Statistical Approaches in the Studies Assessing Associations between Human Milk Immune Composition and Allergic Diseases: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
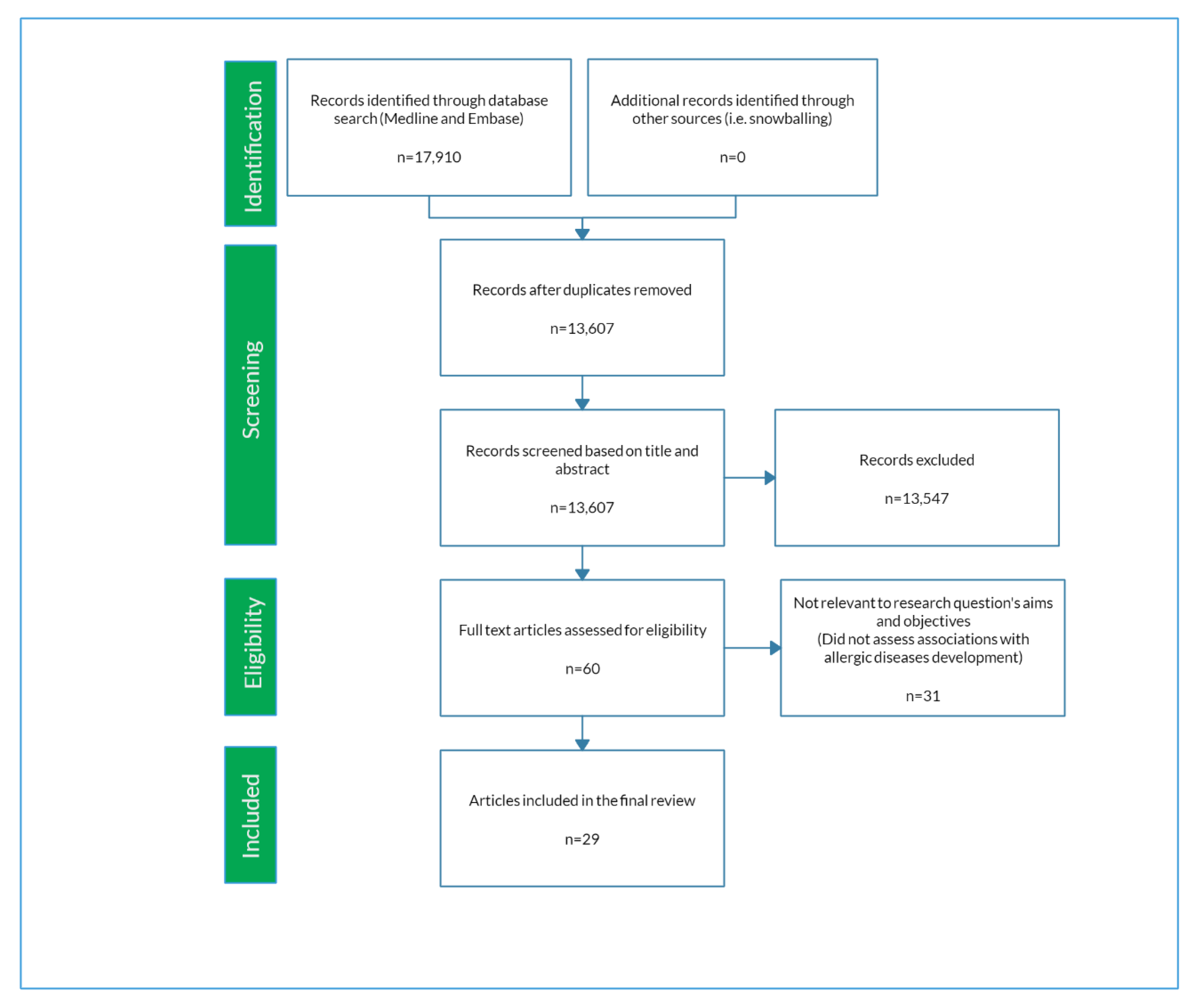
2.2. Eligibility Criteria and Selection of Articles
2.3. Data Extraction
3. Results
3.1. Synthesis
3.2. Sample Collection and Analysis
3.3. Statistical Methods and Confounders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Asher, M.I.; Montefort, S.; Bjorksten, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H.; The ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: Isaac phases one and three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Williams, H.; Robertson, C.; Stewart, A.; Ait-Khaled, N.; Anabwani, G.; Anderson, R.; Asher, I.; Beasley, R.; Bjorksten, B.; Burr, M.; et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the international study of asthma and allergies in childhood. J. Allergy Clin. Immunol. 1999, 103, 125–138. [Google Scholar] [CrossRef]
- Kung, S.J.; Steenhoff, A.P.; Gray, C. Food allergy in Africa: Myth or reality? Clin. Rev. Allergy Immunol. 2014, 46, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Bahl, R.; Barros, A.J.; Franca, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Lodge, C.J.; Tan, D.J.; Lau, M.X.; Dai, X.; Tham, R.; Lowe, A.J.; Bowatte, G.; Allen, K.J.; Dharmage, S.C. Breastfeeding and asthma and allergies: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Scaloni, A.; Zolla, L. Human milk proteins: An interactomics and updated functional overview. J. Proteome Res. 2010, 9, 3339–3373. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Karmaus, W.; Davis, S.; Gangur, V. Immune markers in breast milk and fetal and maternal body fluids: A systematic review of perinatal concentrations. J. Hum. Lact. 2011, 27, 171–186. [Google Scholar] [CrossRef]
- Munblit, D.; Peroni, D.G.; Boix-Amoros, A.; Hsu, P.S.; Land, B.V.; Gay, M.C.L.; Kolotilina, A.; Skevaki, C.; Boyle, R.J.; Collado, M.C.; et al. Human milk and allergic diseases: An unsolved puzzle. Nutrients 2017, 9, 894. [Google Scholar] [CrossRef]
- Seppo, A.E.; Kukkonen, A.K.; Kuitunen, M.; Savilahti, E.; Yonemitsu, C.; Bode, L.; Jarvinen, K.M. Association of maternal probiotic supplementation with human milk oligosaccharide composition. JAMA Pediatr. 2019, 173, 286–288. [Google Scholar] [CrossRef]
- Peroni, D.G.; Pescollderungg, L.; Piacentini, G.L.; Rigotti, E.; Maselli, M.; Watschinger, K.; Piazza, M.; Pigozzi, R.; Boner, A.L. Immune regulatory cytokines in the milk of lactating women from farming and urban environments. Pediatr. Allergy Immunol. 2010, 21, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Holmlund, U.; Amoudruz, P.; Johansson, M.A.; Haileselassie, Y.; Ongoiba, A.; Kayentao, K.; Traore, B.; Doumbo, S.; Schollin, J.; Doumbo, O.; et al. Maternal country of origin, breast milk characteristics and potential influences on immunity in offspring. Clin. Exp. Immunol. 2010, 162, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Boix-Amoros, A.; Collado, M.C.; Van’t Land, B.; Calvert, A.; Le Doare, K.; Garssen, J.; Hanna, H.; Khaleva, E.; Peroni, D.G.; Geddes, D.T.; et al. Reviewing the evidence on breast milk composition and immunological outcomes. Nutr. Rev. 2019, 77, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Khaleva, E.; Gridneva, Z.; Geddes, D.T.; Oddy, W.H.; Colicino, S.; Blyuss, O.; Boyle, R.J.; Warner, J.O.; Munblit, D. Transforming growth factor beta in human milk and allergic outcomes in children: A systematic review. Clin. Exp. Allergy 2019, 49, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Waidyatillake, N.T.; Dharmage, S.C.; Allen, K.J.; Lodge, C.J.; Simpson, J.A.; Bowatte, G.; Abramson, M.J.; Lowe, A.J. Association of breast milk fatty acids with allergic disease outcomes-a systematic review. Allergy 2018, 73, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Doherty, A.M.; Lodge, C.J.; Dharmage, S.C.; Dai, X.; Bode, L.; Lowe, A.J. Human milk oligosaccharides and associations with immune-mediated disease and infection in childhood: A systematic review. Front. Pediatr. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Silberzahn, R.; Uhlmann, E.L. Crowdsourced research: Many hands make tight work. Nature 2015, 526, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Silberzahn, R.; Uhlmann, E.L.; Martin, D.P.; Anselmi, P.; Aust, F.; Awtrey, E.; Bahník, Š.; Bai, F.; Bannard, C.; Bonnier, E.; et al. Many analysts, one data set: Making transparent how variations in analytic choices affect results. Adv. Methods Pract. Psychol. Sci. 2018, 1, 337–356. [Google Scholar] [CrossRef]
- Drescher, C.W.; Shah, C.; Thorpe, J.; O’Briant, K.; Anderson, G.L.; Berg, C.D.; Urban, N.; McIntosh, M.W. Longitudinal screening algorithm that incorporates change over time in ca125 levels identifies ovarian cancer earlier than a single-threshold rule. J. Clin. Oncol. 2013, 31, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, K.M.; Vaarala, O.; Klemetti, P.; Savilahti, E. Transforming growth factor-beta1 in mothers’ colostrum and immune responses to cows’ milk proteins in infants with cows’ milk allergy. J. Allergy Clin. Immunol. 1999, 104, 1093–1098. [Google Scholar] [CrossRef]
- Böttcher, M.F.; Jenmalm, M.C.; Björkstén, B. Cytokine, chemokine and secretory iga levels in human milk in relation to atopic disease and iga production in infants. Pediatr. Allergy Immunol. 2003, 14, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Savilahti, E.; Siltanen, M.; Kajosaari, M.; Vaarala, O.; Saarinen, K.M. Iga antibodies, tgf-beta1 and -beta2, and soluble cd14 in the colostrum and development of atopy by age 4. Pediatr. Res. 2005, 58, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, E.; Piacentini, G.L.; Ress, M.; Pigozzi, R.; Boner, A.L.; Peroni, D.G. Transforming growth factor-1 and interleukin-10 in breast milk and development of atopic diseases in infants. Clin. Exp. Allergy 2006, 36, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, M.F.; Abrahamsson, T.R.; Fredriksson, M.; Jakobsson, T.; Björkstén, B. Low breast milk tgf-β2 is induced by lactobacillus reuteri supplementation and associates with reduced risk of sensitization during infancy. Pediatr. Allergy Immunol. 2008, 19, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, M.; Kallio, M.J.T.; Siimes, M.A.; Savilahti, E.; Ranki, A. Serum immunoglobulin a concentration in infancy, but not human milk immunoglobulin a, is associated with subsequent atopic manifestations in children and adolescents: A 20-year prospective follow-up study. Clin. Exp. Allergy 2011, 41, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Kuitunen, M.; Kukkonen, A.K.; Savilahti, E. Impact of maternal allergy and use of probiotics during pregnancy on breast milk cytokines and food antibodies and development of allergy in children until 5 years. Int. Arch. Allergy Immunol. 2012, 159, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, S.; Shimojo, N.; Morita, Y.; Tomiita, M.; Arima, T.; Inoue, Y.; Nakaya, M.; Uehara, N.; Sato, Y.; Mori, C.; et al. Cytokine biomarker candidates in breast milk associated with the development of atopic dermatitis in 6-month-old infants. Int. Arch. Allergy Immunol. 2013, 160, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.R.; Rø, A.D.B.; Grimstad, Ø.; Johnsen, R.; Storrø, O.; Øien, T. Atopic dermatitis prevention in children following maternal probiotic supplementation does not appear to be mediated by breast milk tslp or tgf-β. Clin. Transl. Allergy 2016, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Munblit, D.; Treneva, M.; Peroni, D.G.; Colicino, S.; Chow, L.Y.; Dissanayeke, S.; Pampura, A.; Boner, A.L.; Geddes, D.T.; Boyle, R.J.; et al. Immune components in human milk are associated with early infant immunological health outcomes: A prospective three-country analysis. Nutrients 2017, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Berdi, M.; de Lauzon-Guillain, B.; Forhan, A.; Castelli, F.A.; Fenaille, F.; Charles, M.A.; Heude, B.; Junot, C.; Adel-Patient, K. Immune components of early breastmilk: Association with maternal factors and with reported food allergy in childhood. Pediatr. Allergy Immunol. 2019, 30, 107–116. [Google Scholar] [CrossRef]
- Morita, Y.; Campos-Alberto, E.; Yamaide, F.; Nakano, T.; Ohnisi, H.; Kawamoto, M.; Kawamoto, N.; Matsui, E.; Kondo, N.; Kohno, Y.; et al. Tgf-β concentration in breast milk is associated with the development of eczema in infants. Front. Pediatr. 2018, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Huurre, A.; Laitinen, K.; Rautava, S.; Korkeamaki, M.; Isolauri, E. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: A double-blind placebo-controlled study. Clin. Exp. Allergy 2008, 38, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Wickens, K.; Westcott, L.; Jung, W.; Currie, H.; Black, P.N.; Stanley, T.V.; Mitchell, E.A.; Fitzharris, P.; Siebers, R.; et al. Supplementation with lactobacillus rhamnosus or bifidobacterium lactis probiotics in pregnancy increases cord blood interferon-gamma and breast milk transforming growth factor-beta and immunoglobin a detection. Clin. Exp. Allergy 2008, 38, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Tomicic, S.; Johansson, G.; Voor, T.; Bjorksten, B.; Bottcher, M.F.; Jenmalm, M.C. Breast milk cytokine and iga composition differ in estonian and swedish mothers-relationship to microbial pressure and infant allergy. Pediatr. Res. 2010, 68, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Soto-Ramírez, N.; Boyd, K.; Zhang, H.; Gangur, V.; Goetzl, L.; Karmaus, W. Maternal serum but not breast milk il-5, il-6, and il-13 immune markers are associated with scratching among infants. Allergy Asthma Clin. Immunol. 2016, 12, 25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soto-Ramírez, N.; Karmaus, W.; Yousefi, M.; Zhang, H.; Liu, J.; Gangur, V. Maternal immune markers in serum during gestation and in breast milk and the risk of asthma-like symptoms at ages 6 and 12 months: A longitudinal study. Allergy Asthma Clin. Immunol. 2012, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Savilahti, E.; Tainio, V.M.; Salmenpera, L.; Arjomaa, P.; Kallio, M.; Perheentupa, J.; Siimes, M.A. Low colostral iga associated with cow’s milk allergy. Acta Paediatr. Scand. 1991, 80, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, K.M.; Laine, S.T.; Järvenpää, A.L.; Suomalainen, H.K. Does low IGA in human milk predispose the infant to development of cow’s milk allergy? Pediatr. Res. 2000, 48, 457–462. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hogendorf, A.; Stańczyk-Przyłuska, A.; Siniewicz-Luzeńczyk, K.; Wiszniewska, M.; Arendarczyk, J.; Banasik, M.; Fendler, W.; Kowalski, M.; Zeman, K. Is there any association between secretory IGA and lactoferrin concentration in mature human milk and food allergy in breastfed children. Med. Wieku Rozwoj. 2013, 17, 47–52. [Google Scholar] [PubMed]
- Joseph, C.L.M.; Havstad, S.; Bobbitt, K.; Woodcroft, K.; Zoratti, E.M.; Nageotte, C.; Misiak, R.; Enberg, R.; Nicholas, C.; Ezell, J.M.; et al. Transforming growth factor beta (tgfβ1) in breast milk and indicators of infant atopy in a birth cohort. Pediatr. Allergy Immunol. 2014, 25, 257–263. [Google Scholar] [CrossRef]
- Machtinger, S.; Moss, R. Cow’s milk allergy in breast-fed infants: The role of allergen and maternal secretory iga antibody. J. Allergy Clin. Immunol. 1986, 77, 341–347. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Ouwehand, A.; Arvilommi, H.; Kero, P.; Isolauri, E. Transforming growth factor-β in breast milk: A potential regulator of atopic disease at an early age. J. Allergy Clin. Immunol. 1999, 104, 1251–1257. [Google Scholar] [CrossRef]
- Ismail, I.H.; Licciardi, P.V.; Oppedisano, F.; Boyle, R.J.; Tang, M.L.K. Relationship between breast milk scd14, tgf-β1 and total iga in the first month and development of eczema during infancy. Pediatr. Allergy Immunol. 2013, 24, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, A.A.; Chawes, B.L.; Carson, C.G.; Schoos, A.M.M.; Thysen, A.H.; Waage, J.; Brix, S.; Bisgaard, H. High breast milk il-1β level is associated with reduced risk of childhood eczema. Clin. Exp. Allergy 2016, 46, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S.; Kalliomaki, M.; Isolauri, E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J. Allergy Clin. Immunol. 2002, 109, 119–121. [Google Scholar] [CrossRef]
- Oddy, W.H.; Halonen, M.; Martinez, F.D.; Lohman, I.C.; Stern, D.A.; Kurzius-Spencer, M.; Guerra, S.; Wright, A.L. Tgf-β in human milk is associated with wheeze in infancy. J. Allergy Clin. Immunol. 2003, 112, 723–728. [Google Scholar] [CrossRef]
- Snijders, B.E.; Damoiseaux, J.G.; Penders, J.; Kummeling, I.; Stelma, F.F.; van Ree, R.; van den Brandt, P.A.; Thijs, C. Cytokines and soluble cd14 in breast milk in relation with atopic manifestations in mother and infant (koala study). Clin. Exp. Allergy. 2006, 36, 1609–1615. [Google Scholar] [CrossRef]
- Orivuori, L.; Loss, G.; Roduit, C.; Dalphin, J.C.; Depner, M.; Genuneit, J.; Lauener, R.; Pekkanen, J.; Pfefferle, P.; Riedler, J.; et al. Soluble immunoglobulin a in breast milk is inversely associated with atopic dermatitis at early age: The pasture cohort study. Clin. Exp. Allergy 2014, 44, 102–112. [Google Scholar] [CrossRef]
- World Health Organization. Infant and Young Child Feeding; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Darragh, A.; Lönnerdal, B. Human milk. In Encyclopedia of Dairy Sciences, 2nd ed.; Elsevier: London, UK, 2011; pp. 581–590. [Google Scholar]
- Blyuss, O.; Burnell, M.; Ryan, A.; Gentry-Maharaj, A.; Marino, I.P.; Kalsi, J.; Manchanda, R.; Timms, J.F.; Parmar, M.; Skates, S.J.; et al. Comparison of longitudinal ca125 algorithms as a first-line screen for ovarian cancer in the general population. Clin. Cancer Res. 2018, 24, 4726–4733. [Google Scholar] [CrossRef]
- Tomasko, L.; Helms, R.W.; Snapinn, S.M. A discriminant analysis extension to mixed models. Stat. Med. 1999, 18, 1249–1260. [Google Scholar] [CrossRef]
- Slate, E.H.; Turnbull, B.W. Statistical models for longitudinal biomarkers of disease onset. Stat. Med. 2000, 19, 617–637. [Google Scholar] [CrossRef]
- Skates, S.J.; Pauler, D.K.; Jacobs, I.J. Screening based on the risk of cancer calculation from bayesian hierarchical changepoint and mixture models of longitudinal markers. J. Am. Stat. Assoc. 2001, 96, 429–439. [Google Scholar] [CrossRef]
- Mariño, I.P.; Blyuss, O.; Ryan, A.; Gentry-Maharaj, A.; Timms, J.F.; Dawnay, A.; Kalsi, J.; Jacobs, I.; Menon, U.; Zaikin, A. Change-point of multiple biomarkers in women with ovarian cancer. Biomed. Signal Process. Control 2017, 33, 169–177. [Google Scholar] [CrossRef]
- Vazquez, M.A.; Marino, I.P.; Blyuss, O.; Ryan, A.; Gentry-Maharaj, A.; Kalsi, J.; Manchanda, R.; Jacobs, I.; Menon, U.; Zaikin, A. A quantitative performance study of two automatic methods for the diagnosis of ovarian cancer. Biomed. Signal Process. Control 2018, 46, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Munblit, D.; Boyle, R.J.; Warner, J.O. Factors affecting breast milk composition and potential consequences for development of the allergic phenotype. Clin. Exp. Allergy 2015, 45, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Gyorffy, B.; Gyorffy, A.; Tulassay, Z. The problem of multiple testing and its solutions for genom-wide studies. Orv. Hetil. 2005, 146, 559–563. [Google Scholar]
| Author, Year | Country | Number of Participants | Number of HM Samples Collected | Serial Sample Collection | Number of Immune Markers Measured | Serial Analysis 1 | Dimension Reduction/ Clustering 2 |
|---|---|---|---|---|---|---|---|
| Machtinger 1986 [41] | USA | 57 | NR▪ | Yes | 1 | No | No |
| Savilahti 1991 [37] | Finland | 161 | 102 C (NR) 204 MM (2,6,9 mo) | Yes | 6 | No | No |
| Kalliomaki 1999 [42] | Finland | 47 | 43 C (NR) 38 MM (3 mo) | Yes | 2 | No | No |
| Saarinen 1999 [20] | Finland | 315 | 315 C (1–4 d) | No | 5 | N/A | No |
| Jarvinen 2000 [38] | Finland | 87 | NR MM (2w, 1, 3, 6 mo) | Yes | 1 | Yes | No |
| Rautava 2002 [45] | Finland | 62 | NR ▪▪ MM (3 mo) | No | 2 | N/A | No |
| Bottcher 2003 [21] | Sweden | 53 | 53 C (4 d) 47 MM (1 mo) | Yes | 13 | No | No |
| Oddy 2003 [46] | USA | 243 | 243 MM (2 w) | No | 4 | N/A | No |
| Savilahti 2005 [22] | Finland | 228 | 228 C (1–4 d) | No | 4 | N/A | No |
| Rigotti 2006 [23] | Italy | 22 | 22 C (3 d) 22 MM (1 mo) | Yes | 2 | No | No |
| Snijders 2006 [47] | Netherlands | 315 | 315 MM (1 mo) | No | 5 | N/A | No |
| Bottcher 2008 [24] | Sweden | 109 | 109 C (<3 d) 109 MM (1 mo) | Yes | 7 | No | No |
| Huurre 2008 [32] | Finland | Between 118 and 126 | 58 С (1 d) 68 (1 mo) | Yes | 7 | No | No |
| Prescott 2008 [33] | New Zealand | 105 | 239 MM (7d, 3, 6 mo) | Yes | 8 | No | No |
| Tomicic 2010 [34] | Estonia, Sweden | 99 | 99 C (0-4 d) 99 MM (1 mo) | Yes | 7 | No | No |
| Pesonen 2011 [25] | Finland | 169 | 169 C (5 d) 286 MM (2, 6 mo) | Yes | 1 | No | No |
| Kuitunen 2012 [26] | Finland | 364 | 364 C (0–3 d) 321 MM (3 mo) | Yes | 7 | No | No |
| Soto-Ramírez 2012 [36] | United States of America | 115 | 115 MM (1–8 w) | No | 13 | N/A | No |
| Hogendorf 2013 [39] | Poland | 84 | 84 MM (NR) | No | 1 | N/A | No |
| Ismail 2013 [43] | Australia, Malaysia, UK | 79 | 158 MM (7, 28d) | Yes | 3 | No | No |
| Ochiai 2013[27] | Japan | 98 | 98 C (4–5 d) 98 MM (1 mo) | Yes | 26 | No | No |
| Orivuori 2013 [48] | Austria, Finland, France, Germany and Switzerland | 610 | 610 MM (2 mo) | No | 2 | N/A | No |
| Joseph 2014 [40] | USA | 304 | 304 MM (1 mo) | No | 1 | N/A | No |
| Jepsen 2016 [44] | Denmark | 223 | 223 MM (1 mo) | No | 14 | N/A | Yes |
| Simpson 2016 [28] | Norway | 259 | 255 MM (10 d) 247 MM (3 mo) | Yes | 4 | No | No |
| Soto-Ramírez 2016 [35] | United States of America | 115 | 115 MM (1–8 w) | No | 13 | N/A | No |
| Munblit 2017 [29] | UK, Italy, Russia | 398 | 398 C (6 d) 153 MM (4–6 w) | Yes | 11 | No | No |
| Morita 2018 [31] | Japan | 96 | 96 C (5 d) 96 MM (1 mo) | Yes | 2 | Yes | No |
| Berdi 2019 [30] | France | 263 | 263 C (2–6 d) | No | 50 | N/A | No |
| Confounding Factors | Frequency | Reference |
|---|---|---|
| Maternal atopy | 11 | [24,28,29,30,31,35,38,44,46,47,48] |
| Child gender | 7 | [25,30,31,35,44,46,48] |
| Maternal smoking | 6 | [25,28,30,35,44,46] |
| Breastfeeding duration | 4 | [22,25,43,44] |
| Maternal age | 4 | [30,35,44,47] |
| Number of siblings | 4 | [25,28,43,47] |
| Family history of atopy | 3 | [22,25,35] |
| Site of collection | 3 | [29,30,48] |
| Exposure to other children | 2 | [44,46] |
| Maternal educational level | 2 | [30,46] |
| Mode of delivery | 2 | [43,44] |
| Probiotics | 2 | [28,47] |
| Sibling atopy | 2 | [29,30] |
| Colostrum collection time/infant age | 2 | [29,38] |
| Birth weight | 1 | [46] |
| BMI before pregnancy | 1 | [30] |
| Breastfeeding by 1 month and Transforming Growth Factor (TGF)β ratio | 1 | [31] |
| C-section | 1 | [30] |
| Gestational age | 1 | [46] |
| Household income | 1 | [44] |
| Household pets | 1 | [43] |
| Introduction of food during first year of life | 1 | [48] |
| Maternal consumption of acetaminophen during pregnancy | 1 | [35] |
| Maternal infection | 1 | [47] |
| Maternal marital status | 1 | [35] |
| Maternal race | 1 | [35] |
| Mother’s alcohol use (3rd trimester) | 1 | [44] |
| Mother’s antibiotic use (3rd trimester) | 1 | [44] |
| Na+/K+ ratios | 1 | [24] |
| Season of birth | 1 | [35] |
| Season of breast milk collection | 1 | [47] |
| Study treatment | 1 | [24] |
| Time interval between births | 1 | [47] |
| Vaginal or urinary infections during pregnancy | 1 | [35] |
| Yoghurt and antibiotic consumption during pregnancy | 1 | [43] |
| Author, Year | Univariable/MultivariableAnalysis | Statistical Method of HM Marker/Outcome Assessment | Confounders 1 | Association Reporting | Discrimination Analysis 2Yes/No | Classification Measures 3 |
|---|---|---|---|---|---|---|
| Machtinger 1986 [41] | Univariable | Chi-squared, Fisher’s | No | Proportions | No | No |
| Savilahti 1991 [20] | Univariable | t-test | No | Mean differences | No | No |
| Kalliomaki 1999 [42] | Univariable | Kruskal–Wallis,Mann–Whitney | No | Median differences | No | No |
| Saarinen 1999 [20] | Univariable | ANOVA | No | Mean differences | No | No |
| Jarvinen 2000 [38] | Univariable | ANOVA for repeated measurement | Yes | Mean difference | No | Yes |
| Rautava 2002 [45] | Univariable | t-test,Mann–Whitney | No | Mean difference | No | No |
| Bottcher 2003 [21] | Univariable | Chi-squared, Fisher’s, Mann–Whitney | No | Median differences | No | No |
| Oddy 2003 [46] | Multivariable | Chi-squared,multivariable logistic regression | Yes | Mean differences, Odds Ratios | No | No |
| Savilahti 2005 [22] | Multivariable | Independent samples t-test, multivariable logistic regression | Yes | Mean differences, Odds Ratios | No | No |
| Rigotti 2006 [23] | Univariable | Mann–Whitney, independent samples, t-test | No | Median differences | No | No |
| Snijders 2006 [47] | Multivariable | Multivariable logistic regression | Yes | Odds Ratios | No | No |
| Bottcher 2008 [24] | Multivariable | Logistic regression | Yes | Odds ratios | No | No |
| Huurre 2008 [32] | Univariable | Descriptive | No | Descriptive | No | No |
| Prescott 2008 [33] | Multivariable | Mann–Whitney, Chi-squared, Fisher’s, logistic regression ▪ | No | NR | No | No |
| Tomicic 2010 [34] | Univariable | Mann–Whitney | No | Median differences | No | No |
| Pesonen 2011 [25] | Multivariable | Two-tailed unpaired t-test | Yes | Mean differences | No | No |
| Kuitunen 2012 [26] | Univariable | Chi-squared, ANOVA, Mantel–Haenszel | No | Geometric means, Odds ratios | No | No |
| Soto-Ramírez 2012 [36] | Multivariable | Log-linear regression,generalized estimating equations | Yes | Risk Ratio | No | No |
| Hogendorf 2013 [39] | Univariable | Mann–Whitney | No | Median differences | No | No |
| Ismail 2013 [43] | Univariable | Mann–Whitney | Yes | Median differences | No | No |
| Ochiai 2013[27] | Multivariable | Fisher’s, t-test, MannWhitney, multivariable logistic regression | Yes | Proportions, Odds Ratios, Median differences | No | No |
| Orivuori 2013 [48] | Multivariable | Multivariable logistic regression | Yes | Odds Ratios | No | No |
| Joseph 2014 [40] | Multivariable | Logistic regression | Yes | Odds ratios | No | No |
| Jepsen 2016 [44] | Multivariable | Cox regression | Yes | Hazard ratios | No | No |
| Simpson 2016 [28] | Multivariable | Logistic regression | Yes | Odds Ratios | No | No |
| Soto-Ramírez 2016 [35] | Multivariable | Generalized estimating equations | Yes | Risk Ratio | No | No |
| Munblit 2017 [29] | Multivariable | Binomial GLmulti, LASSO | Yes | Odds Ratios | No | No |
| Morita 2018 [31] | Multivariable | Mann–Whitney, multivariable logistic regression | Yes | Odds Ratios, Median differences | No | No |
| Berdi 2019 [30] | Multivariable | Cox regression | Yes | Hazard ratios | No | No |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blyuss, O.; Cheung, K.Y.; Chen, J.; Parr, C.; Petrou, L.; Komarova, A.; Kokina, M.; Luzan, P.; Pasko, E.; Eremeeva, A.; et al. Statistical Approaches in the Studies Assessing Associations between Human Milk Immune Composition and Allergic Diseases: A Scoping Review. Nutrients 2019, 11, 2416. https://doi.org/10.3390/nu11102416
Blyuss O, Cheung KY, Chen J, Parr C, Petrou L, Komarova A, Kokina M, Luzan P, Pasko E, Eremeeva A, et al. Statistical Approaches in the Studies Assessing Associations between Human Milk Immune Composition and Allergic Diseases: A Scoping Review. Nutrients. 2019; 11(10):2416. https://doi.org/10.3390/nu11102416
Chicago/Turabian StyleBlyuss, Oleg, Ka Yan Cheung, Jessica Chen, Callum Parr, Loukia Petrou, Alina Komarova, Maria Kokina, Polina Luzan, Egor Pasko, Alina Eremeeva, and et al. 2019. "Statistical Approaches in the Studies Assessing Associations between Human Milk Immune Composition and Allergic Diseases: A Scoping Review" Nutrients 11, no. 10: 2416. https://doi.org/10.3390/nu11102416
APA StyleBlyuss, O., Cheung, K. Y., Chen, J., Parr, C., Petrou, L., Komarova, A., Kokina, M., Luzan, P., Pasko, E., Eremeeva, A., Peshko, D., Eliseev, V. I., Pedersen, S. A., Azad, M. B., Jarvinen, K. M., Peroni, D. G., Verhasselt, V., Boyle, R. J., Warner, J. O., ... Munblit, D. (2019). Statistical Approaches in the Studies Assessing Associations between Human Milk Immune Composition and Allergic Diseases: A Scoping Review. Nutrients, 11(10), 2416. https://doi.org/10.3390/nu11102416







