Enzyme Treatment Alters the Anti-Inflammatory Activity of the Water Extract of Wheat Germ In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of UWG and EWG
2.2. HPLC Analysis of DMBQ
2.3. Animals
2.4. Macrophage Isolation
2.5. Cell Viability Assay
2.6. Western Blotting
2.7. Cytokine Analysis
2.8. Luciferase Assay
2.9. In vivo Experiments
2.10. Real-Time RT PCR
2.11. Statistical Analysis
3. Results
3.1. Analysis of DMBQ
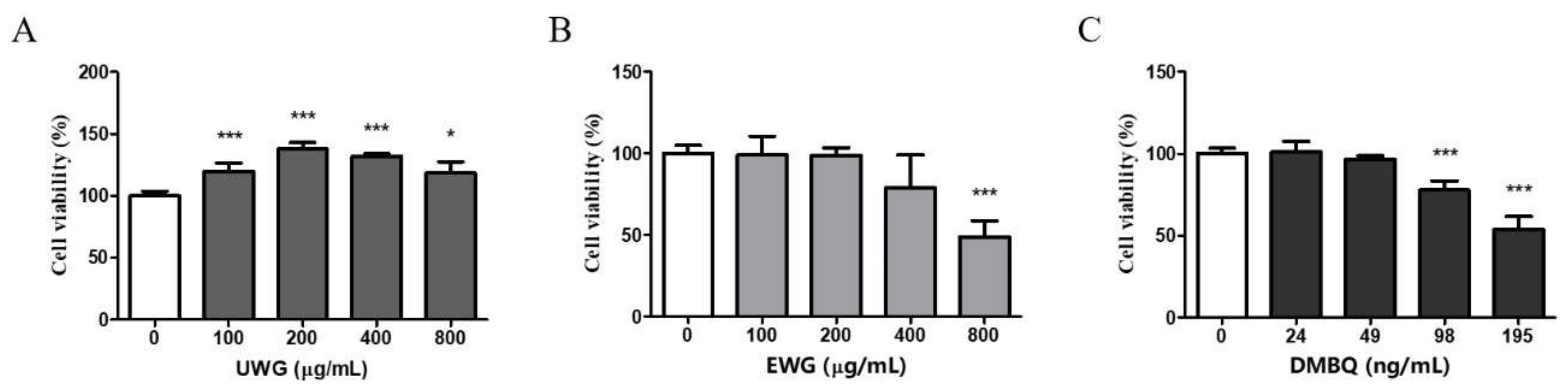
3.2. Effects of UWG and EWG on Viability of Mouse Peritoneal Macrophages
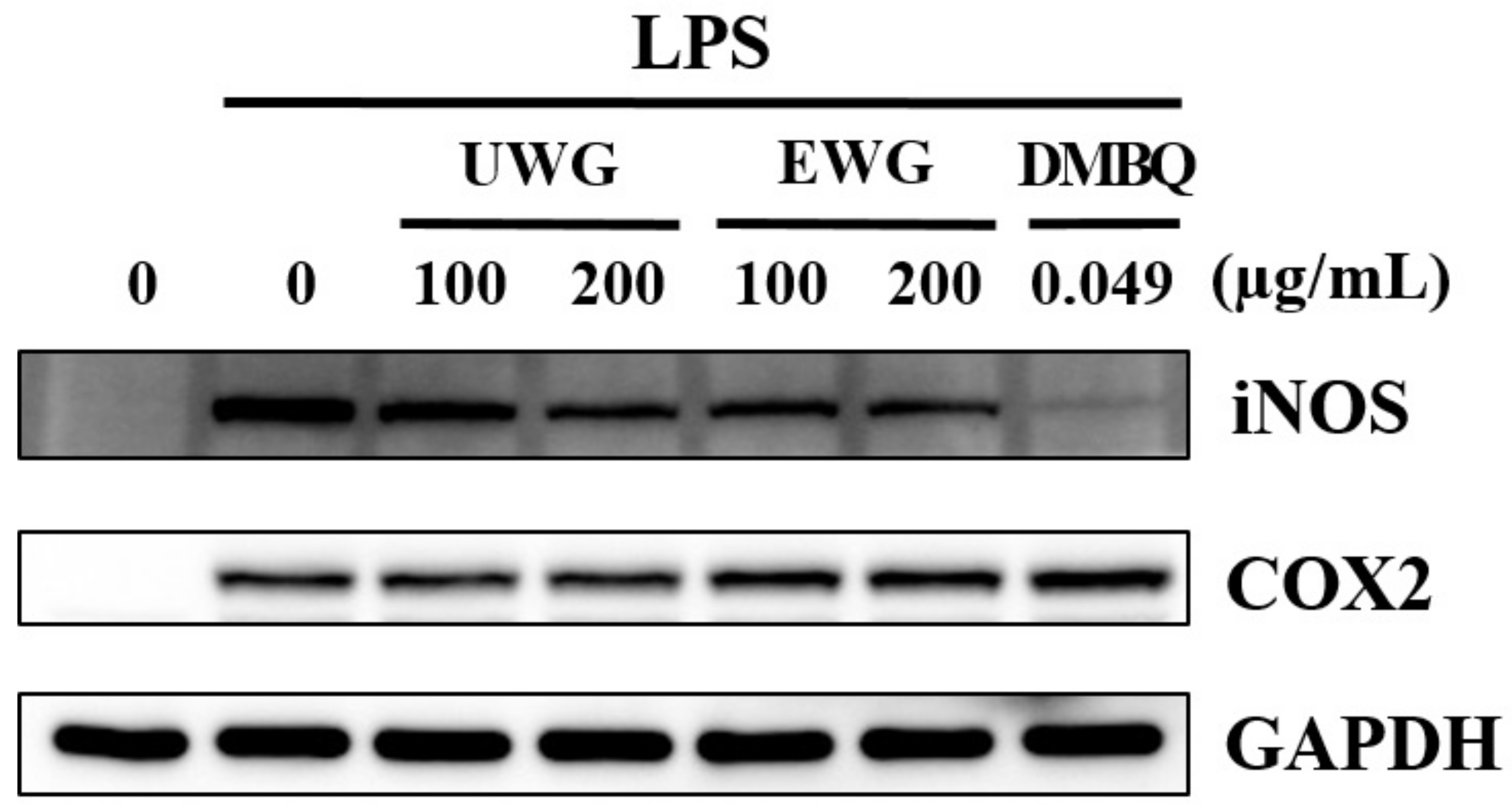
3.3. Effects of UWG and EWG on Inflammatory Enzyme Expression
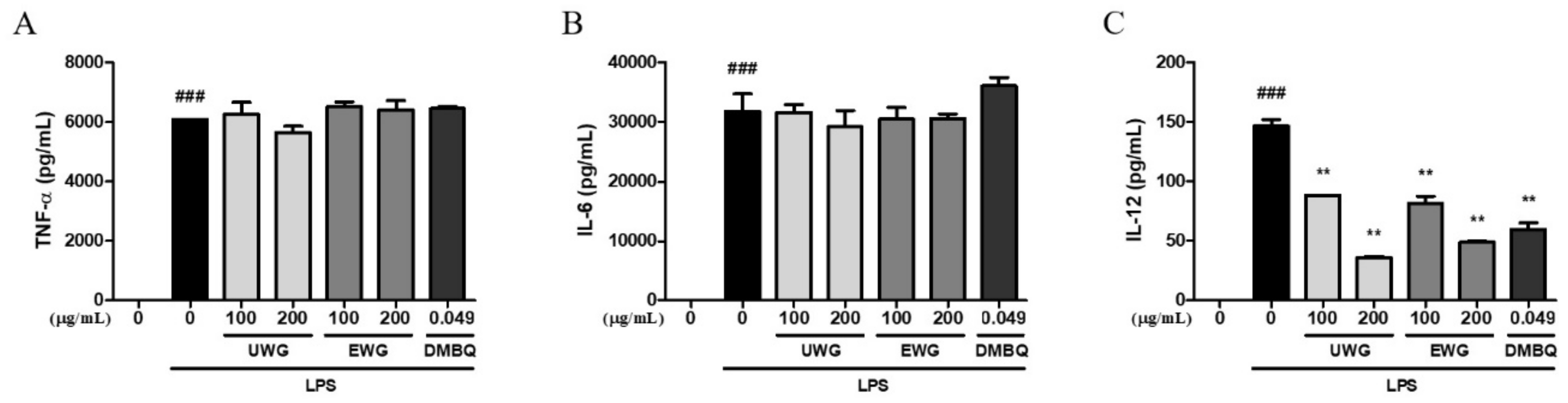
3.4. Effects of UWG and EWG on Pro-Inflammatory Cytokine Secretion
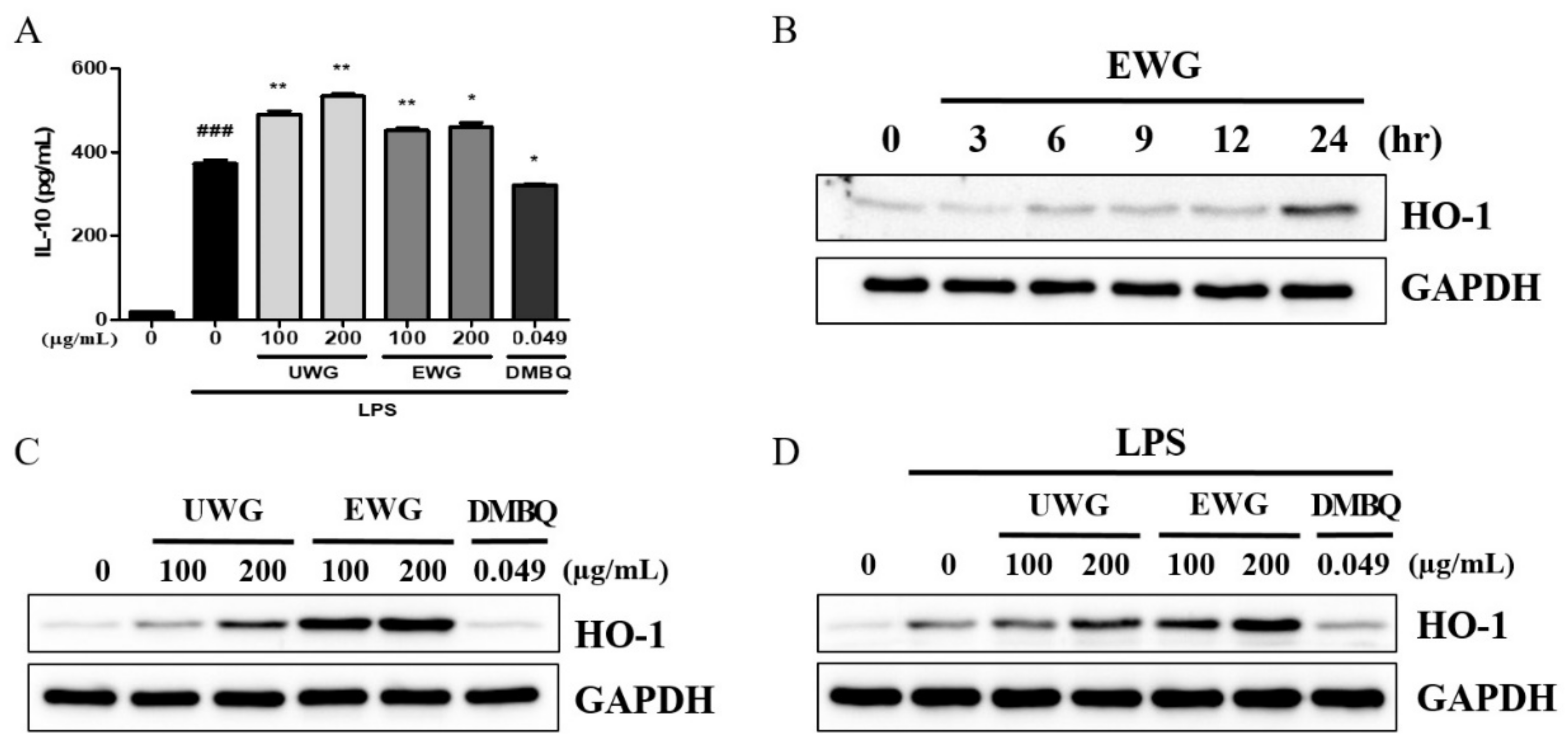
3.5. Effects of UWG and EWG on Anti-Inflammatory Protein Expression
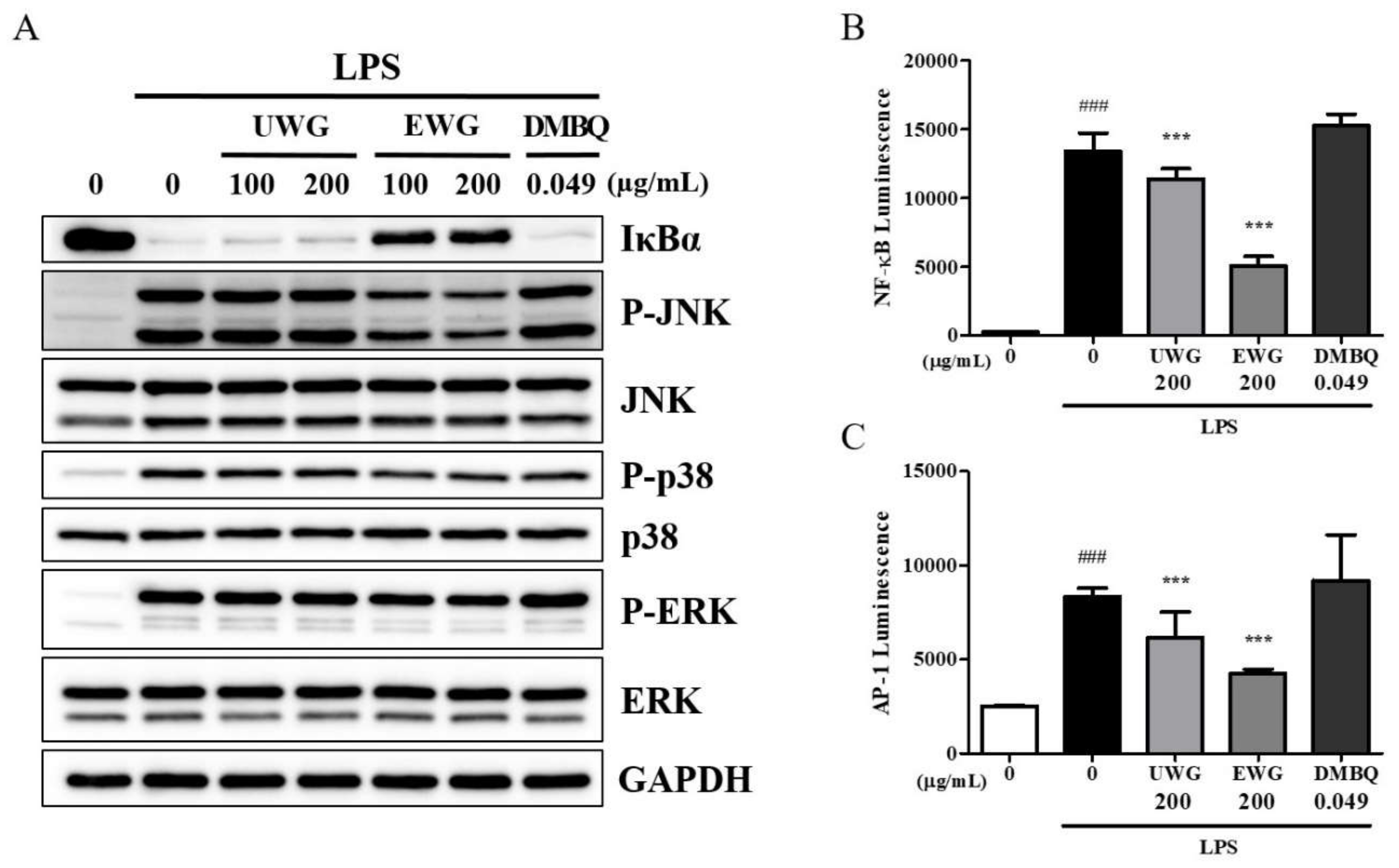
3.6. Effects of UWG and EWG on LPS-Induced Signaling Molecules
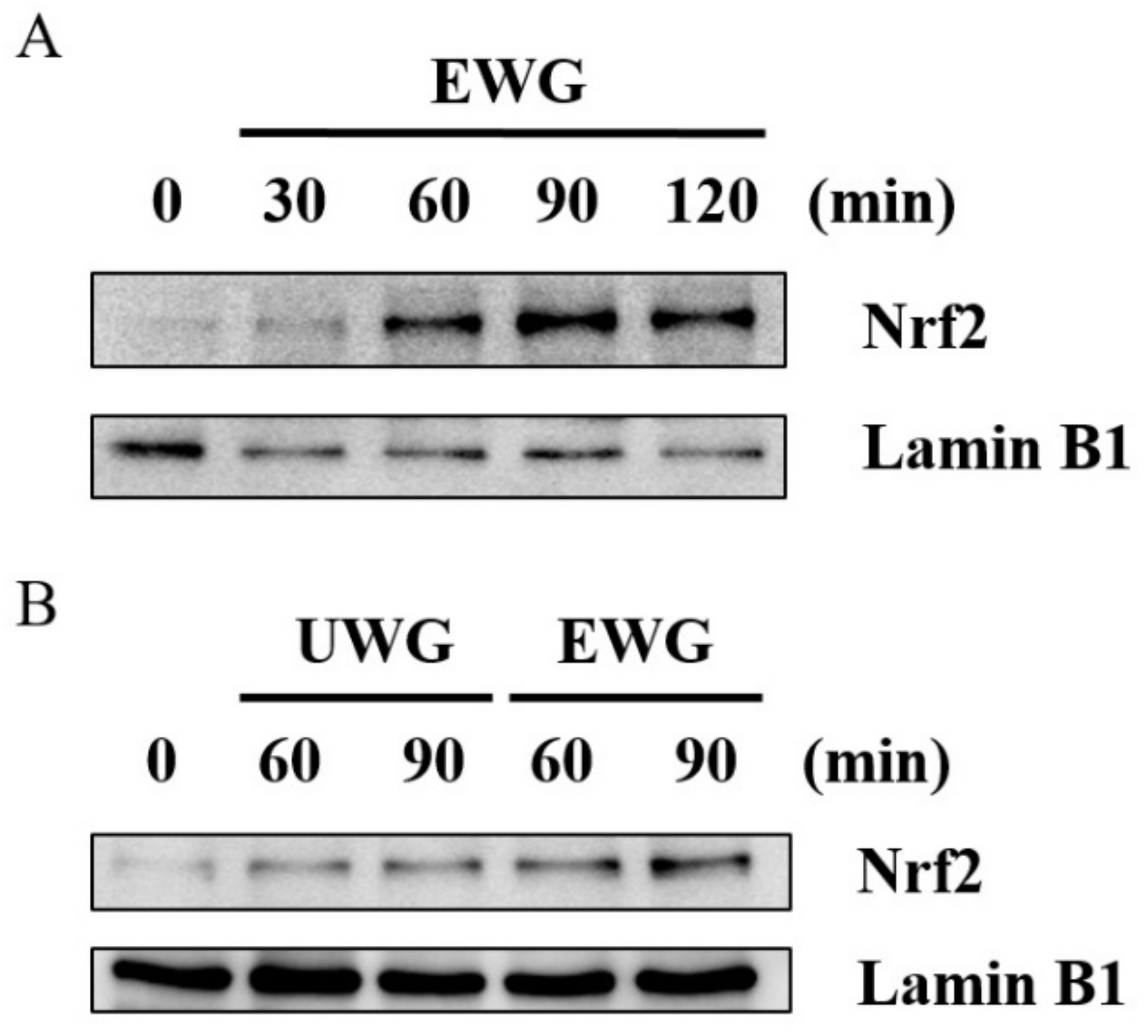
3.7. Effects of UWG and EWG on the Nuclear Translocation of Nrf2
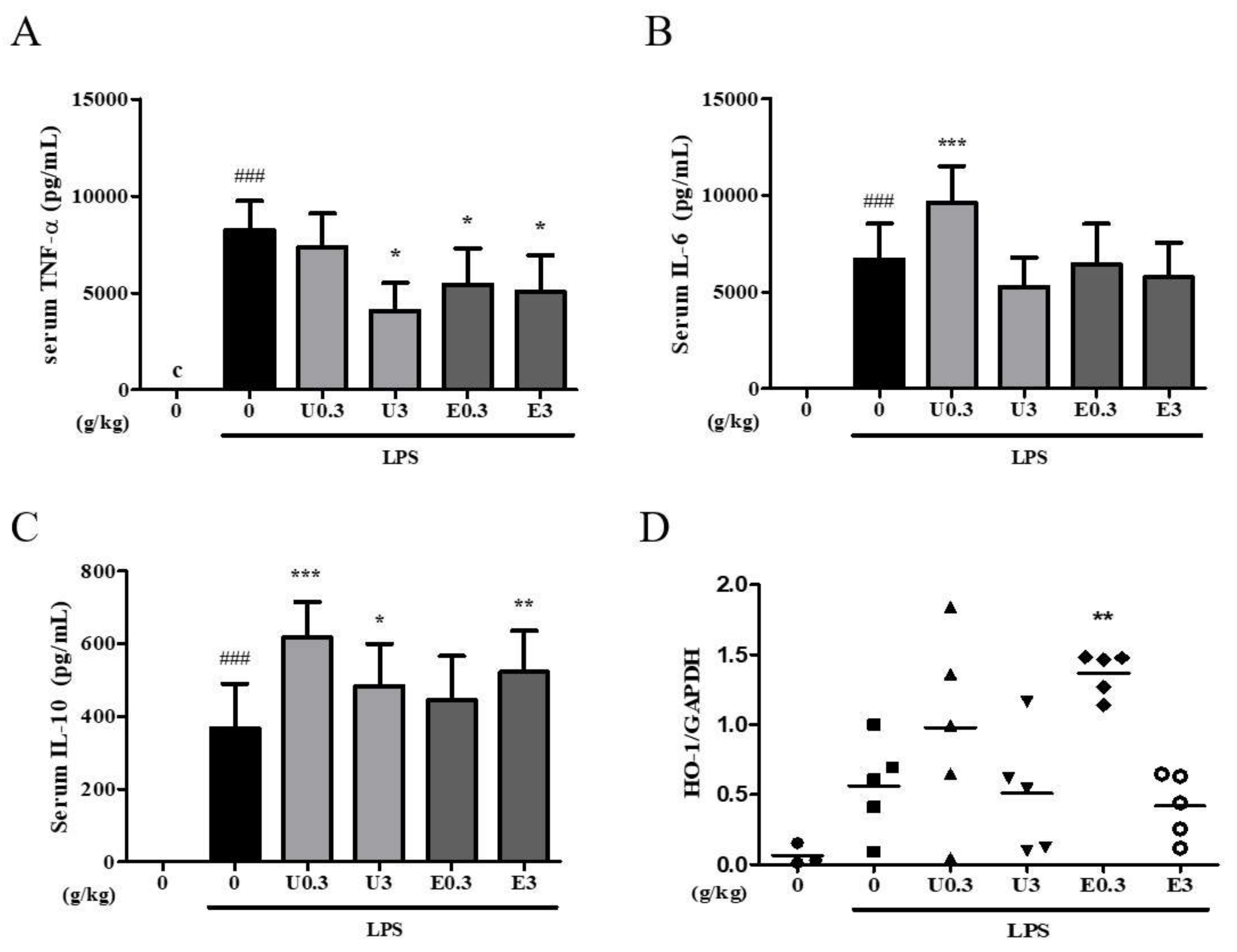
3.8. Effects of UWG and EWG on Systemic Response Following Intraperitoneal Injection of LPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, A.; Hidalgo, A. Wheat germ: Not only a by-product. Int. J. Food Sci. Nutr. 2012, 63, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; De Angelis, M.; Gobbetti, M. Effect of sourdough fermentation on stabilisation, and chemical and nutritional characteristics of wheat germ. Food Chem. 2010, 119, 1079–1089. [Google Scholar] [CrossRef]
- Hidvegi, M.; Raso, E.; Tomoskozi-Farkas, R.; Szende, B.; Paku, S.; Pronai, L.; Bocsi, J.; Lapis, K. MSC, a new benzoquinone-containing natural product with antimetastatic effect. Cancer Biother. Radiopharm. 1999, 14, 277–289. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Mueller, T.; Coda, R.; Reipsch, F.; Nionelli, L.; Curiel, J.A.; Gobbetti, M. Synthesis of 2-methoxy benzoquinone and 2,6-dimethoxybenzoquinone by selected lactic acid bacteria during sourdough fermentation of wheat germ. Microb. Cell Fact. 2013, 12, 105. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xiao, X.; Dong, Y.; Wu, J.; Yao, F.; Zhou, X.H. Effect of fermented wheat germ extract with lactobacillus plantarum dy-1 on HT-29 cell proliferation and apoptosis. J. Agric. Food Chem. 2015, 63, 2449–2457. [Google Scholar] [CrossRef]
- Demidov, L.V.; Manziuk, L.V.; Kharkevitch, G.Y.; Pirogova, N.A.; Artamonova, E.V. Adjuvant fermented wheat germ extract (Avemar) nutraceutical improves survival of high-risk skin melanoma patients: A randomized, pilot, phase II clinical study with a 7-year follow-up. Cancer Biother. Radiopharm. 2008, 23, 477–482. [Google Scholar] [CrossRef]
- Mueller, T.; Jordan, K.; Voigt, W. Promising cytotoxic activity profile of fermented wheat germ extract (Avemar(R)) in human cancer cell lines. J. Exp. Clin. Cancer Res. 2011, 30, 42. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xiao, X.; Dong, Y.; Wu, J.; Zhou, X.H. Antitumor Activities and Apoptosis-regulated Mechanisms of Fermented Wheat Germ Extract in the Transplantation Tumor Model of Human HT-29 Cells in Nude Mice. Biomed. Environ. Sci. 2015, 28, 718–727. [Google Scholar]
- Telekes, A.; Hegedus, M.; Chae, C.H.; Vekey, K. Avemar (wheat germ extract) in cancer prevention and treatment. Nutr. Cancer 2009, 61, 891–899. [Google Scholar] [CrossRef]
- Otto, C.; Hahlbrock, T.; Eich, K.; Karaaslan, F.; Jurgens, C.; Germer, C.T.; Wiegering, A.; Kammerer, U. Antiproliferative and antimetabolic effects behind the anticancer property of fermented wheat germ extract. BMC Complement. Altern. Med. 2016, 16, 160. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide synthase in innate and adaptive immunity: An update. Trends Immunol. 2015, 36, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Chau, L.Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002, 8, 240–246. [Google Scholar] [CrossRef]
- Kang, H.; Lee, M.G.; Lee, J.K.; Choi, Y.H.; Choi, Y.S. Enzymatically-Processed Wheat Bran Enhances Macrophage Activity and Has in Vivo Anti-Inflammatory Effects in Mice. Nutrients 2016, 8, 188. [Google Scholar] [CrossRef]
- Justo, M.L.; Claro, C.; Zeyda, M.; Stulnig, T.M.; Herrera, M.D.; Rodriguez-Rodriguez, R. Rice bran prevents high-fat diet-induced inflammation and macrophage content in adipose tissue. Eur. J. Nutr. 2016, 55, 2011–2019. [Google Scholar] [CrossRef]
- Giriwono, P.E.; Shirakawa, H.; Hokazono, H.; Goto, T.; Komai, M. Fermented barley extract supplementation maintained antioxidative defense suppressing lipopolysaccharide-induced inflammatory liver injury in rats. Biosci. Biotechnol. Biochem. 2011, 75, 1971–1976. [Google Scholar] [CrossRef]
- Lordan, R.; O′Keeffe, E.; Dowling, D.; Mullally, M.; Hefferman, H.; Tsoupras, A.; Zabetakis, I. The in vitro antithrombotic properties of ale, lager, and stout beers. Food Biosci. 2019, 28, 83–88. [Google Scholar] [CrossRef]
- Lordan, R.; O'Keeffe, E.; Tsoupras, A.; Zabetakis, I. Total, neutral, and polar lipids of brewing ingredients, by-products and beer: Evaluation of antithrombotic activities. Foods 2019, 8, 171. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Choi, Y.S.; Lee, J.K.; Lee, B.J.; Kim, W.K.; Kang, H. Anti-Inflammatory Activity of Citric Acid-Treated Wheat Germ Extract in Lipopolysaccharide-Stimulated Macrophages. Nutrients 2017, 9, 730. [Google Scholar] [CrossRef]
- Sorensen, H.R.; Meyer, A.S.; Pedersen, S. Enzymatic hydrolysis of water-soluble wheat arabinoxylan. 1. Synergy between alpha-L-arabinofuranosidases, endo-1,4-beta-xylanases, and beta-xylosidase activities. Biotechnol. Bioeng. 2003, 81, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Comin-Anduix, B.; Boros, L.G.; Marin, S.; Boren, J.; Callol-Massot, C.; Centelles, J.J.; Torres, J.L.; Agell, N.; Bassilian, S.; Cascante, M. Fermented wheat germ extract inhibits glycolysis/pentose cycle enzymes and induces apoptosis through poly(ADP-ribose) polymerase activation in Jurkat T-cell leukemia tumor cells. J. Biol. Chem. 2002, 277, 46408–46414. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Scarfi, S.; Benatti, U.; Giovine, M. Interference in MTT cell viability assay in activated macrophage cell line. Anal. Biochem. 2003, 313, 338–341. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Hidvegi, M.; Raso, E.; Tomoskozi Farkas, R.; Lapis, K.; Szende, B. Effect of MSC on the immune response of mice. Immunopharmacology 1999, 41, 183–186. [Google Scholar] [CrossRef]
- Lin, H.Y.; Juan, S.H.; Shen, S.C.; Hsu, F.L.; Chen, Y.C. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochem. Pharmacol. 2003, 66, 1821–1832. [Google Scholar] [CrossRef]
- Wang, J.; Mazza, G. Effects of anthocyanins and other phenolic compounds on the production of tumor necrosis factor alpha in LPS/IFN-gamma-activated RAW 264.7 macrophages. J. Agric. Food Chem. 2002, 50, 4183–4189. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Wang, Z.; Yu, S.; Long, T.; Zhou, X.; Bao, Y. Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci. Rep. 2017, 7, 44822. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in Research on Immunoregulation of Macrophages by Plant Polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Jeong, H.-Y.; Lee, J.-K.; Choi, Y.-S.; Kim, D.-O.; Jang, D.; Park, C.-S.; Maeng, S.; Kang, H. Enzyme Treatment Alters the Anti-Inflammatory Activity of the Water Extract of Wheat Germ In Vitro and In Vivo. Nutrients 2019, 11, 2490. https://doi.org/10.3390/nu11102490
Song Y, Jeong H-Y, Lee J-K, Choi Y-S, Kim D-O, Jang D, Park C-S, Maeng S, Kang H. Enzyme Treatment Alters the Anti-Inflammatory Activity of the Water Extract of Wheat Germ In Vitro and In Vivo. Nutrients. 2019; 11(10):2490. https://doi.org/10.3390/nu11102490
Chicago/Turabian StyleSong, Youngju, Hee-Young Jeong, Jae-Kang Lee, Yong-Seok Choi, Dae-Ok Kim, Davin Jang, Cheon-Seok Park, Sungho Maeng, and Hee Kang. 2019. "Enzyme Treatment Alters the Anti-Inflammatory Activity of the Water Extract of Wheat Germ In Vitro and In Vivo" Nutrients 11, no. 10: 2490. https://doi.org/10.3390/nu11102490
APA StyleSong, Y., Jeong, H.-Y., Lee, J.-K., Choi, Y.-S., Kim, D.-O., Jang, D., Park, C.-S., Maeng, S., & Kang, H. (2019). Enzyme Treatment Alters the Anti-Inflammatory Activity of the Water Extract of Wheat Germ In Vitro and In Vivo. Nutrients, 11(10), 2490. https://doi.org/10.3390/nu11102490




