Impact of Endurance and Resistance Training on Skeletal Muscle Glucose Metabolism in Older Adults
Abstract
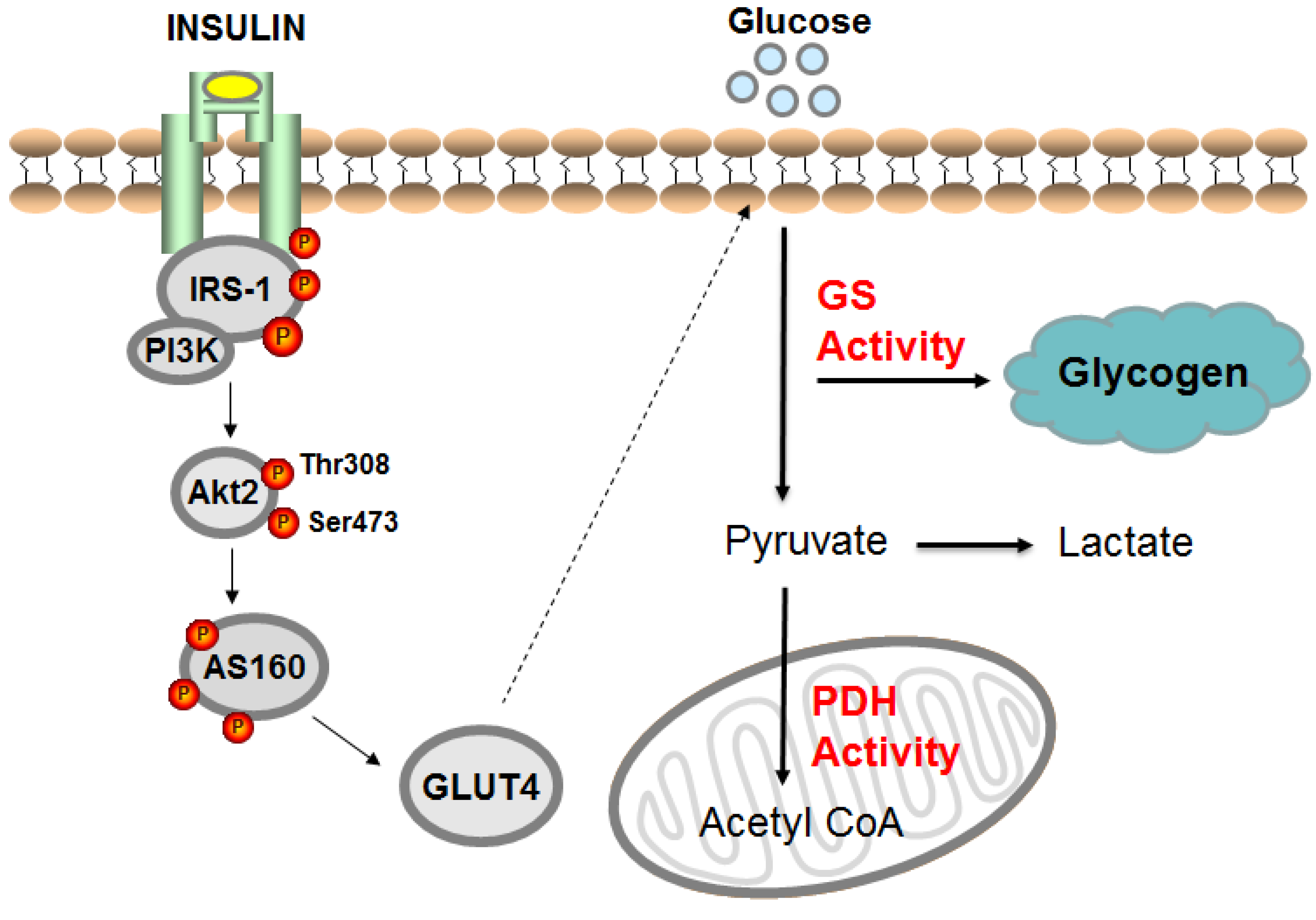
:1. Age and Insulin Resistance
2. Age and Skeletal Muscle Atrophy
3. Age and Skeletal Muscle Insulin Resistance
4. Age and Nonoxidative (Glycogen Synthesis) and Oxidative Pathways
5. Physical Activity, Exercise, and Insulin Sensitivity in Older Adults
6. Endurance Training
6.1. Endurance Training and Skeletal Muscle Insulin Signaling in Older Adults
6.2. Endurance Training and the Glycogen Synthase Pathway in Older Adults
6.3. Endurance Training and Glucose Oxidation Pathway in Older Adults
6.4. Endurance Training and Intramuscular Lipid (IMCL) in Older Adults
7. Resistance Training and Skeletal Muscle Insulin Signaling in Older Adults
7.1. Resistance Training and Skeletal Muscle Insulin Signaling in Older Adults
7.2. Resistance Training and Glycogen Synthase Pathway in Older Adults
7.3. Resistance Training and Glucose Oxidation Pathway in Older Adults
7.4. Resistance Training and IMCL in Older Adults
8. Effects of Exercise Training on Skeletal Muscle Tumor Necrosis Factor (TNF)-Alpha in Older Adults
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cowie, C.C.; Rust, K.F.; Ford, E.S.; Eberhardt, M.S.; Byrd-Holt, D.D.; Li, C.; Williams, D.E.; Gregg, E.W.; Bainbridge, K.E.; Saydah, S.H.; et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 2009, 32, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Consitt, L.A.; Van Meter, J.; Newton, C.A.; Collier, D.N.; Dar, M.S.; Wojtaszewski, J.F.; Treebak, J.T.; Tanner, C.J.; Houmard, J.A. Impairments in site-specific AS160 phosphorylation and effects of exercise training. Diabetes 2013, 62, 3437–3447. [Google Scholar] [CrossRef] [PubMed]
- Dela, F.; Mikines, K.J.; Larsen, J.J.; Galbo, H. Training-induced enhancement of insulin action in human skeletal muscle: The influence of aging. J. Gerontol. A Biol. Sci. Med. Sci. 1996, 51, B247–B252. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Befroy, D.; Dufour, S.; Dziura, J.; Ariyan, C.; Rothman, D.L.; DiPietro, L.; Cline, G.W.; Shulman, G.I. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science 2003, 300, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Fink, R.I.; Kolterman, O.G.; Griffin, J.; Olefsky, J.M. Mechanisms of insulin resistance in aging. J. Clin. Investig. 1983, 71, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Fink, R.I.; Wallace, P.; Olefsky, J.M. Effects of aging on glucose-mediated glucose disposal and glucose transport. J. Clin. Investig. 1986, 77, 2034–2041. [Google Scholar] [CrossRef]
- Rowe, J.W.; Minaker, K.L.; Pallotta, J.A.; Flier, J.S. Characterization of the insulin resistance of aging. J. Clin. Investig. 1983, 71, 1581–1587. [Google Scholar] [CrossRef]
- Petersen, K.F.; Morino, K.; Alves, T.C.; Kibbey, R.G.; Dufour, S.; Sono, S.; Yoo, P.S.; Cline, G.W.; Shulman, G.I. Effect of aging on muscle mitochondrial substrate utilization in humans. Proc. Natl. Acad. Sci. USA 2015, 112, 11330–11334. [Google Scholar] [CrossRef] [Green Version]
- Amati, F.; Dube, J.J.; Coen, P.M.; Stefanovic-Racic, M.; Toledo, F.G.; Goodpaster, B.H. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care 2009, 32, 1547–1549. [Google Scholar] [CrossRef]
- Greiwe, J.S.; Cheng, B.; Rubin, D.C.; Yarasheski, K.E.; Semenkovich, C.F. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 2001, 15, 475–482. [Google Scholar] [CrossRef]
- Kohrt, W.M.; Kirwan, J.P.; Staten, M.A.; Bourey, R.E.; King, D.S.; Holloszy, J.O. Insulin resistance in aging is related to abdominal obesity. Diabetes 1993, 42, 273–281. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. Glucose intolerance and aging. Diabetes Care 1981, 4, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J.; Taylor, C.C.; Sjostrom, M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Consitt, L.A.; Clark, B.C. The Vicious Cycle of Myostatin Signaling in Sarcopenic Obesity: Myostatin Role in Skeletal Muscle Growth, Insulin Signaling and Implications for Clinical Trials. J. Frailty Aging 2018, 7, 21–27. [Google Scholar] [CrossRef]
- Coggan, A.R.; Spina, R.J.; King, D.S.; Rogers, M.A.; Brown, M.; Nemeth, P.M.; Holloszy, J.O. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J. Gerontol. 1992, 47, B71–B76. [Google Scholar] [CrossRef]
- Murgia, M.; Toniolo, L.; Nagaraj, N.; Ciciliot, S.; Vindigni, V.; Schiaffino, S.; Reggiani, C.; Mann, M. Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell Rep. 2017, 19, 2396–2409. [Google Scholar] [CrossRef] [Green Version]
- Gaster, M.; Poulsen, P.; Handberg, A.; Schroder, H.D.; Beck-Nielsen, H. Direct evidence of fiber type-dependent GLUT-4 expression in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E910–E916. [Google Scholar] [CrossRef]
- Gupte, A.A.; Bomhoff, G.L.; Geiger, P.C. Age-related differences in skeletal muscle insulin signaling: The role of stress kinases and heat shock proteins. J. Appl. Physiol. 2008, 105, 839–848. [Google Scholar] [CrossRef]
- Sharma, N.; Arias, E.B.; Sajan, M.P.; MacKrell, J.G.; Bhat, A.D.; Farese, R.V.; Cartee, G.D. Insulin resistance for glucose uptake and Akt2 phosphorylation in the soleus, but not epitrochlearis, muscles of old vs. adult rats. J. Appl. Physiol. 2010, 108, 1631–1640. [Google Scholar] [CrossRef] [Green Version]
- Consitt, L.A.; Saxena, G.; Slyvka, Y.; Clark, B.C.; Friedlander, M.; Zhang, Y.; Nowak, F.V. Paternal high-fat diet enhances offspring whole-body insulin sensitivity and skeletal muscle insulin signaling early in life. Physiol. Rep. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Thorell, A.; Hirshman, M.F.; Nygren, J.; Jorfeldt, L.; Wojtaszewski, J.F.; Dufresne, S.D.; Horton, E.S.; Ljungqvist, O.; Goodyear, L.J. Exercise and insulin cause GLUT-4 translocation in human skeletal muscle. Am. J. Physiol. 1999, 277, E733–E741. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.F.; Witczak, C.A.; Fujii, N.; Jessen, N.; Taylor, E.B.; Arnolds, D.E.; Sakamoto, K.; Hirshman, M.F.; Goodyear, L.J. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 2006, 55, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Kane, S.; Sano, E.; Miinea, C.P.; Asara, J.M.; Lane, W.S.; Garner, C.W.; Lienhard, G.E. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 2003, 278, 14599–14602. [Google Scholar] [CrossRef]
- Sharma, P.; Arias, E.B.; Cartee, G.D. Protein Phosphatase 1-alpha Regulates AS160 Ser588 and Thr642 Dephosphorylation in Skeletal Muscle. Diabetes 2016, 65, 2606–2617. [Google Scholar] [CrossRef]
- Ng, Y.; Ramm, G.; Burchfield, J.G.; Coster, A.C.; Stockli, J.; James, D.E. Cluster analysis of insulin action in adipocytes reveals a key role for Akt at the plasma membrane. J. Biol. Chem. 2010, 285, 2245–2257. [Google Scholar] [CrossRef]
- Geraghty, K.M.; Chen, S.; Harthill, J.E.; Ibrahim, A.F.; Toth, R.; Morrice, N.A.; Vandermoere, F.; Moorhead, G.B.; Hardie, D.G.; MacKintosh, C. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem. J. 2007, 407, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Consitt, L.A.; Koves, T.R.; Muoio, D.M.; Nakazawa, M.; Newton, C.A.; Houmard, J.A. Plasma acylcarnitines during insulin stimulation in humans are reflective of age-related metabolic dysfunction. Biochem. Biophys. Res. Commun. 2016, 479, 868–874. [Google Scholar] [CrossRef] [Green Version]
- Houmard, J.A.; Weidner, M.D.; Dolan, P.L.; Leggett-Frazier, N.; Gavigan, K.E.; Hickey, M.S.; Tyndall, G.L.; Zheng, D.; Alshami, A.; Dohm, G.L. Skeletal muscle GLUT4 protein concentration and aging in humans. Diabetes 1995, 44, 555–560. [Google Scholar] [CrossRef]
- Cox, J.H.; Cortright, R.N.; Dohm, G.L.; Houmard, J.A. Effect of aging on response to exercise training in humans: Skeletal muscle GLUT-4 and insulin sensitivity. J. Appl. Physiol. 1999, 86, 2019–2025. [Google Scholar] [CrossRef]
- Dela, F.; Ploug, T.; Handberg, A.; Petersen, L.N.; Larsen, J.J.; Mikines, K.J.; Galbo, H. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes 1994, 43, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R.; Villar, M.; Gallardo, N.; Carrascosa, J.M.; Martinez, C.; Andres, A. The effect of aging on insulin signalling pathway is tissue dependent: Central role of adipose tissue in the insulin resistance of aging. Mech. Ageing Dev. 2009, 130, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Bryhni, B.; Jenssen, T.G.; Olafsen, K.; Bendikssen, A. Oxidative and nonoxidative glucose disposal in elderly vs younger men with similar and smaller body mass indices and waist circumferences. Metabolism 2005, 54, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, P.; Wojtaszewski, J.F.; Petersen, I.; Christensen, K.; Richter, E.A.; Beck-Nielsen, H.; Vaag, A. Impact of genetic versus environmental factors on the control of muscle glycogen synthase activation in twins. Diabetes 2005, 54, 1289–1296. [Google Scholar] [CrossRef]
- Franssila-Kallunki, A.; Schalin-Jantti, C.; Groop, L. Effect of gender on insulin resistance associated with aging. Am. J. Physiol. 1992, 263, E780–E785. [Google Scholar] [CrossRef]
- Meredith, C.N.; Frontera, W.R.; Fisher, E.C.; Hughes, V.A.; Herland, J.C.; Edwards, J.; Evans, W.J. Peripheral effects of endurance training in young and old subjects. J. Appl. Physiol. 1989, 66, 2844–2849. [Google Scholar] [CrossRef]
- Bienso, R.S.; Olesen, J.; Gliemann, L.; Schmidt, J.F.; Matzen, M.S.; Wojtaszewski, J.F.; Hellsten, Y.; Pilegaard, H. Effects of Exercise Training on Regulation of Skeletal Muscle Glucose Metabolism in Elderly Men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 866–872. [Google Scholar] [CrossRef] [Green Version]
- Pehleman, T.L.; Peters, S.J.; Heigenhauser, G.J.; Spriet, L.L. Enzymatic regulation of glucose disposal in human skeletal muscle after a high-fat, low-carbohydrate diet. J. Appl. Physiol. 2005, 98, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Bonadonna, R.C.; Groop, L.C.; Simonson, D.C.; DeFronzo, R.A. Free fatty acid and glucose metabolism in human aging: Evidence for operation of the Randle cycle. Am. J. Physiol. 1994, 266, E501–E509. [Google Scholar] [CrossRef]
- Gumbiner, B.; Thorburn, A.W.; Ditzler, T.M.; Bulacan, F.; Henry, R.R. Role of impaired intracellular glucose metabolism in the insulin resistance of aging. Metabolism 1992, 41, 1115–1121. [Google Scholar] [CrossRef]
- Consitt, L.A.; Saxena, G.; Saneda, A.; Houmard, J.A. Age-related impairments in skeletal muscle PDH phosphorylation and plasma lactate are indicative of metabolic inflexibility and the effects of exercise training. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E145–E156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantin-Teodosiu, D.; Stephens, F.B.; Greenhaff, P.L. Perpetual muscle PDH activation in PDH kinase knockout mice protects against high-fat feeding-induced muscle insulin resistance. Proc. Natl. Acad. Sci. USA 2015, 112, E824. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.L.; Svendsen, P.F.; Jeppesen, J.F.; Hoeg, L.D.; Andersen, N.R.; Kristensen, J.M.; Nilas, L.; Lundsgaard, A.M.; Wojtaszewski, J.F.P.; Madsbad, S.; et al. Molecular Mechanisms in Skeletal Muscle Underlying Insulin Resistance in Women Who Are Lean with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- Healy, G.N.; Wijndaele, K.; Dunstan, D.W.; Shaw, J.E.; Salmon, J.; Zimmet, P.Z.; Owen, N. Objectively measured sedentary time, physical activity, and metabolic risk: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care 2008, 31, 369–371. [Google Scholar] [CrossRef]
- Lee, I.M.; Sesso, H.D.; Oguma, Y.; Paffenbarger, R.S., Jr. The “weekend warrior” and risk of mortality. Am. J. Epidemiol. 2004, 160, 636–641. [Google Scholar] [CrossRef]
- Bangsbo, J.; Blackwell, J.; Boraxbekk, C.J.; Caserotti, P.; Dela, F.; Evans, A.B.; Jespersen, A.P.; Gliemann, L.; Kramer, A.F.; Lundbye-Jensen, J.; et al. Copenhagen Consensus statement 2019: Physical activity and ageing. Br. J. Sports Med. 2019, 53, 856–858. [Google Scholar] [CrossRef]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Lancet Physical Activity Series Working, G. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Jonker, J.T.; De Laet, C.; Franco, O.H.; Peeters, A.; Mackenbach, J.; Nusselder, W.J. Physical activity and life expectancy with and without diabetes: Life table analysis of the Framingham Heart Study. Diabetes Care 2006, 29, 38–43. [Google Scholar] [CrossRef]
- Balkau, B.; Mhamdi, L.; Oppert, J.M.; Nolan, J.; Golay, A.; Porcellati, F.; Laakso, M.; Ferrannini, E.; Group, E.R.S. Physical activity and insulin sensitivity: The RISC study. Diabetes 2008, 57, 2613–2618. [Google Scholar] [CrossRef]
- Sylow, L.; Richter, E. Current advances in our understanding of exercise as medicine in metabolic disease. Curr. Opin. Physiol. 2019, 12, 12–19. [Google Scholar] [CrossRef]
- Evans, E.M.; Racette, S.B.; Peterson, L.R.; Villareal, D.T.; Greiwe, J.S.; Holloszy, J.O. Aerobic power and insulin action improve in response to endurance exercise training in healthy 77-87 yr olds. J. Appl. Physiol. 2005, 98, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Coker, R.H.; Hays, N.P.; Williams, R.H.; Brown, A.D.; Freeling, S.A.; Kortebein, P.M.; Sullivan, D.H.; Starling, R.D.; Evans, W.J. Exercise-induced changes in insulin action and glycogen metabolism in elderly adults. Med. Sci. Sports Exerc. 2006, 38, 433–438. [Google Scholar] [CrossRef] [PubMed]
- de Guia, R.M.; Agerholm, M.; Nielsen, T.S.; Consitt, L.A.; Sogaard, D.; Helge, J.W.; Larsen, S.; Brandauer, J.; Houmard, J.A.; Treebak, J.T. Aerobic and resistance exercise training reverses age-dependent decline in NAD(+) salvage capacity in human skeletal muscle. Physiol. Rep. 2019, 7, e14139. [Google Scholar] [CrossRef] [PubMed]
- Suominen, H.; Heikkinen, E.; Liesen, H.; Michel, D.; Hollmann, W. Effects of 8 weeks’ endurance training on skeletal muscle metabolism in 56-70-year-old sedentary men. Eur. J. Appl. Physiol. Occup. Physiol. 1977, 37, 173–180. [Google Scholar] [CrossRef]
- Prior, S.J.; Goldberg, A.P.; Ortmeyer, H.K.; Chin, E.R.; Chen, D.; Blumenthal, J.B.; Ryan, A.S. Increased Skeletal Muscle Capillarization Independently Enhances Insulin Sensitivity in Older Adults After Exercise Training and Detraining. Diabetes 2015, 64, 3386–3395. [Google Scholar] [CrossRef] [Green Version]
- Frosig, C.; Rose, A.J.; Treebak, J.T.; Kiens, B.; Richter, E.A.; Wojtaszewski, J.F. Effects of endurance exercise training on insulin signaling in human skeletal muscle: Interactions at the level of phosphatidylinositol 3-kinase, Akt, and AS160. Diabetes 2007, 56, 2093–2102. [Google Scholar] [CrossRef]
- Ryan, A.S.; Katzel, L.I.; Prior, S.J.; McLenithan, J.C.; Goldberg, A.P.; Ortmeyer, H.K. Aerobic exercise plus weight loss improves insulin sensitivity and increases skeletal muscle glycogen synthase activity in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 790–798. [Google Scholar] [CrossRef]
- O’Gorman, D.J.; Karlsson, H.K.; McQuaid, S.; Yousif, O.; Rahman, Y.; Gasparro, D.; Glund, S.; Chibalin, A.V.; Zierath, J.R.; Nolan, J.J. Exercise training increases insulin-stimulated glucose disposal and GLUT4 (SLC2A4) protein content in patients with type 2 diabetes. Diabetologia 2006, 49, 2983–2992. [Google Scholar] [CrossRef] [Green Version]
- Babraj, J.A.; Vollaard, N.B.; Keast, C.; Guppy, F.M.; Cottrell, G.; Timmons, J.A. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr. Disord. 2009, 9, 3. [Google Scholar] [CrossRef]
- Mandrup, C.M.; Egelund, J.; Nyberg, M.; Enevoldsen, L.H.; Kjaer, A.; Clemmensen, A.E.; Christensen, A.N.; Suetta, C.; Frikke-Schmidt, R.; Steenberg, D.E.; et al. Effects of menopause and high-intensity training on insulin sensitivity and muscle metabolism. Menopause 2018, 25, 165–175. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.S.; Kim, C.K. Effect of exercise training on muscle glucose transporter 4 protein and intramuscular lipid content in elderly men with impaired glucose tolerance. Eur. J. Appl. Physiol. 2004, 93, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Hughes, V.A.; Fiatarone, M.A.; Fielding, R.A.; Kahn, B.B.; Ferrara, C.M.; Shepherd, P.; Fisher, E.C.; Wolfe, R.R.; Elahi, D.; Evans, W.J. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am. J. Physiol. 1993, 264, E855–E862. [Google Scholar] [CrossRef] [PubMed]
- Langfort, J.; Viese, M.; Ploug, T.; Dela, F. Time course of GLUT4 and AMPK protein expression in human skeletal muscle during one month of physical training. Scand. J. Med. Sci. Sports 2003, 13, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Dube, J.J.; Amati, F.; Toledo, F.G.; Stefanovic-Racic, M.; Rossi, A.; Coen, P.; Goodpaster, B.H. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 2011, 54, 1147–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, A.S.; Ortmeyer, H.K.; Sorkin, J.D. Exercise with calorie restriction improves insulin sensitivity and glycogen synthase activity in obese postmenopausal women with impaired glucose tolerance. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E145–E152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, C.M.; Goldberg, A.P.; Ortmeyer, H.K.; Ryan, A.S. Effects of aerobic and resistive exercise training on glucose disposal and skeletal muscle metabolism in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.E.; Jubrias, S.A.; Esselman, P.C. Oxidative capacity and ageing in human muscle. J. Physiol. 2000, 526 Pt 1, 203–210. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Hagen, T.M.; Ames, B.N. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. USA 1994, 91, 10771–10778. [Google Scholar] [CrossRef]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef] [Green Version]
- Joseph, A.M.; Pilegaard, H.; Litvintsev, A.; Leick, L.; Hood, D.A. Control of gene expression and mitochondrial biogenesis in the muscular adaptation to endurance exercise. Essays Biochem. 2006, 42, 13–29. [Google Scholar] [CrossRef]
- Konopka, A.R.; Suer, M.K.; Wolff, C.A.; Harber, M.P. Markers of human skeletal muscle mitochondrial biogenesis and quality control: Effects of age and aerobic exercise training. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Lanza, I.R.; Short, D.K.; Short, K.R.; Raghavakaimal, S.; Basu, R.; Joyner, M.J.; McConnell, J.P.; Nair, K.S. Endurance exercise as a countermeasure for aging. Diabetes 2008, 57, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Hattori, M.; Harada, K.; Shirase, R.; Bando, M.; Okano, G. Age-related changes in intramyocellular lipid in humans by in vivo H-MR spectroscopy. Gerontology 2007, 53, 218–223. [Google Scholar] [CrossRef]
- Vessby, B.; Tengblad, S.; Lithell, H. Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia 1994, 37, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef]
- Ngo, K.T.; Denis, C.; Saafi, M.A.; Feasson, L.; Verney, J. Endurance but not resistance training increases intra-myocellular lipid content and beta-hydroxyacyl coenzyme A dehydrogenase activity in active elderly men. Acta Physiol. 2012, 205, 133–144. [Google Scholar] [CrossRef]
- Pruchnic, R.; Katsiaras, A.; He, J.; Kelley, D.E.; Winters, C.; Goodpaster, B.H. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E857–E862. [Google Scholar] [CrossRef] [Green Version]
- Dube, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.; Sauers, S.E.; Goodpaster, B.H. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E882–E888. [Google Scholar] [CrossRef]
- Amati, F.; Dube, J.J.; Alvarez-Carnero, E.; Edreira, M.M.; Chomentowski, P.; Coen, P.M.; Switzer, G.E.; Bickel, P.E.; Stefanovic-Racic, M.; Toledo, F.G.; et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: Another paradox in endurance-trained athletes? Diabetes 2011, 60, 2588–2597. [Google Scholar] [CrossRef]
- Moro, C.; Bajpeyi, S.; Smith, S.R. Determinants of intramyocellular triglyceride turnover: Implications for insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E203–E213. [Google Scholar] [CrossRef]
- Bandet, C.L.; Tan-Chen, S.; Bourron, O.; Le Stunff, H.; Hajduch, E. Sphingolipid Metabolism: New Insight into Ceramide-Induced Lipotoxicity in Muscle Cells. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Sogaard, D.; Baranowski, M.; Larsen, S.; Taulo Lund, M.; Munk Scheuer, C.; Vestergaard Abildskov, C.; Greve Dideriksen, S.; Dela, F.; Wulff Helge, J. Muscle-Saturated Bioactive Lipids Are Increased with Aging and Influenced by High-Intensity Interval Training. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports, M.; Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Bucci, M.; Huovinen, V.; Guzzardi, M.A.; Koskinen, S.; Raiko, J.R.; Lipponen, H.; Ahsan, S.; Badeau, R.M.; Honka, M.J.; Koffert, J.; et al. Resistance training improves skeletal muscle insulin sensitivity in elderly offspring of overweight and obese mothers. Diabetologia 2016, 59, 77–86. [Google Scholar] [CrossRef]
- Dunstan, D.W.; Daly, R.M.; Owen, N.; Jolley, D.; De Courten, M.; Shaw, J.; Zimmet, P. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care 2002, 25, 1729–1736. [Google Scholar] [CrossRef]
- Castaneda, C.; Layne, J.E.; Munoz-Orians, L.; Gordon, P.L.; Walsmith, J.; Foldvari, M.; Roubenoff, R.; Tucker, K.L.; Nelson, M.E. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 2002, 25, 2335–2341. [Google Scholar] [CrossRef]
- Craig, B.W.; Everhart, J.; Brown, R. The influence of high-resistance training on glucose tolerance in young and elderly subjects. Mech. Ageing Dev. 1989, 49, 147–157. [Google Scholar] [CrossRef]
- Smutok, M.A.; Reece, C.; Kokkinos, P.F.; Farmer, C.M.; Dawson, P.K.; DeVane, J.; Patterson, J.; Goldberg, A.P.; Hurley, B.F. Effects of exercise training modality on glucose tolerance in men with abnormal glucose regulation. Int. J. Sports Med. 1994, 15, 283–289. [Google Scholar] [CrossRef]
- Dela, F.; Kjaer, M. Resistance training, insulin sensitivity and muscle function in the elderly. Essays Biochem. 2006, 42, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Verdijk, L.B.; Gleeson, B.G.; Jonkers, R.A.; Meijer, K.; Savelberg, H.H.; Dendale, P.; van Loon, L.J. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 332–339. [Google Scholar] [CrossRef]
- Trappe, S.; Williamson, D.; Godard, M.; Porter, D.; Rowden, G.; Costill, D. Effect of resistance training on single muscle fiber contractile function in older men. J. Appl. Physiol. 2000, 89, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, J.L.; Schjerling, P.; Andersen, L.L.; Dela, F. Resistance training and insulin action in humans: Effects of de-training. J. Physiol. 2003, 551, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Holten, M.K.; Zacho, M.; Gaster, M.; Juel, C.; Wojtaszewski, J.F.; Dela, F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes 2004, 53, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.P.; Pratley, R.E.; Goldberg, A.P.; Gordon, P.; Rubin, M.; Treuth, M.S.; Ryan, A.S.; Hurley, B.F. Strength training increases insulin action in healthy 50- to 65-yr-old men. J. Appl. Physiol. 1994, 77, 1122–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart, C.A.; Lee, M.L.; South, M.A.; Howell, M.E.A.; Stone, M.H. Muscle hypertrophy in prediabetic men after 16 wk of resistance training. J. Appl. Physiol. 2017, 123, 894–901. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Page, R.A.; Sukala, W.R.; Giri, M.; Ghimbovschi, S.D.; Hayat, I.; Cheema, B.S.; Lys, I.; Leikis, M.; Sheard, P.W.; et al. Multi-omic integrated networks connect DNA methylation and miRNA with skeletal muscle plasticity to chronic exercise in Type 2 diabetic obesity. Physiol. Genom. 2014, 46, 747–765. [Google Scholar] [CrossRef]
- Eriksson, J.; Tuominen, J.; Valle, T.; Sundberg, S.; Sovijarvi, A.; Lindholm, H.; Tuomilehto, J.; Koivisto, V. Aerobic endurance exercise or circuit-type resistance training for individuals with impaired glucose tolerance? Horm. Metab. Res. 1998, 30, 37–41. [Google Scholar] [CrossRef]
- Porter, C.; Reidy, P.T.; Bhattarai, N.; Sidossis, L.S.; Rasmussen, B.B. Resistance Exercise Training Alters Mitochondrial Function in Human Skeletal Muscle. Med. Sci. Sports Exerc. 2015, 47, 1922–1931. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Hikida, R.S.; Staron, R.S.; Simoneau, J.A. Muscle fiber types of women after resistance training--quantitative ultrastructure and enzyme activity. Pflugers Arch. 1993, 424, 494–502. [Google Scholar] [CrossRef]
- Alway, S.E.; MacDougall, J.D.; Sale, D.G.; Sutton, J.R.; McComas, A.J. Functional and structural adaptations in skeletal muscle of trained athletes. J. Appl. Physiol. 1988, 64, 1114–1120. [Google Scholar] [CrossRef]
- MacDougall, J.D.; Sale, D.G.; Moroz, J.R.; Elder, G.C.; Sutton, J.R.; Howald, H. Mitochondrial volume density in human skeletal muscle following heavy resistance training. Med. Sci. Sports 1979, 11, 164–166. [Google Scholar] [PubMed]
- Frank, P.; Andersson, E.; Ponten, M.; Ekblom, B.; Ekblom, M.; Sahlin, K. Strength training improves muscle aerobic capacity and glucose tolerance in elderly. Scand. J. Med. Sci. Sports 2016, 26, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, V.S.; Rao, M.; Menon, V.; Gordon, P.L.; Pilichowska, M.; Castaneda, F.; Castaneda-Sceppa, C. Resistance training increases muscle mitochondrial biogenesis in patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, T.G.; Monteiro, M.A.; Lamont, L.S.; Singh, M.F.; Foldvari, M.; White, S.; Cosmas, A.C.; Urso, M.L. Postmenopausal effects of resistance training on muscle damage and mitochondria. J. Strength Cond. Res. 2013, 27, 556–561. [Google Scholar] [CrossRef]
- Jubrias, S.A.; Esselman, P.C.; Price, L.B.; Cress, M.E.; Conley, K.E. Large energetic adaptations of elderly muscle to resistance and endurance training. J. Appl. Physiol. 2001, 90, 1663–1670. [Google Scholar] [CrossRef] [Green Version]
- Sparks, L.M.; Johannsen, N.M.; Church, T.S.; Earnest, C.P.; Moonen-Kornips, E.; Moro, C.; Hesselink, M.K.; Smith, S.R.; Schrauwen, P. Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. J. Clin. Endocrinol. Metab. 2013, 98, 1694–1702. [Google Scholar] [CrossRef]
- Mueller, M.; Breil, F.A.; Lurman, G.; Klossner, S.; Fluck, M.; Billeter, R.; Dapp, C.; Hoppeler, H. Different molecular and structural adaptations with eccentric and conventional strength training in elderly men and women. Gerontology 2011, 57, 528–538. [Google Scholar] [CrossRef]
- Abbatecola, A.M.; Ferrucci, L.; Grella, R.; Bandinelli, S.; Bonafe, M.; Barbieri, M.; Corsi, A.M.; Lauretani, F.; Franceschi, C.; Paolisso, G. Diverse effect of inflammatory markers on insulin resistance and insulin-resistance syndrome in the elderly. J. Am. Geriatr. Soc. 2004, 52, 399–404. [Google Scholar] [CrossRef]
- Del Aguila, L.F.; Claffey, K.P.; Kirwan, J.P. TNF-alpha impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am. J. Physiol. 1999, 276, E849–E855. [Google Scholar] [CrossRef]
- Plomgaard, P.; Bouzakri, K.; Krogh-Madsen, R.; Mittendorfer, B.; Zierath, J.R.; Pedersen, B.K. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 2005, 54, 2939–2945. [Google Scholar] [CrossRef]
- Olesen, J.; Gliemann, L.; Bienso, R.; Schmidt, J.; Hellsten, Y.; Pilegaard, H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J. Physiol. 2014, 592, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.P.; Wright, N.R.; Finck, B.N.; Villareal, D.T. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J. Appl. Physiol. 2008, 105, 473–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Endurance Training | Resistance Training | |
|---|---|---|
| Whole-Body Insulin Sensitivity | ↑ | ↑ |
| Skeletal Muscle Insulin Signaling | ||
| AS160 Insulin Signaling | ↑, ↔ (obesity) | ↑ |
| GLUT4 | ↑ | ↔, ↑ (obesity) |
| Glycogen Synthase Activity | ↑ | ↔, ↑ (type 2 diabetic) |
| PDH Regulation | ↑ | ↑ |
| Skeletal Muscle Mitochondria | ↑ | ↑ |
| Skeletal Muscle IMCL | ↑ | ↔ or ↓ |
| Skeletal Muscle TNF-alpha | ↓ | ↓ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Consitt, L.A.; Dudley, C.; Saxena, G. Impact of Endurance and Resistance Training on Skeletal Muscle Glucose Metabolism in Older Adults. Nutrients 2019, 11, 2636. https://doi.org/10.3390/nu11112636
Consitt LA, Dudley C, Saxena G. Impact of Endurance and Resistance Training on Skeletal Muscle Glucose Metabolism in Older Adults. Nutrients. 2019; 11(11):2636. https://doi.org/10.3390/nu11112636
Chicago/Turabian StyleConsitt, Leslie A., Courtney Dudley, and Gunjan Saxena. 2019. "Impact of Endurance and Resistance Training on Skeletal Muscle Glucose Metabolism in Older Adults" Nutrients 11, no. 11: 2636. https://doi.org/10.3390/nu11112636
APA StyleConsitt, L. A., Dudley, C., & Saxena, G. (2019). Impact of Endurance and Resistance Training on Skeletal Muscle Glucose Metabolism in Older Adults. Nutrients, 11(11), 2636. https://doi.org/10.3390/nu11112636




