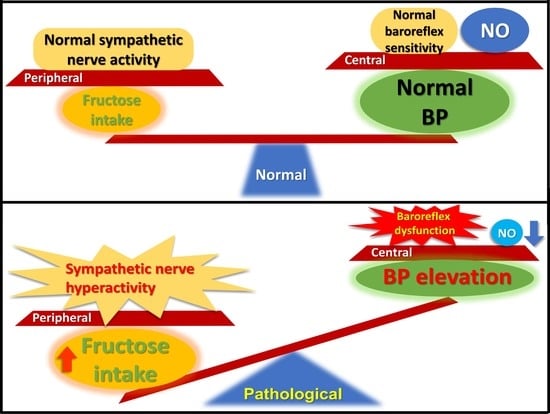
Excessive Fructose Intake Impairs Baroreflex Sensitivity and Led to Elevated Blood Pressure in Rats
Abstract
:1. Introduction
2. Materials and Methods
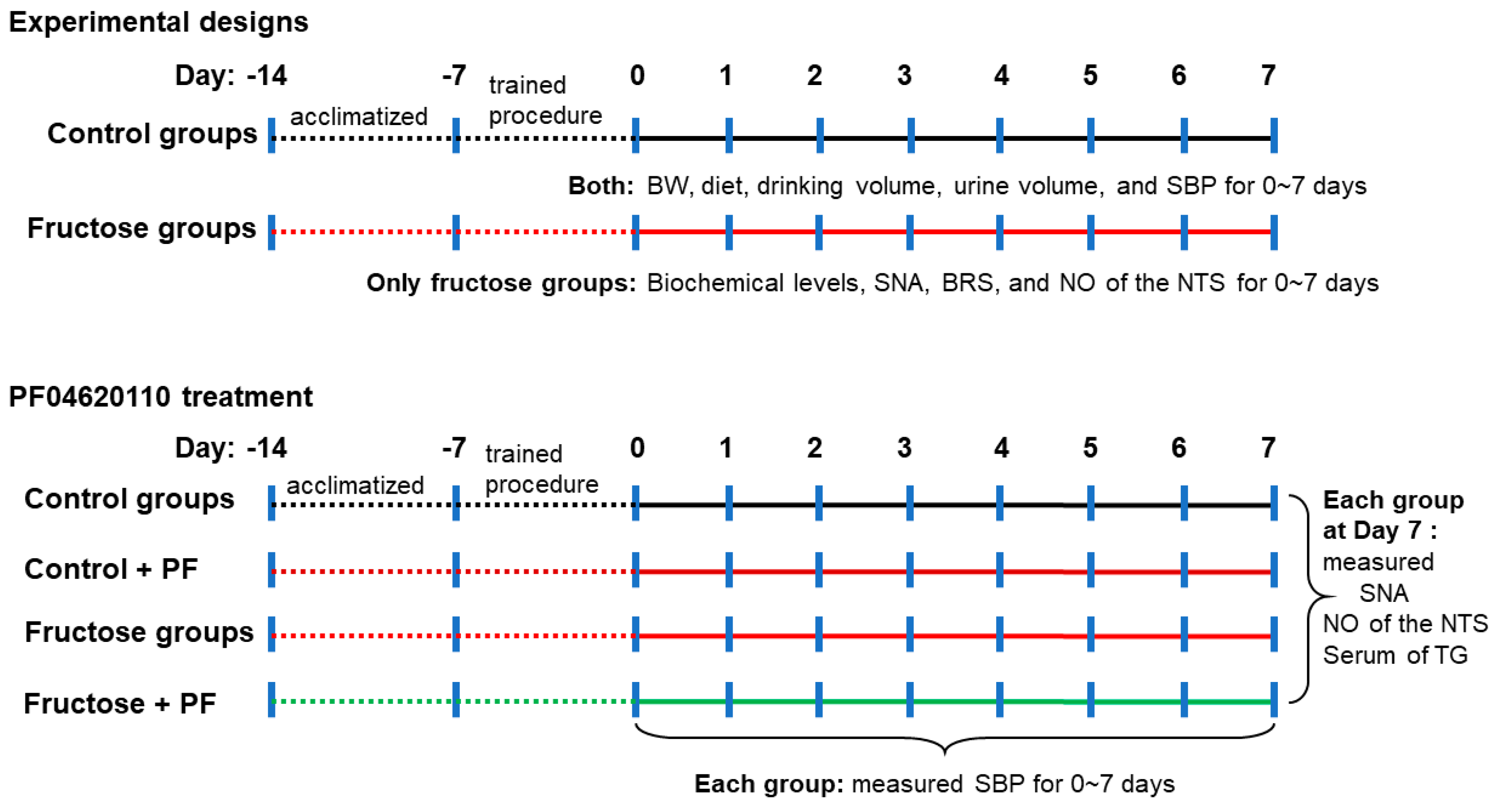
2.1. Animal Care and Experiments
2.2. Blood Pressure Measurement
2.3. Determination of Nitric Oxide Analysis in the NTS
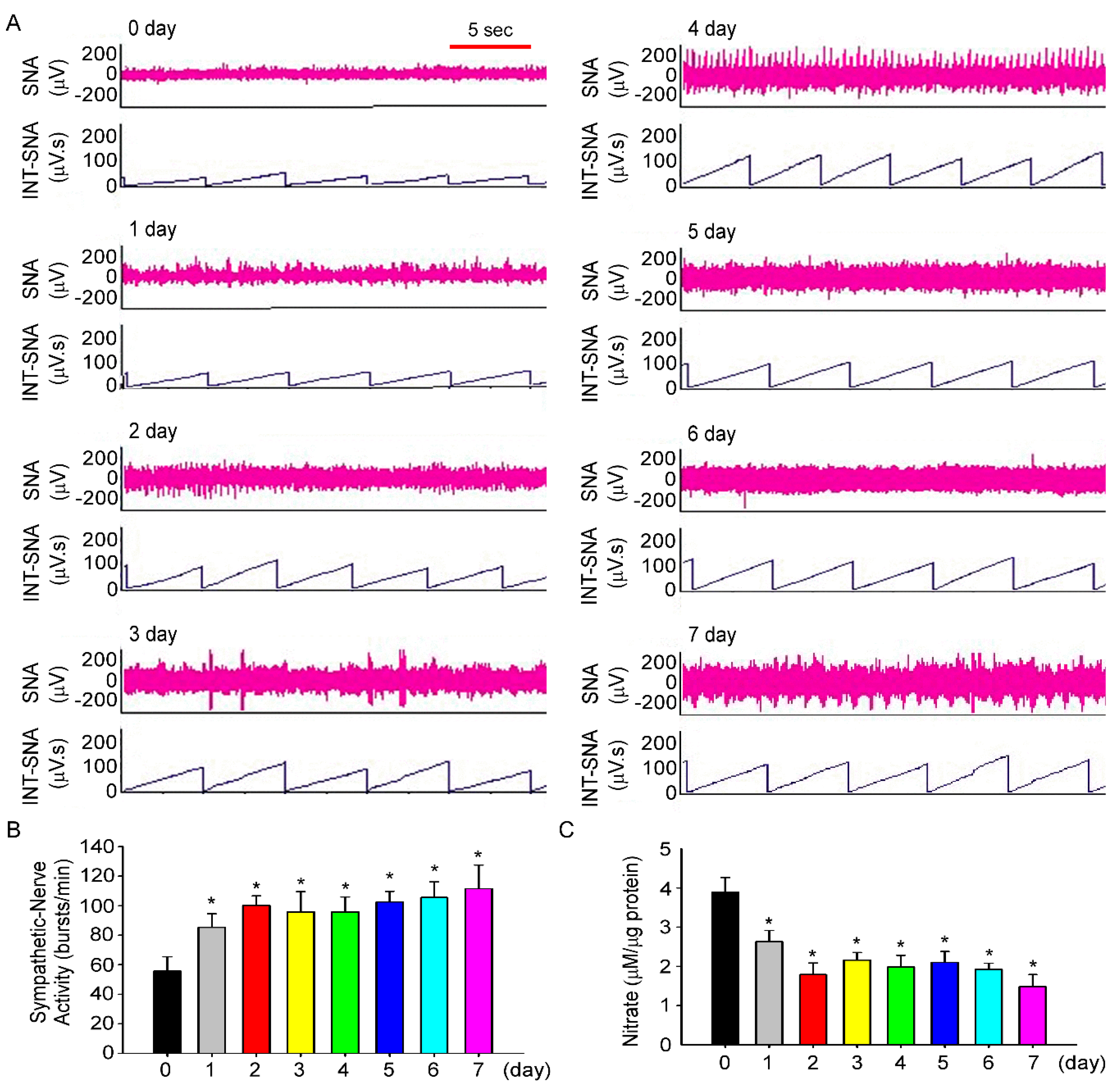
2.4. Renal Sympathetic Nerve Activity Recording
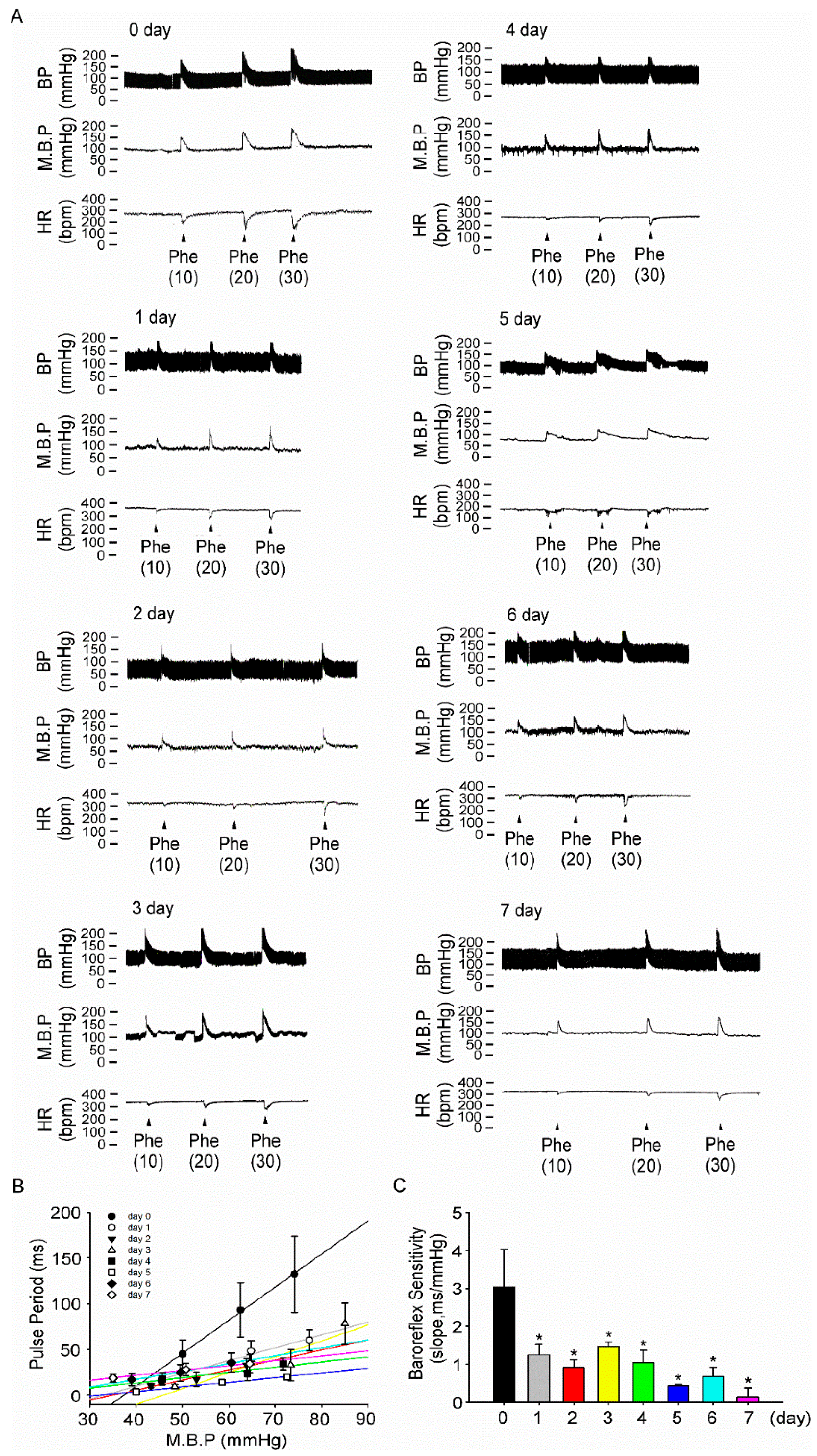
2.5. Baroreflex Sensitivity Recording
2.6. Assay of Serum Biochemical Parameters
2.7. Statistical Analysis
3. Results
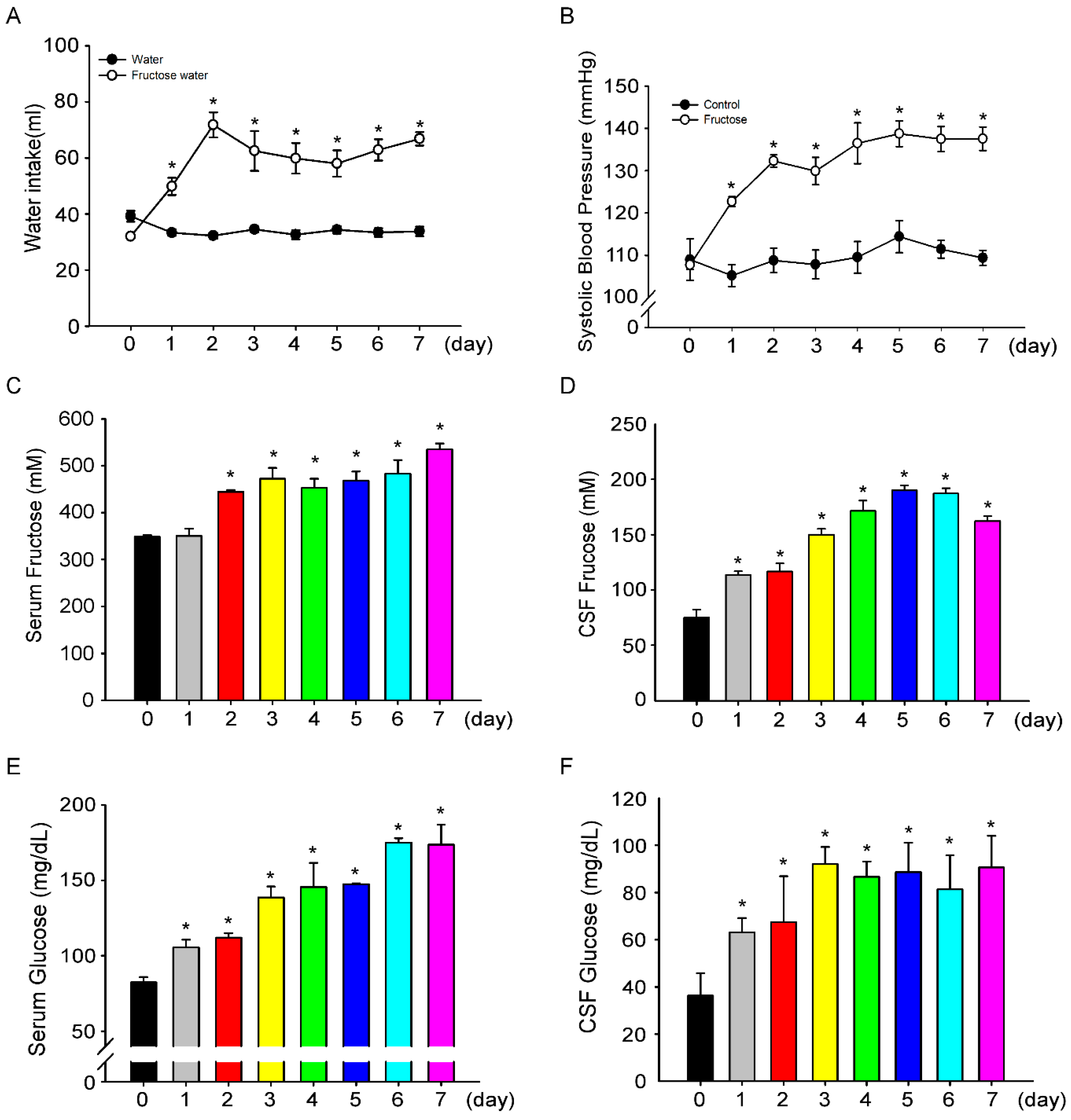
3.1. Fructose Intake for One Week Significantly Increases Blood Pressure in Rats
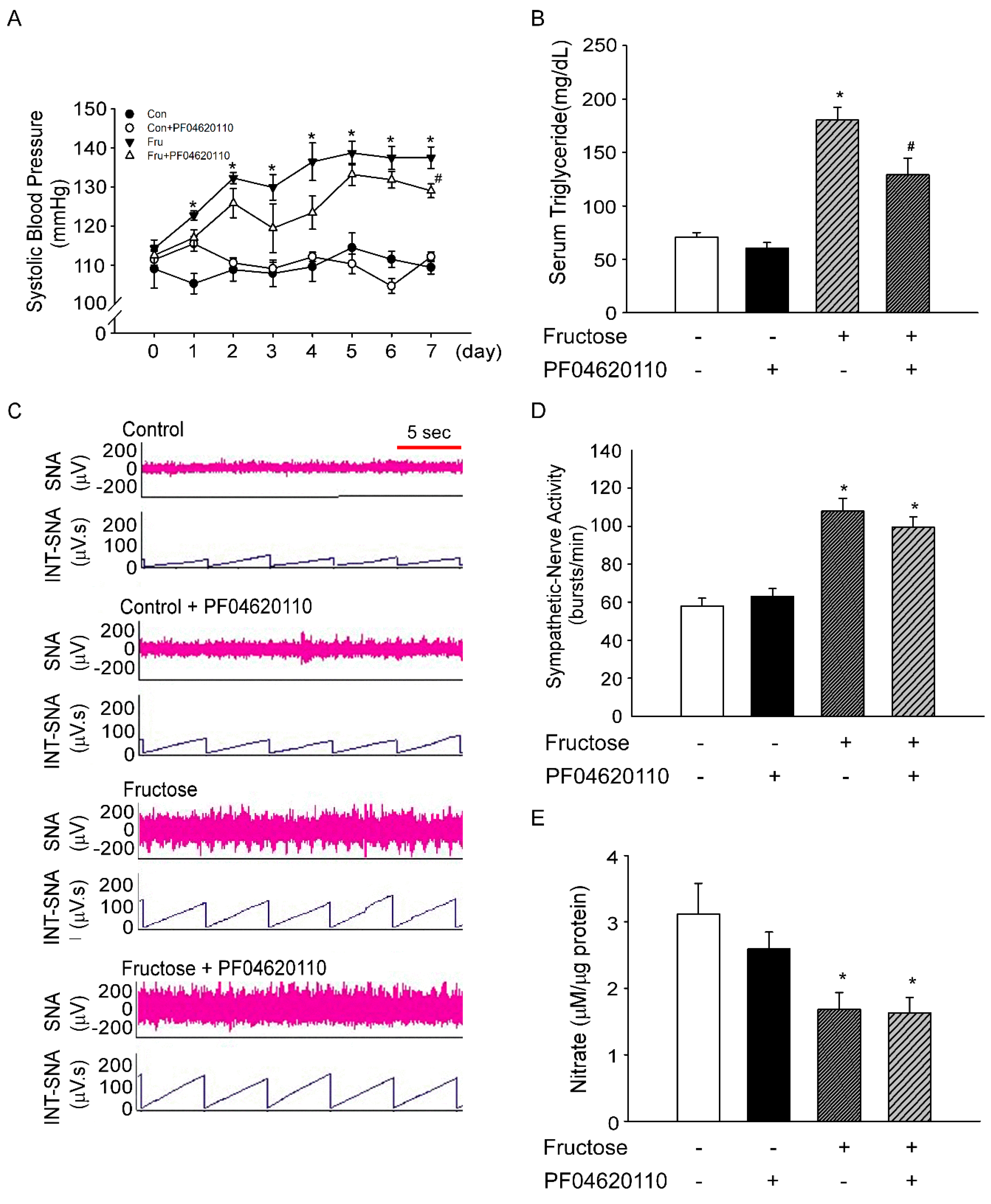
3.2. Inhibition of Triglyceride does not Decrease Sympathetic-Nerve Activity after 1 Week of Fructose Intake
3.3. Fructose Intake Induces Sympathetic Hyperactivity and Decreases Nitric Oxide Level in Rats Fed Fructose for One Week
3.4. Fructose Intake for One Week Impairs Baroreflex Response Sensitivity in Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BRS | baroreflex responses sensitivity |
| CSF | cerebrospinal fluid |
| DGAT1 | diacylglycerol acyltransferase 1 |
| NTS | nucleus tractus solitarii |
| NO | nitric oxide |
| ROS | reactive oxygen species |
| SNA | sympathetic nerve activity |
| WKY | Wistar Kyoto |
References
- Hajjar, I.; Kotchen, T. Trends in prevalence, awareness, treatment and control of hypertension in the United States, 1988–2000. JAMA 2003, 290, 199–206. [Google Scholar] [CrossRef]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar]
- Park, Y.K.; Yetley, E.A. Intakes and food sources of fructose in the United States. Am. J. Clin. Nutr. 1993, 58, 737–747. [Google Scholar] [CrossRef]
- Marriott, B.P.; Cole, N.; Lee, E. National Estimates of Dietary Fructose Intake Increased from 1977 to 2004 in the United States. J. Nutr. 2009, 139, 1228–1235. [Google Scholar] [CrossRef]
- Tappy, L.; Lê, K.A. Metabolic Effects of Fructose and the Worldwide Increase in Obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [Green Version]
- Pepin, A.; Stanhope, K.L.; Imbeault, P. Are Fruit Juices Healthier Than Sugar-Sweetened Beverages? A Review. Nutrients 2019, 11, 1006. [Google Scholar] [CrossRef]
- Baena, M.; Sangüesa, G.; Hutter, N.; Beltrán, J.M.; Sánchez, R.M.; Roglans, N.; Alegret, M.; Laguna, J.C. Liquid fructose in Western-diet-fed mice impairs liver insulin signaling and causes cholesterol and triglyceride loading without changing calorie intake and body weight. J. Nutr. Biochem. 2017, 40, 105–115. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; Tapia, E.; Jimenez, A.; Bautista, P.; Cristóbal, M.; Nepomuceno, T.; Soto, V.; Avila-Casado, C.; Nakagawa, T.; Johnson, R.J.; et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am. J. Physiol. Physiol. 2007, 292, 423–429. [Google Scholar] [CrossRef]
- Van Opstal, A.; Kaal, I.; Berg-Huysmans, A.V.D.; Hoeksma, M.; Blonk, C.; Pijl, H.; Rombouts, S.; Van Der Grond, J. Dietary sugars and non-caloric sweeteners elicit different homeostatic and hedonic responses in the brain. Nutrition 2019, 60, 80–86. [Google Scholar] [CrossRef]
- Klein, A.V.; Kiat, H. The mechanisms underlying fructose-induced hypertension: A review. J. Hypertens. 2015, 33, 912–920. [Google Scholar] [CrossRef]
- Tran, L.T.; MacLeod, K.M.; McNeill, J.H. Selective alpha1-adrenoceptor blockade prevents fructose-induced hypertension. Mol. Cell. Biochem. 2014, 392, 205–211. [Google Scholar] [CrossRef]
- Verma, S.; Bhanot, S.; McNeill, J.H. Sympathectomy prevents fructose-induced hyperinsulinemia and hypertension. Eur. J. Pharmacol. 1999, 373, R1–R4. [Google Scholar] [CrossRef]
- Mark, A.L.; Agassandian, K.; Morgan, D.A.; Liu, X.; Cassell, M.D.; Rahmouni, K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension 2009, 53, 375–380. [Google Scholar] [CrossRef]
- Chen, H.H.; Cheng, P.W.; Ho, W.Y.; Lu, P.J.; Lai, C.C.; Tseng, Y.M.; Fang, H.C.; Sun, G.C.; Hsiao, M.; Liu, C.P.; et al. Renal Denervation Improves the Baroreflex and GABA System in Chronic Kidney Disease-induced Hypertension. Sci. Rep. 2016, 6, 38447. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.H.; Hsieh, K.S.; Lu, P.J.; Wu, Y.S.; Ho, W.Y.; Lai, C.C.; Wang, J.S.; Ger, L.P.; Hsiao, M.; Tseng, C.J. Hexamethonium reverses the lethal cardiopulmonary damages in a rat model of brainstem lesions mimicking fatal enterovirus 71 encephalitis. Crit. Care Med. 2013, 41, 1276–1285. [Google Scholar] [CrossRef]
- Biaggioni, I.; Whetsell, W.O.; Jobe, J.; Nadeau, J.H. Baroreflex failure in a patient with central nervous system lesions involving the nucleus tractus solitarii. Hypertension 1994, 23, 491–495. [Google Scholar] [CrossRef]
- Zavaroni, I.; Chen, Y.D.I.; Reaven, G.M. Studies of the mechanism of fructose-induced hypertriglyceridemia in the rat. Metabolism 1982, 31, 1077–1083. [Google Scholar] [CrossRef]
- Yeh, T.C.; Liu, C.P.; Cheng, W.H.; Chen, B.R.; Lu, P.J.; Cheng, P.W.; Ho, W.Y.; Sun, G.C.; Liou, J.C.; Tseng, C.J. Caffeine Intake Improves Fructose-Induced Hypertension and Insulin Resistance by Enhancing Central Insulin Signaling. Hypertension 2014, 63, 535–541. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academy Press: Washington, DC, USA, 2011. [Google Scholar]
- Dow, R.L.; Li, J.C.; Pence, M.P.; Gibbs, E.M.; Laperle, J.L.; Litchfield, J.; Piotrowski, D.W.; Munchhof, M.J.; Manion, T.B.; Zavadoski, W.J.; et al. Discovery of PF-04620110, a Potent, Selective, and Orally Bioavailable Inhibitor of DGAT-1. ACS Med. Chem. Lett. 2011, 2, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Wilde, E.; Aubdool, A.A.; Thakore, P.; Baldissera, L.; Alawi, K.M.; Keeble, J.; Nandi, M.; Brain, S.D. Tail-Cuff Technique and Its Influence on Central Blood Pressure in the Mouse. J. Am. Heart Assoc. 2017, 6, e005204. [Google Scholar] [CrossRef]
- Daugherty, A.; Rateri, D.L.; Hong, L.; Balakrishnan, A. Measuring Blood Pressure in Mice using Volume Pressure Recording, a Tail-cuff Method. J. Vis. Exp. 2009. [Google Scholar] [CrossRef]
- Feng, M.; Whitesall, S.; Zhang, Y.; Beibel, M.; Alecy, L.D.; DiPetrillo, K. Validation of Volume-Pressure Recording Tail-Cuff Blood Pressure Measurements. Am. J. Hypertens. 2008, 21, 1288–1291. [Google Scholar] [CrossRef]
- Lo, W.C.; Jan, C.R.; Chiang, H.T.; Tseng, C.J. Modulatory Effects of Carbon Monoxide on Baroreflex Activation in Nucleus Tractus Solitarii of Rats. Hypertension 2000, 35, 1253–1257. [Google Scholar] [CrossRef] [Green Version]
- Jalal, D.I.; Smits, G.; Johnson, R.J.; Chonchol, M. Increased fructose associates with elevated blood pressure. J. Am. Soc. Nephrol. 2010, 21, 1543–1549. [Google Scholar] [CrossRef]
- Nguyen, S.; Choi, H.K.; Lustig, R.H.; Hsu, C.Y. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J. Pediatr. 2009, 154, 807–813. [Google Scholar] [CrossRef]
- Chen, L.; Caballero, B.; Mitchell, D.C.; Loria, C.; Lin, P.H.; Champagne, C.M.; Elmer, P.J.; Ard, J.D.; Batch, B.C.; Anderson, C.A.M.; et al. Reducing consumption of sugar-sweetened beverages is associated with reduced blood pressure: A prospective study among United States adults. Circulation 2010, 121, 2398–2406. [Google Scholar] [CrossRef]
- Kelishadi, R.; Mansourian, M.; Heidari-Beni, M. Association of fructose consumption and components of metabolic syndrome in human studies: A systematic review and meta-analysis. Nutrition 2014, 30, 503–510. [Google Scholar] [CrossRef]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef]
- Chen, D.G.; Dai, X.Z.; Bache, R.J. Postsynaptic adrenoceptor-mediated vasoconstriction in coronary and femoral vascular beds. Am. J. Physiol. Circ. Physiol. 1988, 254, 984–992. [Google Scholar] [CrossRef]
- Grassi, G.; Dell’Oro, R.; Quarti-Trevano, F.; Scopelliti, F.; Seravalle, G.; Paleari, F.; Gamba, P.L.; Mancia, G. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia 2005, 48, 1359–1365. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, K.; Hagelin, E.; Hansén, N.; Lind, L. Baroreceptor sensitivity is impaired in elderly subjects with metabolic syndrome and insulin resistance. J. Hypertens. 2006, 24, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Soncrant, T.; Komnenov, D.; Beierwaltes, W.H.; Chen, H.; Wu, M.; Rossi, N.F. Bilateral renal cryodenervation decreases arterial pressure and improves insulin sensitivity in fructose-fed Sprague-Dawley rats. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R529–R538. [Google Scholar] [CrossRef]
- Komnenov, D.; Levanovich, P.E.; Rossi, N.F. Hypertension Associated with Fructose and High Salt: Renal and Sympathetic Mechanisms. Nutrients 2019, 11, 569. [Google Scholar] [CrossRef]
- Dos Santos, F.; Moraes-Silva, I.C.; Moreira, E.D.; Irigoyen, M.C. The role of the baroreflex and parasympathetic nervous system in fructose-induced cardiac and metabolic alterations. Sci. Rep. 2018, 8, 10970. [Google Scholar] [CrossRef] [Green Version]
- Matsukawa, K.; Ninomiya, I. Anesthetic effects on tonic and reflex renal sympathetic nerve activity in awake cats. Am. J. Physiol. Integr. Comp. Physiol. 1989, 256, 371–378. [Google Scholar] [CrossRef]
- Bencze, M.; Behuliak, M.; Zicha, J. The impact of four different classes of anesthetics on the mechanisms of blood pressure regulation in normotensive and spontaneously hypertensive rats. Physiol. Res. 2013, 62, 471–478. [Google Scholar]
- Staff, P.O.; Staff, T.P.O. Correction: Effects of anesthetics on the renal sympathetic response to anaphylactic hypotension in rats. PLoS ONE 2015, 10, e0118042. [Google Scholar]
- Fisher, M.T.; Fisher, J.L. Activation of alpha6-containing GABAA receptors by pentobarbital occurs through a different mechanism than activation by GABA. Neurosci. Lett. 2010, 471, 195–199. [Google Scholar] [CrossRef]
- Lo, W.J.; Liu, H.W.; Lin, H.C.; Ger, L.P.; Tung, C.S.; Tseng, C.J. Modulatory effects of nitric oxide on baroreflex activation in the brainstem nuclei of rats. Chin. J. Physiol. 1996, 39, 57–62. [Google Scholar]
- Tseng, C.J.; Liu, H.Y.; Lin, H.C.; Ger, L.P.; Tung, C.S.; Yen, M.H. Cardiovascular Effects of Nitric Oxide in the Brain Stem Nuclei of Rats. Hypertension 1996, 27, 36–42. [Google Scholar] [CrossRef]
- Cheng, P.W.; Ho, W.Y.; Su, Y.T.; Lu, P.J.; Chen, B.Z.; Cheng, W.H.; Lu, W.H.; Sun, G.C.; Yeh, T.C.; Hsiao, M.; et al. Resveratrol decreases fructose-induced oxidative stress, mediated by NADPH oxidase via an AMPK-dependent mechanism. Br. J. Pharmacol. 2014, 171, 2739–2750. [Google Scholar] [CrossRef] [Green Version]
- Cheng, P.W.; Lin, Y.T.; Ho, W.Y.; Lu, P.J.; Chen, H.H.; Lai, C.C.; Sun, G.C.; Yeh, T.C.; Hsiao, M.; Tseng, C.J.; et al. Fructose induced neurogenic hypertension mediated by overactivation of p38 MAPK to impair insulin signaling transduction caused central insulin resistance. Free Radic. Biol. Med. 2017, 112, 298–307. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-H.; Chu, C.-H.; Wen, S.-W.; Lai, C.-C.; Cheng, P.-W.; Tseng, C.-J. Excessive Fructose Intake Impairs Baroreflex Sensitivity and Led to Elevated Blood Pressure in Rats. Nutrients 2019, 11, 2581. https://doi.org/10.3390/nu11112581
Chen H-H, Chu C-H, Wen S-W, Lai C-C, Cheng P-W, Tseng C-J. Excessive Fructose Intake Impairs Baroreflex Sensitivity and Led to Elevated Blood Pressure in Rats. Nutrients. 2019; 11(11):2581. https://doi.org/10.3390/nu11112581
Chicago/Turabian StyleChen, Hsin-Hung, Chih-Hsun Chu, Shu-Wei Wen, Chi-Cheng Lai, Pei-Wen Cheng, and Ching-Jiunn Tseng. 2019. "Excessive Fructose Intake Impairs Baroreflex Sensitivity and Led to Elevated Blood Pressure in Rats" Nutrients 11, no. 11: 2581. https://doi.org/10.3390/nu11112581
APA StyleChen, H.-H., Chu, C.-H., Wen, S.-W., Lai, C.-C., Cheng, P.-W., & Tseng, C.-J. (2019). Excessive Fructose Intake Impairs Baroreflex Sensitivity and Led to Elevated Blood Pressure in Rats. Nutrients, 11(11), 2581. https://doi.org/10.3390/nu11112581