Is N-Carbamoyl Putrescine, the Decarboxylation Derivative of Citrulline, a Regulator of Muscle Protein Metabolism in Rats?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Sample Treatment
2.4. Nitrogen Metabolism
2.5. Expression/Activation of Anabolic and Catabolic Signaling Protein
2.6. Biological Assessment
2.7. Calculations and Statistical Analysis
3. Results
3.1. Study 1
3.1.1. Tolerance
3.1.2. Nitrogen and Muscle Protein Metabolism
3.1.3. Polyamine Metabolism
3.2. Study 2
3.2.1. Tolerance
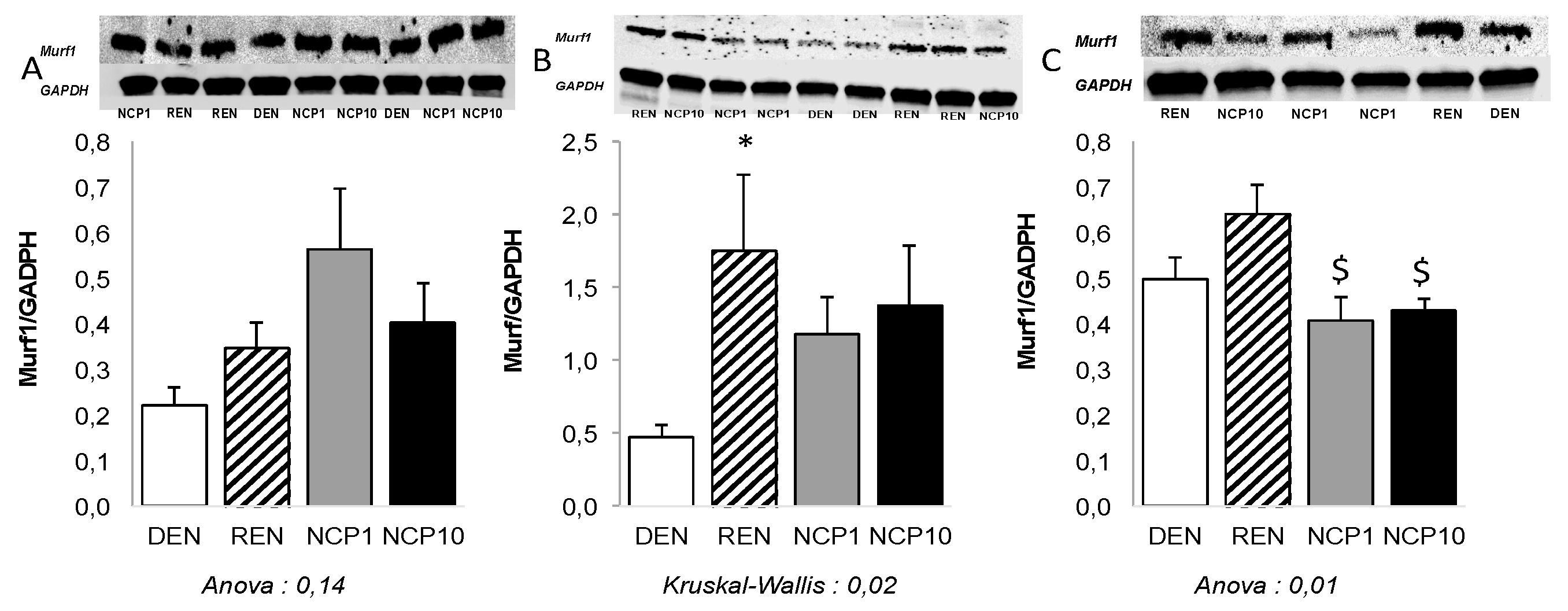
3.2.2. Nitrogen and Muscle Protein Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cynober, L.; Moinard, C.; De Bandt, J.-P. The 2009 ESPEN Sir David Cuthbertson. Citrulline: A new major signaling molecule or just another player in the pharmaconutrition game? Clin. Nutr. 2010, 29, 545–551. [Google Scholar] [CrossRef]
- Moinard, C.; Cynober, L.; de Bandt, J.-P. Polyamines: Metabolism and implications in human diseases. Clin. Nutr. 2005, 24, 184–197. [Google Scholar] [CrossRef]
- Janowitz, T.; Kneifel, H.; Piotrowski, M. Identification and characterization of plant agmatine iminohydrolase, the last missing link in polyamine biosynthesis of plants. FEBS Lett. 2003, 544, 258–261. [Google Scholar] [CrossRef]
- Nakada, Y.; Itoh, Y. Identification of the putrescine biosynthetic genes in Pseudomonas aeruginosa and characterization of agmatine deiminase and N-carbamoylputrescine amidohydrolase of the arginine decarboxylase pathway. Microbiology (Read. Engl.) 2003, 149, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.E.; Landete, J.M.; Manca de Nadra, M.C.; Pardo, I.; Ferrer, S. Factors affecting the production of putrescine from agmatine by Lactobacillus hilgardii XB isolated from wine. J. Appl. Microbiol. 2008, 105, 158–165. [Google Scholar] [CrossRef]
- Landete, J.M.; Arena, M.E.; Pardo, I.; Manca de Nadra, M.C.; Ferrer, S. Comparative survey of putrescine production from agmatine deamination in different bacteria. Food Microbiol. 2008, 25, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Brodal, B.P.; Eliassen, K.A.; Rönning, H.; Osmundsen, H. Effects of dietary polyamines and clofibrate on metabolism of polyamines in the rat. J. Nutr. Biochem. 1999, 10, 700–708. [Google Scholar] [CrossRef]
- Bardocz, S. Polyamines in Health and Nutrition; White, A., Ed.; Kluwer Academic Publishers: Boston, MA, USA, 1999. [Google Scholar]
- Til, H.P.; Falke, H.E.; Prinsen, M.K.; Willems, M.I. Acute and subacute toxicity of tyramine, spermidine, spermine, putrescine and cadaverine in rats. Food Chem. Toxicol. 1997, 35, 337–348. [Google Scholar] [CrossRef]
- Ramani, D.; Nakib, S.; Chen, H.; Garbay, C.; Loukaci, A.; Cynober, L.; De Bandt, J. N-Carbamoylputrescine, a citrulline-derived polyamine, is not a significant citrulline metabolite in rats. Anal. Biochem. 2012, 423, 54–60. [Google Scholar] [CrossRef]
- Osowska, S.; Duchemann, T.; Walrand, S.; Paillard, A.; Boirie, Y.; Cynober, L.; Moinard, C. Citrulline modulates muscle protein metabolism in old malnourished rats. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E582–E586. [Google Scholar] [CrossRef] [Green Version]
- Osowska, S.; Moinard, C.; Neveux, N.; Loï, C.; Cynober, L. Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection. Gut 2004, 53, 1781–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neveux, N.; David, P.; Cynober, L. Measurement of amino acid concentration in biological fluids and tissues using ion-exchange chromatography. In Metabolic and Therapeutic Aspects of Amino Acids in Clinical Nutrition, 2nd ed.; Cynober, L., Ed.; CRC: Boca Raton, FL, USA, 2004; pp. 17–28. [Google Scholar]
- Le Plénier, S.; Goron, A.; Sotiropoulos, A.; Archambault, E.; Guihenneuc, C.; Walrand, S.; Salles, J.; Jourdan, M.; Neveux, N.; Cynober, L.; et al. Citrulline directly modulates muscle protein synthesis via the PI3K/MAPK/4E-BP1 pathway in a malnourished state: Evidence from in vivo, ex vivo, and in vitro studies. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E27–E36. [Google Scholar] [CrossRef] [PubMed]
- Seiler, N. Use of the dansyl reaction in biochemical analysis. Methods Biochem. Anal. 1970, 18, 259–337. [Google Scholar] [PubMed]
- Kovacevic, Z.; Day, S.H.; Collett, V.; Brosnan, J.T.; Brosnan, M.E. Activation of hepatic glutaminase by spermine. Biochem. J. 1995, 305, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.A.; Kotecki, J.A.; Jacobs, B.L.; Hornberger, T.A. Muscle fiber type-dependent differences in the regulation of protein synthesis. PLoS ONE 2012, 7, e37890. [Google Scholar] [CrossRef]
- Satriano, J.; Matsufuji, S.; Murakami, Y.; Lortie, M.J.; Schwartz, D.; Kelly, C.J.; Hayashi, S.-I.; Blantz, R.C. Agmatine suppresses proliferation by frameshift induction of antizyme and attenuation of cellular polyamine levels. J. Biol. Chem. 1998, 273, 15313–15316. [Google Scholar] [CrossRef] [PubMed]
- Dudkowska, M.; Lai, J.; Gardini, G.; Stachurska, A.; Grzelakowska-Sztabert, B.; Colombatto, S.; Manteuffel-Cymborowska, M. Agmatine modulates the in vivo biosynthesis and interconversion of polyamines and cell proliferation. Biochim. Biophys. Acta 2003, 1619, 159–166. [Google Scholar] [CrossRef]
- Vargiu, C.; Cabella, C.; Belliardo, S.; Cravanzola, C.; Grillo, M.A.; Colombatto, S. Agmatine modulates polyamine content in hepatocytes by inducing spermidine/spermine acetyltransferase. Eur. J. Biochem. 1999, 259, 933–938. [Google Scholar] [CrossRef]
- Watanabe, S.; Sato, S.; Nagase, S.; Shimosato, K.; Ohkuma, S. Chemotherapeutic targeting of etoposide to various tissues on the basis of polyamine level. J. Drug Target. 2004, 12, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Sato, S.; Nagase, S.; Shimosato, K.; Ohkuma, S. Effects of methotrexate and cyclophosphamide on polyamine levels in various tissues of rats. J. Drug Target. 1999, 7, 197–205. [Google Scholar] [CrossRef]
- Marcelino, H.; Veyrat-Durebex, C.; Summermatter, S.; Sarafian, D.; Miles-Chan, J.; Arsenijevic, D.; Zani, F.; Montani, J.P.; Seydoux, J.; Solinas, G.; et al. A role for adipose tissue de novo lipogenesis in glucose homeostasis during catch-up growth: A Randle cycle favoring fat storage. Diabetes 2013, 62, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Calonne, J.; Isacco, L.; Miles-Chan, J.; Arsenijevic, D.; Montani, J.-P.; Guillet, C.; Boirie, Y.; Dulloo, A.G. Reduced Skeletal Muscle Protein Turnover and Thyroid Hormone Metabolism in Adaptive Thermogenesis That Facilitates Body Fat Recovery During Weight Regain. Front. Endocrinol. (Lausanne) 2019, 10, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| C | NCP5 | NCP50 | p Values | |
|---|---|---|---|---|
| Weight (g) | 314 ± 4 | 313 ± 7 | 316 ± 5 | 0.93 |
| Weight gain (g) | 51 ± 3 | 48 ± 3 | 51 ± 3 | 0.76 |
| Metabolism | ||||
| Glucose (mmol/l) | 6.6 ± 0.3 | 7.1 ± 0.3 | 7.0 ± 0.3 | 0.37 |
| Cholesterol (mmol/l) | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 0.79 |
| Triglycerides (mmol/l) | 0.5 ± 0.1 | 0.5 ± 0.0 | 0.6 ± 0.1 | 0.22 |
| Liver, muscle and kidney function | ||||
| AST (IU/L) | 152 ± 6 | 163 ± 14 | 162 ± 13 | 0.78 |
| ALT (IU/L) | 46 ± 2 | 46 ± 3 | 45 ± 3 | 0.93 |
| Creatinine (µmol/l) | 13 ± 1 | 12 ± 1 | 13 ± 1 | 0.28 |
| Creatine kinase (UI/L) | 11 ± 1 | 11 ± 2 | 11 ± 1 | 0.99 |
| Bilirubin (µmol/l) | 1.0 ± 0.3 | 1.1 ± 0.1 | 1.0 ± 0.0 | 0.76 |
| C | NCP5 | NCP50 | p Values | |
|---|---|---|---|---|
| Soleus | ||||
| Weight (g) | 0.14 ± 0.00 | 0.15 ± 0.01 | 0.14 ± 0.00 | 0.31 |
| Protein content (mg/g) | 127 ± 7 | 145 ± 3 * | 140 ± 3 | 0.05 |
| Protein content (mg) | 17 ± 1 | 21 ± 1 * | 20 ± 0.5 | 0.03 |
| Tibialis | ||||
| Weight (g) | 0.59 ± 0.01 | 0.62 ± 0.02 | 0.60 ± 0.01 | 0.43 |
| Protein content (mg/g) | 174 ± 3 | 175 ± 3 | 177 ± 2 | 0.32 |
| Protein content (mg) | 52 ± 1 | 52 ± 1 | 53 ± 1 | 0.62 |
| Liver | ||||
| Weight (g) | 9.20 ± 0.23 | 9.50 ± 0.28 | 9.10 ± 0.13 | 0.49 |
| Protein content (mg/g) | 205 ± 7 | 211 ± 2 | 208 ± 5 | 0.90 |
| Plasma amino acids | ||||
| ΣBCAA (µmol/l) | 463 ± 39 | 422 ± 23 | 393 ± 32 | 0.32 |
| ΣEAA (µmol/l) | 916 ± 69 | 843 ± 30 | 806 ± 50 | 0.33 |
| ΣNEAA (µmol/l) | 2369 ± 158 | 2234 ± 103 | 2131 ± 95 | 0.39 |
| ΣAA (µmol/l) | 3285 ± 227 | 3077 ± 130 | 2937 ± 143 | 0.36 |
| C | NCP5 | NCP50 | p Values | |
|---|---|---|---|---|
| Liver (nmol/g) | ||||
| Putrescine | 5.95 ± 0.65 | 5.70 ± 0.83 | 4.79 ± 0.67 | 0.51 |
| Spermidine | 790 ± 32 | 805 ± 32 | 781 ± 30 | 0.86 |
| Spermine | 918 ± 86 | 930 ± 105 | 1103 ± 81 | 0.31 |
| Plasma (μmol/L) | ||||
| Putrescine | 0.40 ± 0.05 | 0.69 ± 0.16 | 0.56 ± 0.12 | 0.39 |
| Spermidine | 7.65 ± 0.68 | 7.83 ± 0.36 | 9.27 ± 0.86 | 0.19 |
| Spermine | 0.63 ± 0.03 | 0.67 ± 0.06 | 0.74 ± 0.04 | 0.23 |
| Jejunum (nmol/g) | ||||
| Putrescine | 84 ± 9 | 87 ± 10 | 97 ± 6 | 0.54 |
| Spermidine | 1884 ± 352 | 1551 ± 101 | 1677 ± 114 | 0.53 |
| Spermine | 1508 ± 135 | 1490 ± 119 | 1598 ± 167 | 0.85 |
| Ileum (nmol/g) | ||||
| Putrescine | 66 ± 7 | 79 ± 10 | 68 ± 7 | 0.51 |
| Spermidine | 1198 ± 85 | 1385 ± 85 | 1210 ± 70 | 0.20 |
| Spermine | 1109 ± 80 | 1175 ± 137 | 1112 ± 103 | 0.90 |
| Kidney (nmol/g) | ||||
| Putrescine | 13 ± 2 | 16 ± 2 | 20 ± 3 | 0.22 |
| Spermidine | 948 ± 32 | 954 ± 45 | 846 ± 69 | 0.28 |
| Spermine | 848 ± 85 | 881 ± 71 | 555 ± 59 *,# | 0.01 |
| Soleus (nmol/g) | ||||
| Putrescine | 11 ± 2 | 8 ± 1 | 13 ± 2 | 0.10 |
| Spermidine | 205 ± 13 | 223 ± 9 | 210 ± 9 | 0.47 |
| Spermine | 164 ± 43 | 235 ± 35 | 206 ± 46 | 0.48 |
| Tibialis (nmol/g) | ||||
| Putrescine | 2 ± 0 | 1 ± 0 | 2 ± 0 | 0.10 |
| Spermidine | 57 ± 3 | 53 ± 2 | 54 ± 3 | 0.47 |
| Spermine | 430 ± 32 | 325 ± 29 | 392 ± 29 | 0.07 |
| DEN | REN | NCP1 | NCP10 | p Values | |
|---|---|---|---|---|---|
| Weight (g) | 550 ± 21 | 604 ± 21 | 577 ± 19 | 592 ± 15 | 0.24 |
| Weight gain (g) | 18 ± 2 * | 20 ± 3 * | 13 ± 3 * | <0.0001 | |
| Metabolism | |||||
| Glucose (mmol/l) | 11.8 ± 1.1 | 15.5 ± 1.6 | 17.1 ± 1.5 * | 17.2 ± 1.1 * | 0.04 |
| Cholesterol (mmol/l) | 3.6 ± 0.2 | 3.0 ± 0.2 | 3.0 ± 0.2 | 3.2 ± 0.2 | 0.11 |
| Triglycerides (mmol/l) | 0.68 ± 0.1 | 0.95 ± 0.09 * | 1.13 ± 0.07 * | 1.14 ± 0.08 * | 0.002 |
| Liver, muscle and kidney function | |||||
| AST (IU/L) | 148 ± 18 | 179 ± 21 | 172 ± 60 | 196 ± 35 | 0.86 |
| ALT (IU/L) | 53 ± 7 | 98 ± 21 | 64 ± 16 | 95 ± 29 | 0.30 |
| Bilirubin (µmol/l) | 1.3 ± 0.2 | 0.9 ± 0.1 | 1.2 ± 0.3 | 1.4 ± 0.2 | 0.38 |
| Creatinine (µmol/l) | 29 ± 1 | 28 ± 2 | 27 ± 3 | 27 ± 1 | 0.87 |
| Urinary Creatinine (µmol/24h) | 91 ± 9 | 91 ± 11 | 74 ± 5 | 96 ± 7 | 0.31 |
| Creatinine clearance (mL/h) | 131 ± 16 | 140 ± 20 | 122 ± 14 | 148 ± 13 | 0.70 |
| DEN | REN | NCP1 | NCP10 | p Values | |
|---|---|---|---|---|---|
| Nitrogen metabolism | |||||
| Nitrogen balance a (mg/24h) | 172 ± 32 | 483 ± 22 * | 471 ± 27 * | 418 ± 44 * | <0.001 |
| 3MH/creatinine a (µmol/mmol) | 19 ± 3 | 40 ± 3 * | 35 ± 4 * | 32 ± 2 * | <0.003 |
| EDL | |||||
| Weight (g) | 0.23 ± 0.01 | 0.25 ± 0.01 | 0.26 ± 0.02 | 0.24 ± 0.01 | 0.40 |
| Protein content (mg/g) | 158 ± 8 | 164 ± 6 | 152 ± 6 | 179 ± 8 | 0.07 |
| Protein content (mg) | 36 ± 2 | 35 ± 2 | 35 ± 2 | 40 ± 2 | 0.28 |
| Soleus | |||||
| Weight (g) | 0.23 ± 0.02 | 0.23 ± 0.01 | 0.25 ± 0.02 | 0.23 ± 0.01 | 0.69 |
| Protein content (mg/g) | 165 ± 24 | 151 ± 9 | 170 ± 15 | 180 ± 38 | 0.80 |
| Protein content (mg) | 31 ± 3 | 34 ± 2 | 42 ± 4 | 39 ± 4 | 0.07 |
| Tibialis | |||||
| Weight (g) | 0.94 ± 0.03 | 0.99 ± 0.03 | 1.07 ± 0.06 * | 1.11 ± 0.05 * | 0.058 |
| Protein content (mg/g) | 177 ± 5 | 162 ± 7 | 179 ± 7 | 187 ± 9 | 0.13 |
| Protein content (mg) | 167 ± 7 | 164 ± 7 | 190 ± 14 | 199 ± 19 | 0.13 |
| Liver | |||||
| Weight (g) | 13.1 ± 0.8 | 17.3 ± 0.9 * | 16.5 ± 0.8 * | 16.0 ± 0.8 * | 0.01 |
| Protein content (mg/g) | 140 ± 20 | 160 ± 10 | 160 ± 10 | 190 ± 10 | 0.11 |
| Plasma amino acids | |||||
| ΣBCAA (µmol/l) | 526 ± 47 | 587 ± 32 | 599 ± 16 | 551 ± 37 | 0.43 |
| ΣEAA (µmol/l) | 874 ± 53 | 940 ± 46 | 978 ± 29 | 910 ± 44 | 0.39 |
| ΣNEAA (µmol/l) | 2968 ± 75 | 2872 ± 155 | 2781 ± 147 | 2757 ± 99 | 0.66 |
| ΣAA (µmol/l) | 3842 ± 101 | 3811 ± 195 | 3759 ± 161 | 3667 ± 104 | 0.86 |
| DEN | REN | NCP1 | NCP10 | p Values | |
|---|---|---|---|---|---|
| EDL | |||||
| Gln | 4.90 ± 0.20 | 4.70 ± 0.10 | 5.50 ± 0.20 $ | 4.70 ± 0.20 # | <0.05 |
| Val | 0.17 ± 0.01 | 0.22 ± 0.01 * | 0.22 ± 0.01 * | 0.20 ± 0.01 | <0.05 |
| BCAA | 0.43 ± 0.03 | 0.52 ± 0.02 | 0.52 ± 0.02 | 0.49 ± 0.03 | 0.099 |
| Phe | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 $ | 0.06 ± 0.00 # | <0.05 |
| Cys | 0.040 ± 0.000 | 0.036 ± 0.002 * | 0.040 ± 0.000 $ | 0.039 ± 0.001 $ | <0.02 |
| Ser | 1.16 ± 0.06 | 0.95 ± 0.03 | 1.12 ± 0.05 | 1.07 ± 0.08 | 0.067 |
| Pro | 0.22 ± 0.01 | 0.30 ± 0.02 * | 0.30 ± 0.02 * | 0.29 ± 0.02 * | <0.05 |
| Gly | 3.12 ± 0.17 | 2.49 ± 0.11 * | 2.45 ± 0.16 * | 2.44 ± 0.22 * | <0.05 |
| Soleus | |||||
| Met | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.04 ± 0.00 | 0.077 |
| Val | 0.16 ± 0.01 | 0.22 ± 0.01 | 0.21 ± 0.01 | 0.20 ± 0.01 | 0.071 |
| Ile | 0.08 ± 0.00 | 0.11 ± 0.06 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.087 |
| Leu | 0.17 ± 0.01 | 0.22 ± 0.01 * | 0.21 ± 0.01 * | 0.21 ± 0.01 * | <0.05 |
| BCAA | 0.42 ± 0.02 | 0.55 ± 0.03 * | 0.52 ± 0.03 * | 0.51 ± 0.03 * | <0.05 |
| Glu | 4.21 ± 0.52 | 3.14 ± 0.49 | 2.22 ± 0.32 * | 3.29 ± 0.45 | <0.05 |
| Ala | 1.94 ± 0.26 | 2.48 ± 0.11 | 2.35 ± 0.08 | 2.55 ± 0.20 | 0.08 |
| Pro | 0.23 ± 0.02 | 0.31 ± 0.02 * | 0.28 ± 0.02 | 0.30 ± 0.01 * | <0.05 |
| Gly | 188 ± 30 | 177 ± 22 * | 197 ± 34 * | 174 ± 24 * | <0.05 |
| Tibialis | |||||
| Val | 0.17 ± 0.01 | 0.23 ± 0.02 * | 0.21 ± 0.01 | 0.19 ± 0.02 $ | <0.05 |
| Leu | 0.18 ± 0.01 | 0.22 ± 0.02 | 0.20 ± 0.01 | 0.17 ± 0.02 | 0.098 |
| BCAA | 0.44 ± 0.03 | 0.57 ± 0.04 * | 0.51 ± 0.02 | 0.45 ± 0.04 $ | <0.05 |
| Tyr | 0.09 ± 0.01 | 0.11 ± 0.01 | 0.09 ± 0.05 | 0.08 ± 0.01 | 0.084 |
| Glu | 0.84 ± 0.06 | 1.19 ± 0.08 * | 1.14 ± 0.07 * | 0.92 ± 0.06 $,# | <0.05 |
| Pro | 0.24 ± 0.01 | 0.34 ± 0.02 * | 0.31 ± 0.02 * | 0.27 ± 0.02 $ | <0.05 |
| Gly | 3.52 ± 0.20 | 2.90 ± 0.20 | 2.70 ± 0.30 * | 2.50 ± 0.20 * | <0.05 |
| Liver | |||||
| Orn | 0.45 ± 0.04 | 0.31 ± 0.03 * | 0.34 ± 0.03 | 0.32 ± 0.02 * | <0.05 |
| Gln | 4.90 ± 0.20 | 4.80 ± 0.30 | 5.10 ± 0.20 $ | 4.90 ± 0.30 # | <0.05 |
| Val | 0.31 ± 0.04 | 0.29 ± 0.03 * | 0.32 ± 0.01 * | 0.31 ± 0.02 | <0.05 |
| Cys | 0.102 ± 0.001 | 0.098 ± 0.002 * | 0.100 ± 0.001 $ | 0.099 ± 0.001 $ | <0.04 |
| Ser | 0.60 ± 0.10 | 0.50 ± 0.10 | 0.60 ± 0.10 | 0.60 ± 0.10 | 0.067 |
| Ala | 2.10 ± 0.20 | 2.80 ± 0.20 * | 3.20 ± 0.30 * | 3.20 ± 0.20 * | <0.05 |
| His | 0.60 ± 0.03 | 0.67 ± 0.05 | 0.72 ± 0.03 | 0.68 ± 0.03 | 0.092 |
| Gly | 2.90 ± 0.20 | 2.10 ± 0.20 * | 2.00 ± 0.20 * | 2.00 ± 0.20 * | <0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jegatheesan, P.; Ramani, D.; Lhuillier, M.; El-Hafaia, N.; Ramassamy, R.; Aboubacar, M.; Nakib, S.; Chen, H.; Garbay, C.; Neveux, N.; et al. Is N-Carbamoyl Putrescine, the Decarboxylation Derivative of Citrulline, a Regulator of Muscle Protein Metabolism in Rats? Nutrients 2019, 11, 2637. https://doi.org/10.3390/nu11112637
Jegatheesan P, Ramani D, Lhuillier M, El-Hafaia N, Ramassamy R, Aboubacar M, Nakib S, Chen H, Garbay C, Neveux N, et al. Is N-Carbamoyl Putrescine, the Decarboxylation Derivative of Citrulline, a Regulator of Muscle Protein Metabolism in Rats? Nutrients. 2019; 11(11):2637. https://doi.org/10.3390/nu11112637
Chicago/Turabian StyleJegatheesan, Prasanthi, David Ramani, Mickael Lhuillier, Naouel El-Hafaia, Radji Ramassamy, Mohamed Aboubacar, Samir Nakib, Huixiong Chen, Christiane Garbay, Nathalie Neveux, and et al. 2019. "Is N-Carbamoyl Putrescine, the Decarboxylation Derivative of Citrulline, a Regulator of Muscle Protein Metabolism in Rats?" Nutrients 11, no. 11: 2637. https://doi.org/10.3390/nu11112637
APA StyleJegatheesan, P., Ramani, D., Lhuillier, M., El-Hafaia, N., Ramassamy, R., Aboubacar, M., Nakib, S., Chen, H., Garbay, C., Neveux, N., Loï, C., Cynober, L., & de Bandt, J.-P. (2019). Is N-Carbamoyl Putrescine, the Decarboxylation Derivative of Citrulline, a Regulator of Muscle Protein Metabolism in Rats? Nutrients, 11(11), 2637. https://doi.org/10.3390/nu11112637






