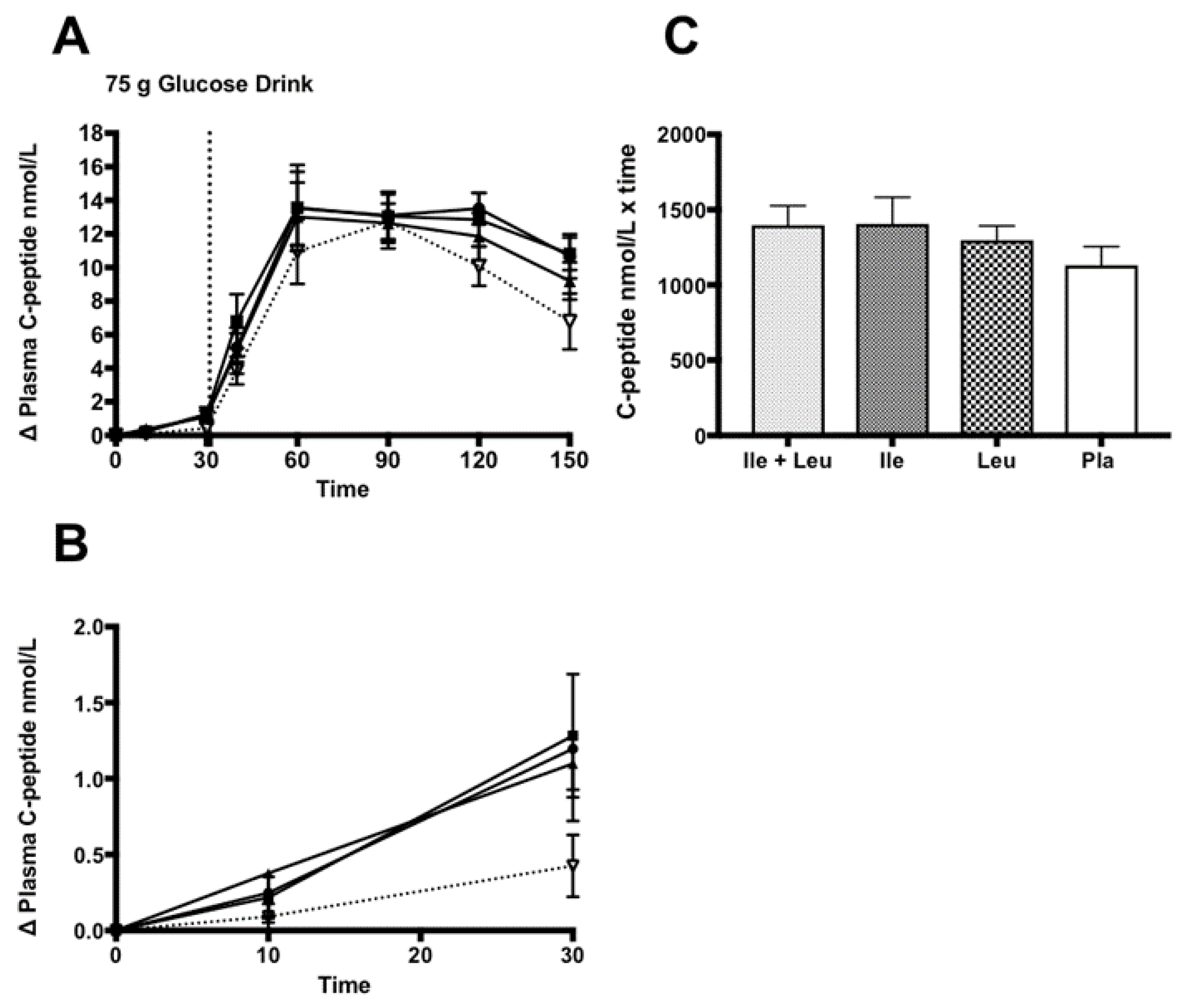
3.4.2. Insulin and C-peptide
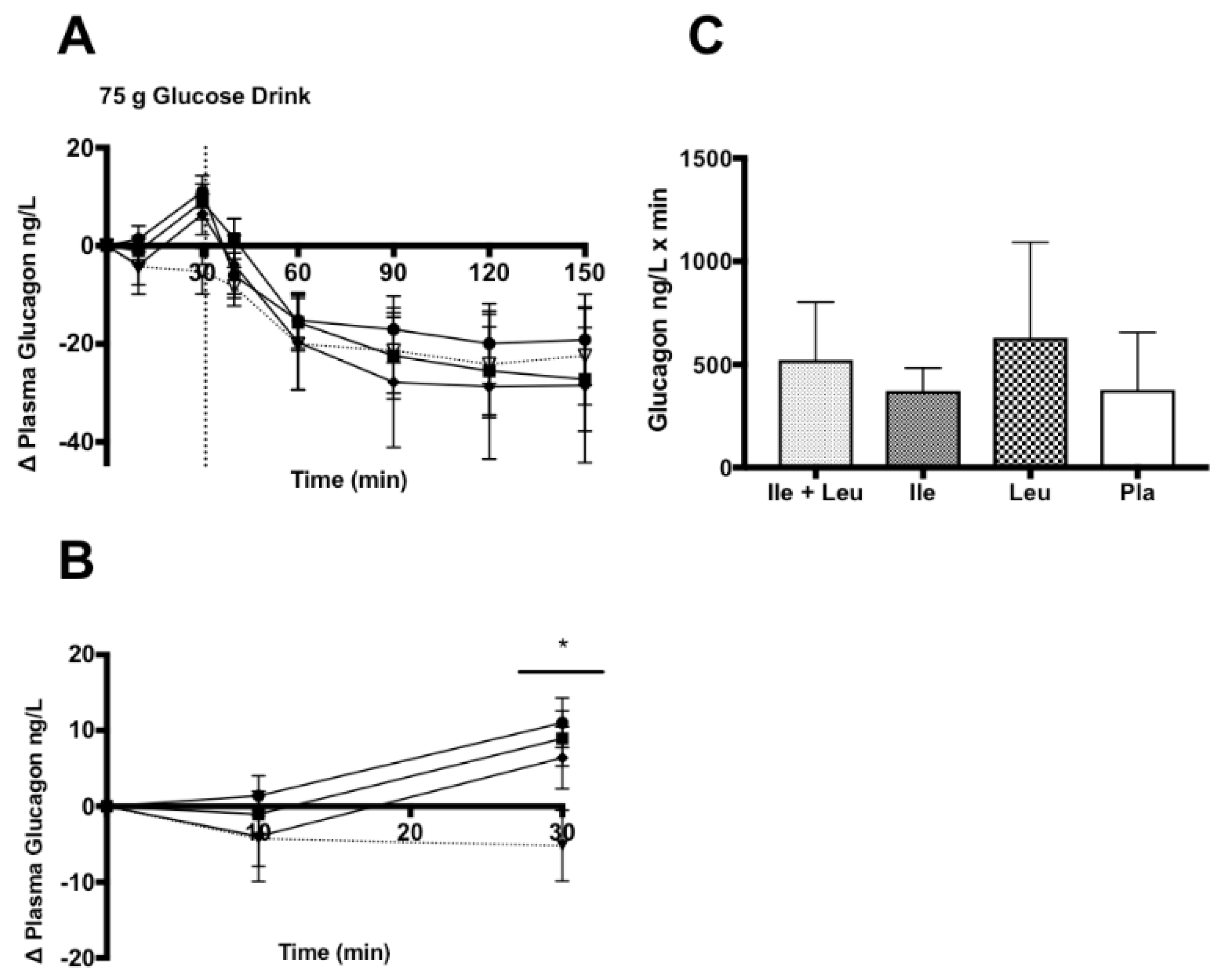
3.4.3. Glucagon
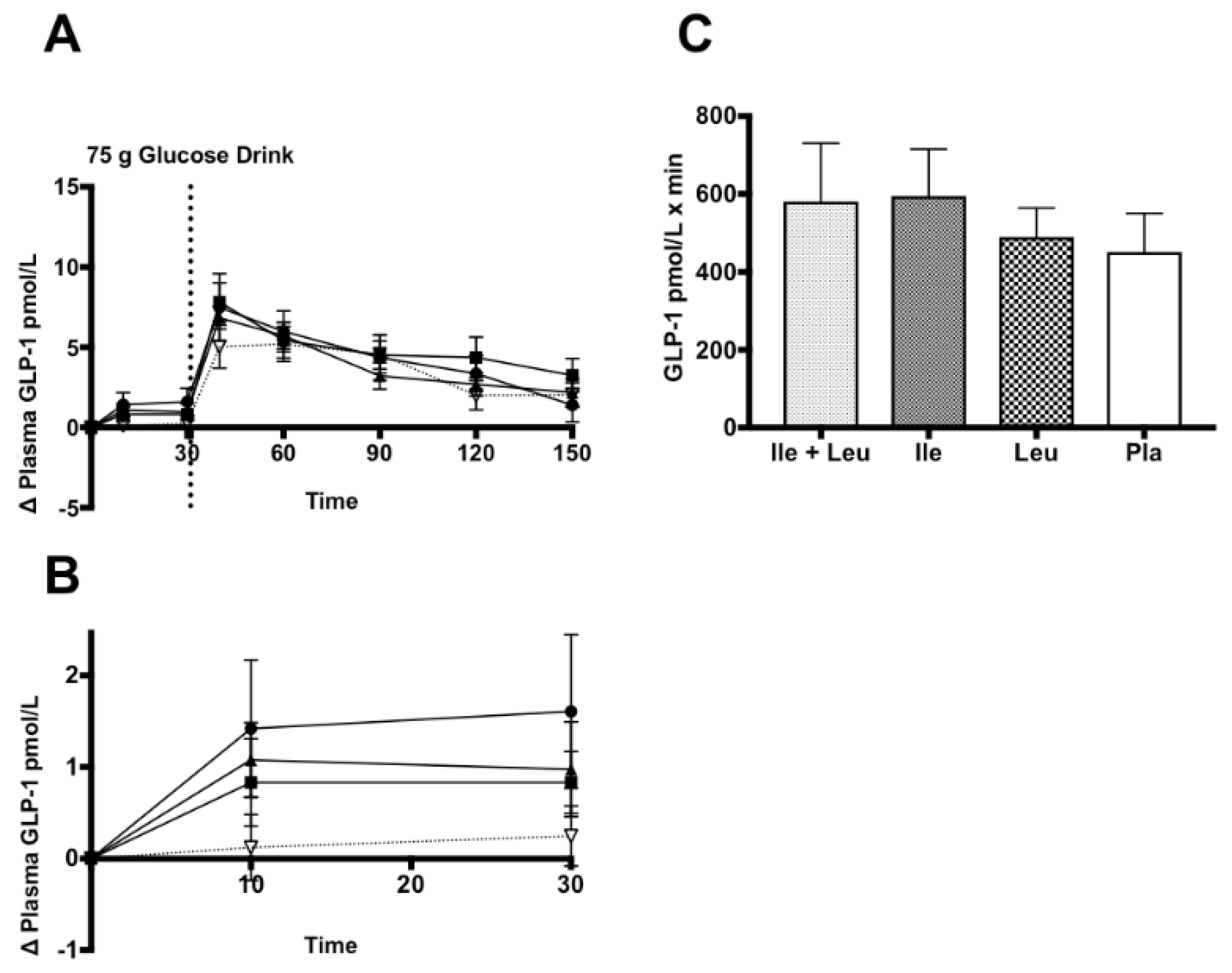
3.4.4. GLP-1
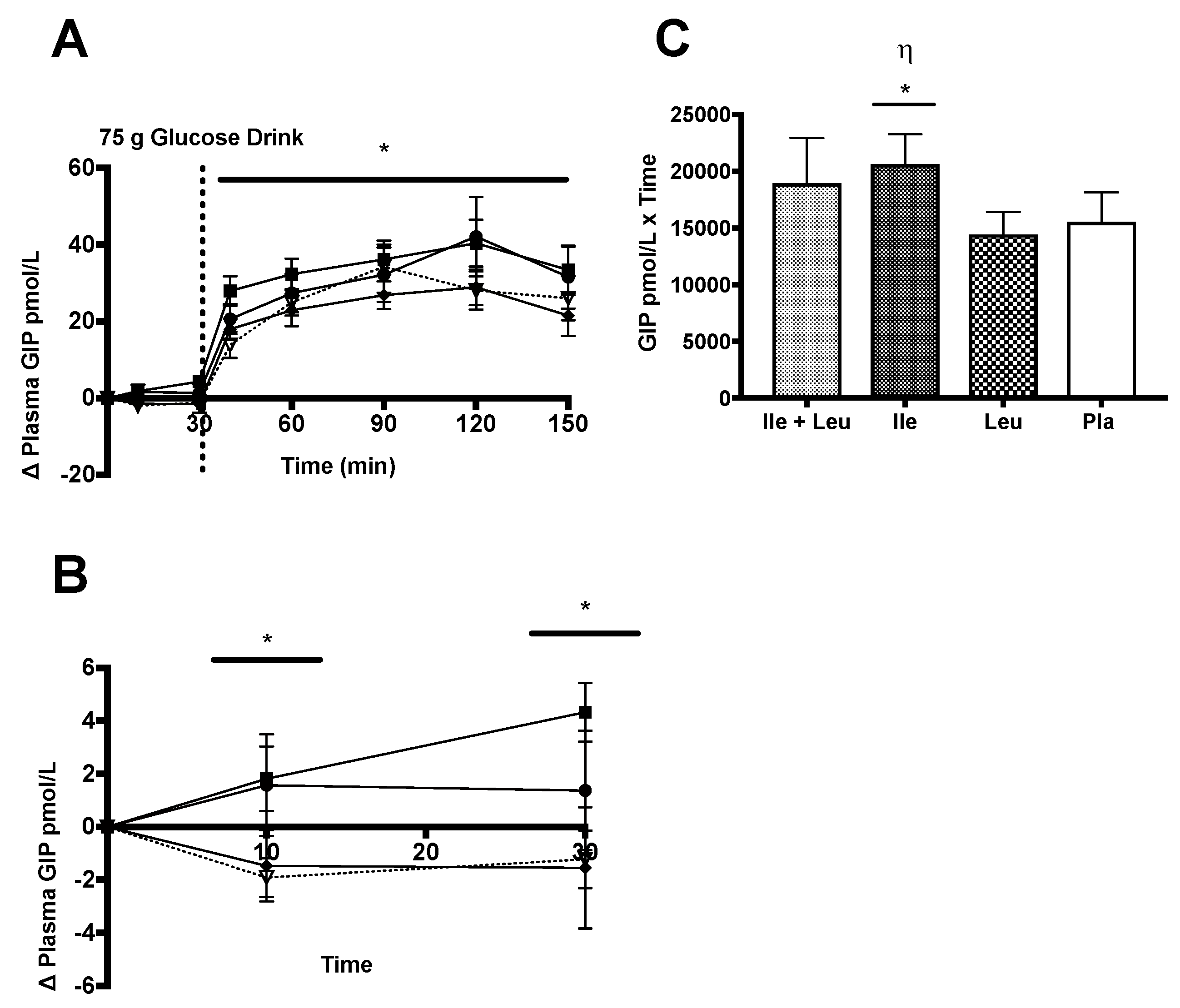
3.4.5. GIP
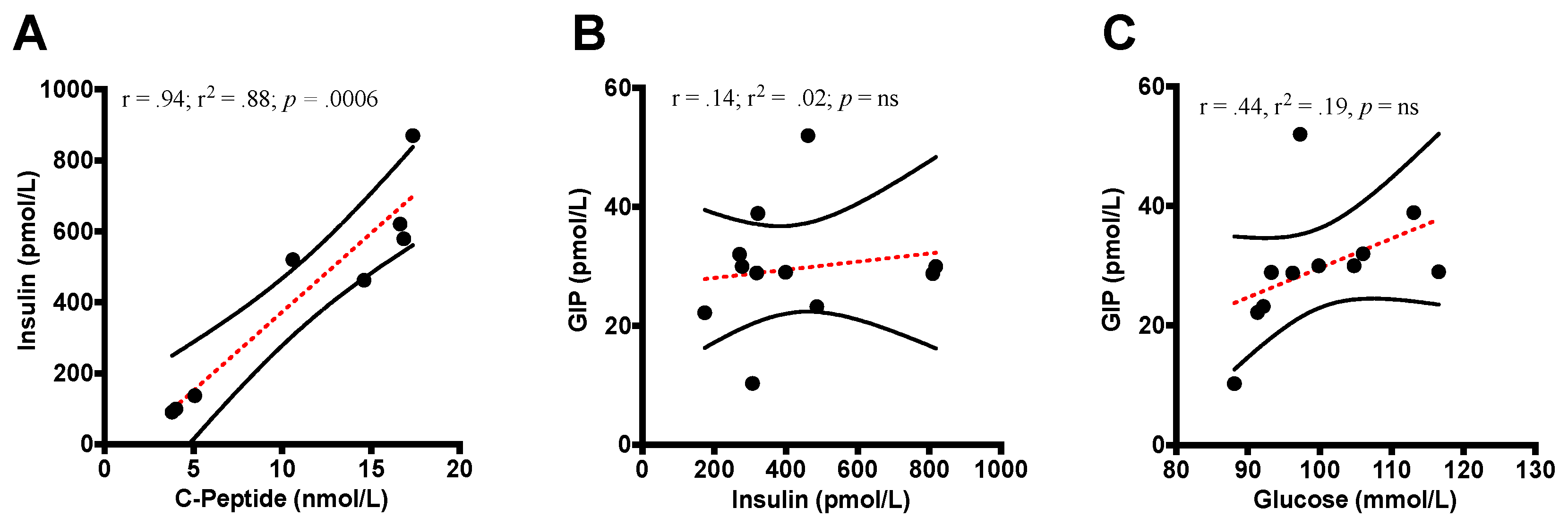
3.4.6. GIP Correlations
4. Discussion
4.1. Glucose
4.2. Insulin and C-peptide
4.3. Glucagon
4.4. GLP-1
4.5. GIP
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
References
- Aguiree, F.; Brown, A.; Cho, N.H.; Dahlquist, G.; Dodd, S.; Dunning, T.; Hirst, M.; Hwang, C.; Magliano, D.; Patterson, C. IDF Diabetes Atlas; International Diabetes Federation: Basel, Switzerland, 2013. [Google Scholar]
- Association, A.D. Economic costs of diabetes in the US in 2012. Diabetes Care 2013, 36, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017.
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B.; American College of Sports, M.; et al. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q.; Lane, J.T.; Burmeister, L.A. Metabolic response to cottage cheese or egg white protein, with or without glucose, in type II diabetic subjects. Metab. Clin. Exp. 1992, 41, 1137–1145. [Google Scholar] [CrossRef]
- Gannon, M.C.; Nuttall, F.Q.; Saeed, A.; Jordan, K.; Hoover, H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am. J. Clin. Nutr. 2003, 78, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, J.A.; Damberg, G.; Gupta, V.; Nuttall, F.Q. Effect of protein ingestion on the glucose appearance rate in people with type 2 diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 1040–1047. [Google Scholar] [CrossRef]
- Khan, M.A.; Gannon, M.C.; Nuttall, F.Q. Glucose appearance rate following protein ingestion in normal subjects. J. Am. Coll. Nutr. 1992, 11, 701–706. [Google Scholar] [CrossRef]
- Nuttall, F.Q.; Gannon, M.C. The metabolic response to a high-protein, low-carbohydrate diet in men with type 2 diabetes mellitus. Metab. Clin. Exp. 2006, 55, 243–251. [Google Scholar] [CrossRef]
- Nuttall, F.Q.; Gannon, M.C. Metabolic response to egg white and cottage cheese protein in normal subjects. Metab. Clin. Exp. 1990, 39, 749–755. [Google Scholar] [CrossRef]
- Westphal, S.A.; Gannon, M.C.; Nuttall, F.Q. Metabolic response to glucose ingested with various amounts of protein. Am. J. Clin. Nutr. 1990, 52, 267–272. [Google Scholar] [CrossRef]
- Gunnerud, U.J.; Ostman, E.M.; Bjorck, I.M. Effects of whey proteins on glycaemia and insulinaemia to an oral glucose load in healthy adults; a dose-response study. Eur. J. Clin. Nutr. 2013, 67, 749–753. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Froy, O.; Ahren, B.; Boaz, M.; Landau, Z.; Bar-Dayan, Y.; Ganz, T.; Barnea, M.; Wainstein, J. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: A randomised clinical trial. Diabetologia 2014, 57, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Frid, A.H.; Nilsson, M.; Holst, J.J.; Björck, I.M. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am. J. Clin. Nutr. 2005, 82, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.; Arfuso, F.; Keane, K. Nutrient regulation of insulin secretion and action. J. Endocrinol. 2014, 221, R105–R120. [Google Scholar] [CrossRef]
- Gaudel, C.; Nongonierma, A.; Maher, S.; Flynn, S.; Krause, M.; Murray, B.; Kelly, P.; Baird, A.; Fitzgerald, R.; Newsholme, P.; et al. A whey protein hydrolysate promotes insulinotropic activity in a clonal pancreatic β-cell line and enhances glycemic function in ob/ob mice. J. Nutr. 2013, 143, 1109–1114. [Google Scholar] [CrossRef]
- Newsholme, P.; Krause, M.; Newsholme, P.; Krause, M. Nutritional regulation of insulin secretion: Implications for diabetes. Clin. Biochem. Rev. 2012, 33, 35–47. [Google Scholar]
- Yoshizawa, F. Effects of leucine and isoleucine on glucose metabolism. In Branched Chain Amino Acids in Clinical Nutrition; Humana Press: New York, NY, USA, 2015; pp. 63–73. [Google Scholar]
- Doi, M.; Yamaoka, I.; Fukunaga, T.; Nakayama, M. Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochem. Biophys. Res. Commun. 2003, 312, 1111–1117. [Google Scholar] [CrossRef]
- Lindgren, O.; Pacini, G.; Tura, A.; Holst, J.J.; Deacon, C.F.; Ahren, B. Incretin effect after oral amino acid ingestion in humans. J. Clin. Endocrinol. Metab. 2015, 100, 1172–1176. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Gutierrez-Aguilar, R.; Woods, S.C. Nutrition and L and K-enteroendocrine cells. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18. [Google Scholar] [CrossRef]
- Gupta, V.; Kalra, S. Choosing a gliptin. Indian J. Endocrinol. Metab. 2011, 15, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, P.; Chepurny, O.G.; Holz, G.G. Regulation of glucose homeostasis by GLP-1. In Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2014; Volume 121, pp. 23–65. [Google Scholar] [CrossRef]
- Ahrén, B.; Schweizer, A.; Dejager, S.; Villhauer, E.; Dunning, B.; Foley, J. Mechanisms of action of the dipeptidyl peptidase-4 inhibitor vildagliptin in humans. Diabetes Obes. Metab. 2011, 13, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Foley, J. Vildagliptin-mode of action. Med. Chem. 2014, 4, 439–440. [Google Scholar]
- Nauck, M.; Stöckmann, F.; Ebert, R.; Creutzfeldt, W. Reduced incretin effect in Type 2 (non-insulin-dependent) diabetes. Diabetologia 1986, 29, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Manders, R.J.; Wagenmakers, A.J.; Koopman, R.; Zorenc, A.H.; Menheere, P.P.; Schaper, N.C.; Saris, W.H.; van Loon, L.J. Co-ingestion of a protein hydrolysate and amino acid mixture with carbohydrate improves plasma glucose disposal in patients with type 2 diabetes. Am. J. Clin. Nutr. 2005, 82, 76–83. [Google Scholar] [CrossRef]
- Kalogeropoulou, D.; Lafave, L.; Schweim, K.; Gannon, M.C.; Nuttall, F.Q. Leucine, when ingested with glucose, synergistically stimulates insulin secretion and lowers blood glucose. Metab. Clin. Exp. 2008, 57, 1747–1752. [Google Scholar] [CrossRef]
- Yang, J.; Chi, Y.; Burkhardt, B.R.; Guan, Y.; Wolf, B.A. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr. Rev. 2010, 68, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Dolinger, M.; Ritaccio, G.; Conti, D.; Zhu, X.; Huang, Y. Leucine as a stimulant of insulin. In Branched Chain Amino Acids in Clinical Nutrition; Humana Press: New York, NY, USA, 2015; pp. 49–62. [Google Scholar]
- Doi, M.; Yamaoka, I.; Nakayama, M.; Mochizuki, S.; Sugahara, K.; Yoshizawa, F. Isoleucine, a blood glucose-lowering amino acid, increases glucose uptake in rat skeletal muscle in the absence of increases in AMP-activated protein kinase activity. J. Nutr. 2005, 135, 2103–2108. [Google Scholar] [CrossRef]
- Doi, M.; Yamaoka, I.; Nakayama, M.; Sugahara, K.; Yoshizawa, F. Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1683–E1693. [Google Scholar] [CrossRef]
- Nuttall, F.Q.; Schweim, K.; Gannon, M.C. Effect of orally administered isoleucine with and without glucose on insulin, glucagon and glucose concentrations in non-diabetic subjects. e-SPEN Eur. E J. Clin. Nutr. Metab. 2008, 3, e152–e158. [Google Scholar] [CrossRef] [Green Version]
- Steinert, R.E.; Landrock, M.F.; Ullrich, S.S.; Standfield, S.; Otto, B.; Horowitz, M.; Feinle-Bisset, C. Effects of intraduodenal infusion of the branched-chain amino acid leucine on ad libitum eating, gut motor and hormone functions, and glycemia in healthy men. Am. J. Clin. Nutr. 2015, 102, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.S.; Fitzgerald, P.C.; Schober, G.; Steinert, R.E.; Horowitz, M.; Feinle-Bisset, C. Intragastric administration of leucine or isoleucine lowers the blood glucose response to a mixed-nutrient drink by different mechanisms in healthy, lean volunteers. Am. J. Clin. Nutr. 2016, 104, 1274–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef] [PubMed]
- McClung, H.L.; Sigrist, L.D.; Smith, T.J.; Karl, J.P.; Rood, J.C.; Young, A.J.; Bathalon, G.P. Monitoring energy intake: A hand-held personal digital assistant provides accuracy comparable to written records. J. Am. Diet. Assoc. 2009, 109, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F.; Holst, J.J. Immunoassays for the incretin hormones GIP and GLP-1. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E. Sex differences in the control of glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Toth, E.; Suthijumroon, A.; Crockford, P.; Ryan, E. Insulin action does not change during the menstrual cycle in normal women. J. Clin. Endocrinol. Metab. 1987, 64, 74–80. [Google Scholar] [CrossRef]
- Yki-Järvinenf, H. Insulin sensitivity during the menstrual cycle. J. Clin. Endocrinol. Metab. 1984, 59, 350–353. [Google Scholar] [CrossRef]
- World Health Organisation. Diabetes Mellitus: Report of a WHO Study Group [Meeting Held in Geneva from 11 to 16 February 1985]; World Health Organisation: Geneva, Switzerland, 1985. [Google Scholar]
- McGuire, E.; Helderman, J.; Tobin, J.; Andres, R.; Berman, M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J. Appl. Physiol. 1976, 41, 565–573. [Google Scholar] [CrossRef]
- Nauck, M.; Liess, H.; Siegel, E.; Niedmann, P.; Creutzfeldt, W. Critical evaluation of the ‘heated-hand-technique’for obtaining ‘arterialized’venous blood: Incomplete arterialization and alterations in glucagon responses. Clin. Physiol. 1992, 12, 537–552. [Google Scholar] [CrossRef]
- Kjems, L.L.; Holst, J.J.; Vølund, A.; Madsbad, S. The influence of GLP-1 on glucose-stimulated insulin secretion effects on β-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 2003, 52, 380–386. [Google Scholar] [CrossRef] [PubMed]
- McFarlin, B.K.; Johnson, C.A.; Moreno, J.P.; Foreyt, J.P. Mexican American children have differential elevation of metabolic biomarkers proportional to obesity status. J. Pediatric Gastroenterol. Nutr. 2013, 57, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Jenkins, D.J.; Jenkins, A.L.; Josse, R.G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 1991, 54, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage: Thousand Oaks, CA, USA, 2013. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Earlbaum Associates: Hilsdale, NJ, USA, 1988; Volume 2. [Google Scholar]
- Olejnik, S.; Algina, J. Measures of effect size for comparative studies: Applications, interpretations, and limitations. Contemp. Educ. Psychol. 2000, 25, 241–286. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg-Hasson, Y.; Hansmann, L.; Liedtke, M.; Herschmann, I.; Maecker, H.T. Effects of serum and plasma matrices on multiplex immunoassays. Immunol. Res. 2014, 58, 224–233. [Google Scholar] [CrossRef]
- Hamburg, N.M.; McMackin, C.J.; Huang, A.L.; Shenouda, S.M.; Widlansky, M.E.; Schulz, E.; Gokce, N.; Ruderman, N.B.; Keaney, J.F., Jr.; Vita, J.A. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2650–2656. [Google Scholar] [CrossRef]
- Mari, A.; Pacini, G.; Murphy, E.; Ludvik, B.; Nolan, J.J. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001, 24, 539–548. [Google Scholar] [CrossRef]
- Sandoval, D.A.; D’Alessio, D.A. Physiology of proglucagon peptides: Role of glucagon and GLP-1 in health and disease. Physiol. Rev. 2015, 95, 513–548. [Google Scholar] [CrossRef]
- Nilsson, M.; Holst, J.J.; Bjorck, I.M. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: Studies using glucose-equivalent drinks. Am. J. Clin. Nutr. 2007, 85, 996–1004. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides 2013, 39, 157–163. [Google Scholar] [CrossRef]
- Matsumura, K.; Miki, T.; Jhomori, T.; Gonoi, T.; Seino, S. Possible role of PEPT1 in gastrointestinal hormone secretion. Biochem. Biophys. Res. Commun. 2005, 336, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Toft-Nielsen, M.-B.; Madsbad, S.; Holst, J. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 3853–3860. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.T. Metabolism of BCAAs. In Branched Chain Amino Acids in Clinical Nutrition; Humana Press: New York, NY, USA, 2015; pp. 13–24. [Google Scholar]
- Have, G.T.; Engelen, M.; Luiking, Y.C.; Deutz, N. Absorption kinetics of amino acids, peptides, and intact proteins. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, S23–S36. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Reimer, R.A. Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro. Nutrition 2009, 25, 340–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulipano, G.; Sibilia, V.; Caroli, A.M.; Cocchi, D. Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides 2011, 32, 835–838. [Google Scholar] [CrossRef]
- Lauritsen, K.; Moody, A. The association between plasma GIP and insulin after oral glucose. Scand. J. Gastroenterol. 1980, 15, 953–957. [Google Scholar] [CrossRef]
- Edholm, T.; Degerblad, M.; Grybäck, P.; Hilsted, L.; Holst, J.J.; Jacobsson, H.; Efendic, S.; Schmidt, P.; Hellström, P.M. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol. Motil. 2010, 22, 1191-e315. [Google Scholar] [CrossRef]
- Wachters-Hagedoorn, R.E.; Priebe, M.G.; Heimweg, J.A.; Heiner, A.M.; Englyst, K.N.; Holst, J.J.; Stellaard, F.; Vonk, R.J. The rate of intestinal glucose absorption is correlated with plasma glucose-dependent insulinotropic polypeptide concentrations in healthy men. J. Nutr. 2006, 136, 1511–1516. [Google Scholar] [CrossRef]
- Parker, H.; Habib, A.; Rogers, G.; Gribble, F.; Reimann, F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 2009, 52, 289. [Google Scholar] [CrossRef]
- Reimann, F.; Tolhurst, G.; Gribble, F.M. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012, 15, 421–431. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Enteroendocrine cells: Chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 2016, 78, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, S.; Bizais, Y.; des Varannes, S.B.; Murat, A.; Pouliquen, B.; Galmiche, J. Inter-and intrasubject variability of solid and liquid gastric emptying parameters. Dig. Dis. Sci. 1994, 39, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Pilichiewicz, A.N.; Chaikomin, R.; Brennan, I.M.; Wishart, J.M.; Rayner, C.K.; Jones, K.L.; Smout, A.J.; Horowitz, M.; Feinle-Bisset, C. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E743–E753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Number of Participants (M/F) | 12 (6/6) |
| Age (years) | 27.3 ± 2.0 |
| Height (cm) | 167.4 ± 2.2 |
| Weight (kg) | 77.3 ± 3.7 |
| BMI (kg/m2) | 26.3 ± 2.1 |
| LBM (kg) | 48.6 ± 1.8 |
| Body Fat (%) | 34.1 ± 2.9 |
| Glucose (mmol∙L−1) | 4.97 ± 0.09 |
| Glucose (mg∙dL−1) | 89.5 ± 1.7 |
| Insulin (pmol∙L−1) | 96.8 ± 13.3 |
| Glucagon (ng∙L−1) | 54.1 ± 9.7 |
| GIPTotal (pmol∙L−1) | 12.3 ± 2.1 |
| GLP-1Active (pmol∙L−1) | 1.9 ± 0.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newmire, D.E.; Rivas, E.; Deemer, S.E.; Willoughby, D.S.; Ben-Ezra, V. The Impact of a Large Bolus Dose of l-leucine and l-isoleucine on Enteroendocrine and Pancreatic Hormones, and Glycemia in Healthy, Inactive Adults. Nutrients 2019, 11, 2650. https://doi.org/10.3390/nu11112650
Newmire DE, Rivas E, Deemer SE, Willoughby DS, Ben-Ezra V. The Impact of a Large Bolus Dose of l-leucine and l-isoleucine on Enteroendocrine and Pancreatic Hormones, and Glycemia in Healthy, Inactive Adults. Nutrients. 2019; 11(11):2650. https://doi.org/10.3390/nu11112650
Chicago/Turabian StyleNewmire, Daniel E., Eric Rivas, Sarah E. Deemer, Darryn S. Willoughby, and Victor Ben-Ezra. 2019. "The Impact of a Large Bolus Dose of l-leucine and l-isoleucine on Enteroendocrine and Pancreatic Hormones, and Glycemia in Healthy, Inactive Adults" Nutrients 11, no. 11: 2650. https://doi.org/10.3390/nu11112650
APA StyleNewmire, D. E., Rivas, E., Deemer, S. E., Willoughby, D. S., & Ben-Ezra, V. (2019). The Impact of a Large Bolus Dose of l-leucine and l-isoleucine on Enteroendocrine and Pancreatic Hormones, and Glycemia in Healthy, Inactive Adults. Nutrients, 11(11), 2650. https://doi.org/10.3390/nu11112650






