Severe Weight Loss and Its Association with Fatigue in Old Patients at Discharge from a Geriatric Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Recruited Patients
2.2. Anthropometric Measurements and Detection of Severe Weight Loss
2.3. Assessment of Fatigue
2.4. Data Analysis
3. Results
3.1. Participant Characteristics and Weight Loss at Hospital Discharge
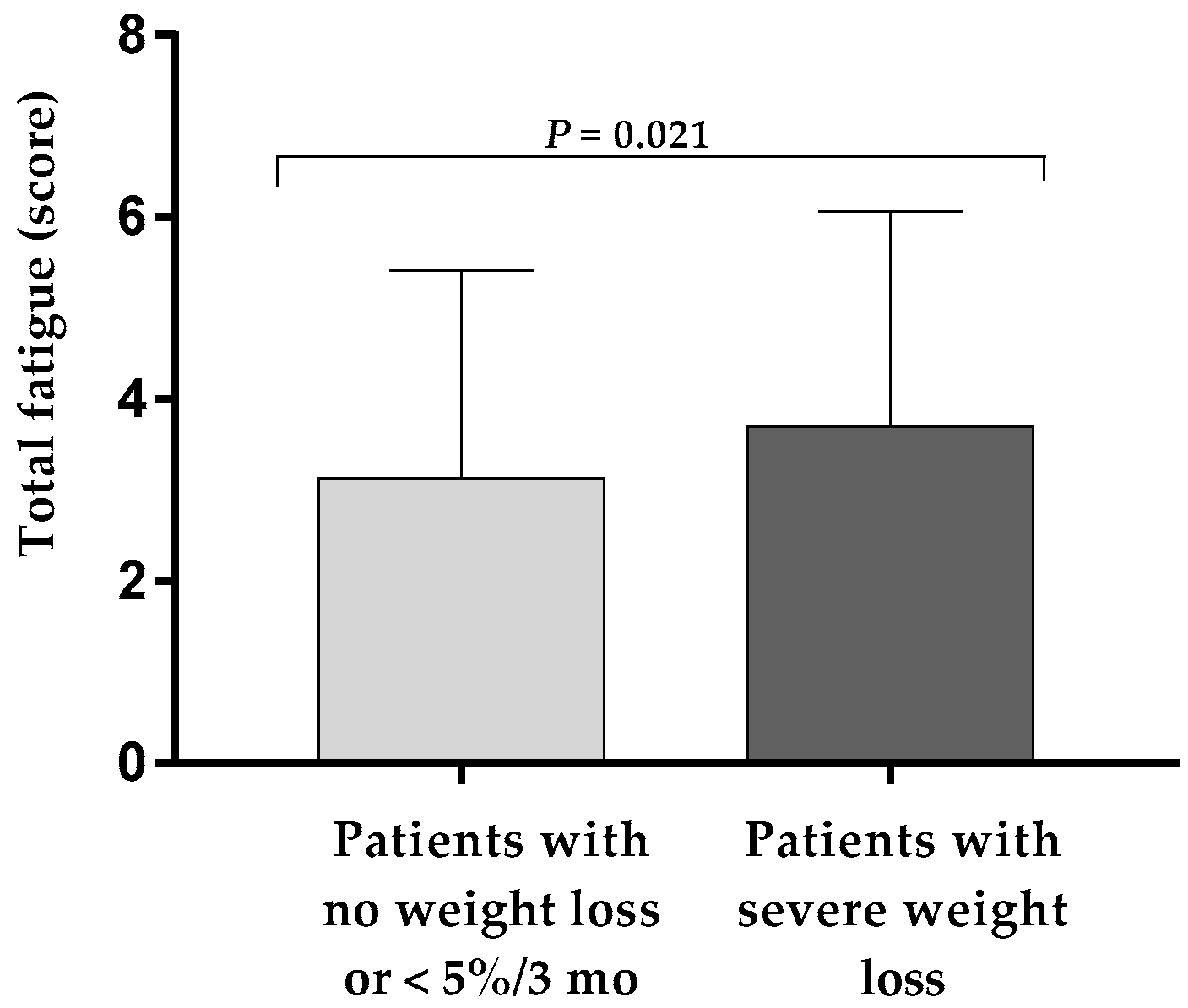
3.2. Higher Fatigue in Patients with Severe Weight Loss at Hospital Discharge
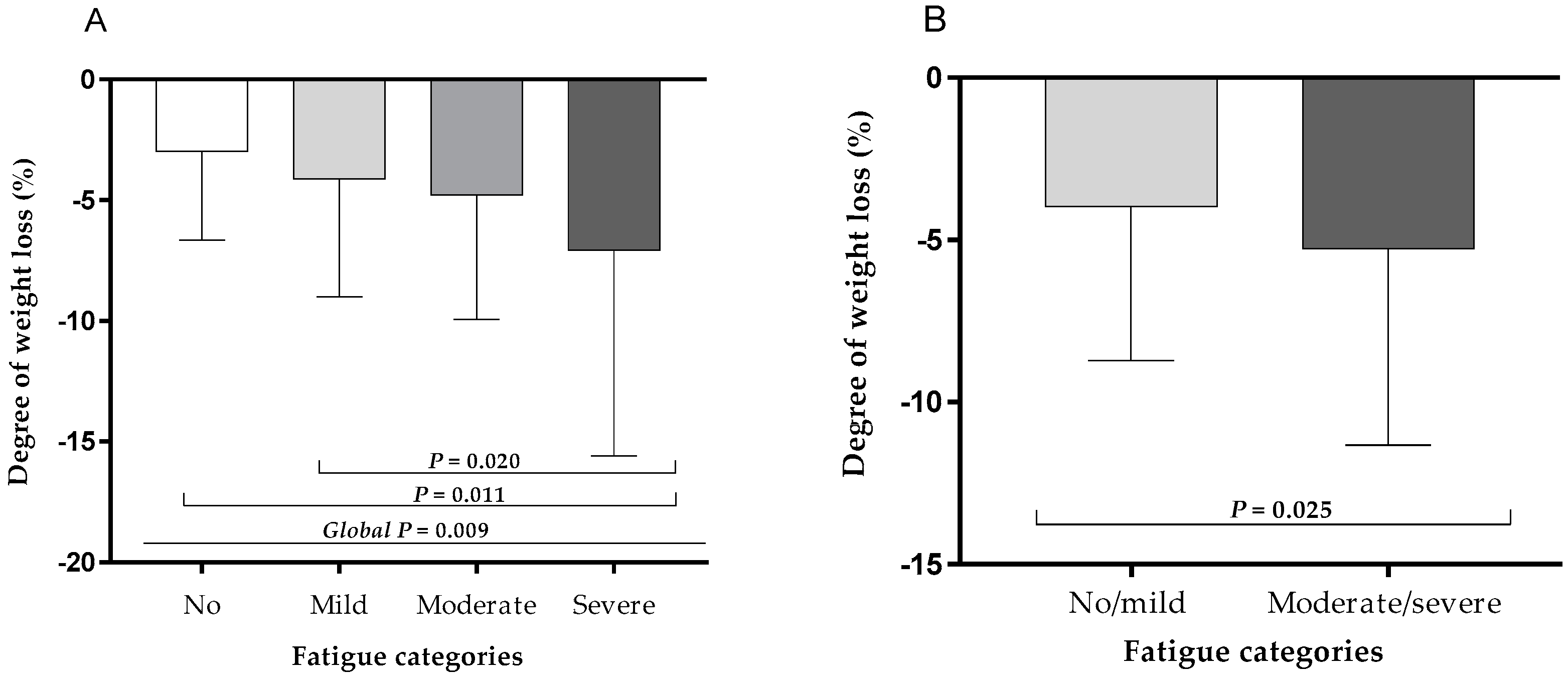
3.3. Association of Severe Weight Loss with Fatigue Severity at Hospital Discharge
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Donini, L.M.; Dominguez, L.J.; Barbagallo, M.; Savina, C.; Castellaneta, E.; Cucinotta, D.; Fiorito, A.; Inelmen, E.M.; Sergi, G.; Enzi, G.; et al. Senile anorexia in different geriatric settings in Italy. J. Nutr. Health Aging 2011, 15, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Sisto, A.; Marzetti, E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients 2016, 8, 69. [Google Scholar] [CrossRef]
- Jensen, G.L.; Mirtallo, J.; Compher, C.; Dhaliwal, R.; Forbes, A.; Grijalba, R.F.; Hardy, G.; Kondrup, J.; Labadarios, D.; Nyulasi, I.; et al. Adult starvation and disease-related malnutrition: A proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. Clin. Nutr. 2010, 29, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.; Correia, M.; Gonzalez, M.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachex. Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Ashurst, I.; Ballesteros, M.D.; Bear, D.E.; Cruz-Jentoft, A.J.; Genton, L.; Landi, F.; Laviano, A.; Norman, K.; Prado, C.M. The Underappreciated Role of Low Muscle Mass in the Management of Malnutrition. J. Am. Med Dir. Assoc. 2019, 20, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Pichard, C.; Lochs, H.; Pirlich, M. Prognostic impact of disease-related malnutrition. Clin. Nutr. 2008, 27, 5–15. [Google Scholar] [CrossRef]
- Pirlich, M.; Schütz, T.; Norman, K.; Gastell, S.; Lübke, H.J.; Bischoff, S.C.; Bolder, U.; Frieling, T.; Güldenzoph, H.; Hahn, K.; et al. The German hospital malnutrition study. Clin. Nutr. 2006, 25, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Kruizenga, H.M.; Van Tulder, M.W.; Seidell, J.C.; Thijs, A.; Ader, H.J.; Schueren, M.A. Effectiveness and cost-effectiveness of early screening and treatment of malnourished patients. Am. J. Clin. Nutr. 2005, 82, 1082–1089. [Google Scholar] [CrossRef]
- Correia, M.I.T.D. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin. Nutr. 2003, 22, 235–239. [Google Scholar] [CrossRef]
- Zengarini, E.; Ruggiero, C.; Pérez-Zepeda, M.U.; Hoogendijk, E.O.; Vellas, B.; Mecocci, P.; Cesari, M. Fatigue: Relevance and implications in the aging population. Exp. Gerontol. 2015, 70, 78–83. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.R.; Andrews, P.L.; Hickok, J.T.; Roscoe, J.A.; Matteson, S. Fatigue associated with cancer and its treatment. Support Care Cancer 2002, 10, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, M.; Suchner, U.; Schäpers, B.; Duerr, E.-M.; Alteheld, B.; Zwingers, T.; Stehle, P.; Zimmer, H.-G. Is glutamine deficiency the link between inflammation, malnutrition, and fatigue in cancer patients? Clin. Nutr. 2015, 34, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.M.; Ghazi, L.; Shafieesabet, M.; Shahidi, G.A.; Delbari, A.; Lokk, J. Motor, psychiatric and fatigue features associated with nutritional status and its effects on quality of life in Parkinson’s disease patients. PLoS ONE 2014, 9, e91153. [Google Scholar] [CrossRef] [PubMed]
- Moreh, E.; Jacobs, J.M.; Stessman, J. Fatigue, function, and mortality in older adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2010, 65, 887–895. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- National Research Council. Diet and Health: Implications for Reducing Chronic Disease Risk; National Academies Press: Washington, DC, USA, 1989. [Google Scholar]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Radbruch, L.; Sabatowski, R.; Elsner, F.; Everts, J.; Mendoza, T.; Cleeland, C. Validation of the German version of the brief fatigue inventory. J. Pain Symptom Manag. 2003, 25, 449–458. [Google Scholar] [CrossRef]
- Shuman-Paretsky, M.J.; Belser-Ehrlich, J.; Holtzer, R. Psychometric properties of the Brief Fatigue Inventory in community-dwelling older adults. Arch. Phys. Med. Rehabil. 2014, 95, 1533–1539. [Google Scholar] [CrossRef]
- Mendoza, T.R.; Wang, X.S.; Cleeland, C.S.; Morrissey, M.; Johnson, B.A.; Wendt, J.K.; Huber, S.L. The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer 1999, 85, 1186–1196. [Google Scholar] [CrossRef]
- Demling, R.H. Nutrition, Anabolism, and the Wound Healing Process: An Overview. Eplasty 2009, 9, e9. [Google Scholar] [PubMed]
- Vigano, A.; Donaldson, N.; Higginson, I.J.; Bruera, E.; Mahmud, S.; Suarez-Almazor, M. Quality of life and survival prediction in terminal cancer patients: A multicenter study. Cancer 2004, 101, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.H.; Liu, L.; Roberson, P.K.; Bopp, M.M.; Rees, J.C. Body Weight Change and Mortality in a Cohort of Elderly Patients Recently Discharged from the Hospital. J. Am. Geriatr. Soc. 2004, 52, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.H.; Johnson, L.E.; Bopp, M.M.; Roberson, P.K. Prognostic significance of monthly weight fluctuations among older nursing home residents. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2004, 59, M633–M639. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Haboubi, N. Assessment and management of nutrition in older people and its importance to health. Clin. Interv. Aging 2010, 5, 207–216. [Google Scholar] [PubMed]
- Gingrich, A.; Volkert, D.; Kiesswetter, E.; Thomanek, M.; Bach, S.; Sieber, C.C.; Zopf, Y. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. 2019, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Ream, E.; Richardson, A. Fatigue: A concept analysis. Int. J. Nurs. Stud. 1996, 33, 519–529. [Google Scholar] [CrossRef]
- Aaronson, L.S.; Teel, C.S.; Cassmeyer, V.; Neuberger, G.B.; Pallikkathayil, L.; Pierce, J.; Press, A.N.; Williams, P.D.; Wingate, A. Defining and measuring fatigue. Image J. Nurs. Sch. 1999, 31, 45–50. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Yang, Y.H. Relationship Between Fatigue and Nutritional Status in Patients with Cancer Undergoing Radiotherapy. J. Korean Acad. Nurs. 2003, 33, 478–487. [Google Scholar] [CrossRef]
- Schulz, K.H.; Patra, S.; Spielmann, H.; Klapdor, S.; Schluter, K.; van Eckert, S. Physical condition, nutritional status, fatigue, and quality of life in oncological out-patients. SAGE Open Med. 2017, 5, 2050312117743674. [Google Scholar] [CrossRef] [PubMed]
- Stobaus, N.; Muller, M.J.; Kupferling, S.; Schulzke, J.D.; Norman, K. Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutr. Cancer 2015, 67, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.B.; Taffet, G.E.; Horne, F.M.; Eldadah, B.A.; Ferrucci, L.; Nayfield, S.; Studenski, S. Bedside-to-Bench conference: Research agenda for idiopathic fatigue and aging. J. Am. Geriatr. Soc. 2010, 58, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.H.; Walls, R.C. Protein-energy undernutrition and the risk of mortality within six years of hospital discharge. J. Am. Coll. Nutr. 1998, 17, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, J.; Hansson, L.O.; Ortqvist, A. Short-and long-term prognosis for middle-aged and elderly patients hospitalized with community-acquired pneumonia: Impact of nutritional and inflammatory factors. Scand. J. Infect. Dis. 1995, 27, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, H.M. Post-hospital syndrome—An acquired, transient condition of generalized risk. N. Engl. J. Med. 2013, 368, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Avlund, K. Fatigue in older adults: An early indicator of the aging process? Aging Clin. Exp. Res. 2010, 22, 100–115. [Google Scholar] [CrossRef]
- Finsterer, J.; Mahjoub, S.Z. Fatigue in healthy and diseased individuals. Am. J. Hosp. Palliat. Med. 2014, 31, 562–575. [Google Scholar] [CrossRef]
| Parameter | All Patients n = 424 | Patients with No Weight Loss or < 5%/3 mo n = 284 | Patients with Severe Weight Loss n = 140 | * p |
|---|---|---|---|---|
| Age (years) | 77.9 ± 6.8 | 78.7 ± 6.6 | 76.7 ± 7.0 | 0.004 |
| Sex (men/women, %) | 40.1/59.9 | 36.9/63.1 | 45.7/54.3 | 0.084 |
| BMI (kg/m2) | 25.4 ± 5.3 | 25.8 ± 5.1 | 24.8 ± 5.7 | 0.062 |
| Activities of daily living (score) | 83.1 ± 19.3 | 84.9 ± 17.6 | 79.6 ± 22.2 | 0.01 |
| Isometric handgrip strength (kg) | 22.9 ± 8.2 | 23.2 ± 8.1 | 22.3 ± 8.4 | 0.285 |
| Gait speed (cm/s) | 69.9 ± 26.1 | 71.7 ± 26.9 | 66.7 ± 24.5 | 0.103 |
| Age ≤ 65 y | n = 16 | n = 10 | n = 6 | |
| Type of principal diagnosis (%) † | ||||
| Orthopedic | 40.0 | 44.4 | 33.3 | |
| Cardiac | 20.0 | 22.2 | 16.7 | |
| Oncologic | 20.0 | 22.2 | 16.7 | |
| Neurologic | 0 | 0 | 0 | |
| Pulmonary | 6.7 | 0 | 16.7 | |
| Gastrointestinal | 13.3 | 11.1 | 16.7 | |
| Renal | 0 | 0 | 0 | |
| Other diseases | 0 | 0 | 0 | |
| Number of comorbidities (n) | 7.6 ± 4.0 | 7.7 ± 4.1 | 7.5 ± 4.3 | |
| Number of medications (drugs/day) | 10.2 ± 3.4 | 10.8 ± 3.5 | 9.8 ± 3.5 | |
| Length of hospital stay (d) | 22.2 ± 5.3 | 21.3 ± 4.3 | 23.3 ± 7.2 | |
| Age > 65 y | n = 408 | n = 274 | n = 134 | |
| Type of principal diagnosis (%) | <0.001 | |||
| Orthopedic | 44.2 | 50.4 | 32.1 | |
| Cardiac | 16.5 | 15.8 | 17.9 | |
| Oncologic | 7.4 | 5.0 | 11.9 | |
| Neurologic | 8.6 | 10.4 | 5.2 | |
| Pulmonary | 6.9 | 6.5 | 7.5 | |
| Gastrointestinal | 6.6 | 5.4 | 9.0 | |
| Renal | 3.6 | 2.7 | 5.2 | |
| Other diseases | 6.3 | 3.8 | 11.2 | |
| Number of comorbidities (n) | 6.8 ± 3.8 | 6.7 ± 3.9 | 7.1 ± 3.5 | 0.243 |
| Number of medications (drugs/day) | 9.9 ± 3.8 | 9.4 ± 3.8 | 10.9 ± 3.6 | <0.001 |
| Length of hospital stay (d) | 19.3 ± 4.8 | 18.7 ± 4.4 | 20.1 ± 5.4 | 0.006 |
| BFI Fatigue Items | All Patients n = 424 | Patients with No Weight Loss or < 5%/3 mo n = 284 | Patients with Severe Weight Loss n = 140 | * p |
|---|---|---|---|---|
| Fatigue right now (score) | 3.9 ± 2.6 | 3.8 ± 2.5 | 4.2 ± 2.6 | 0.15 |
| Usual fatigue (score) | 4.2 ± 2.5 | 4.0 ± 2.4 | 4.5 ± 2.5 | 0.028 |
| Worst fatigue (score) | 5.2 ± 2.9 | 5.0 ± 2.9 | 5.7 ± 2.8 | 0.038 |
| Fatigue-related impairment (score) | ||||
| Activity | 3.0 ± 3.1 | 2.8 ± 3.0 | 3.4 ± 3.2 | 0.062 |
| Mood | 2.6 ± 3.0 | 2.4 ± 2.8 | 2.9 ± 3.2 | 0.111 |
| Walking ability | 3.5 ± 3.2 | 3.3 ± 3.1 | 3.9 ± 3.2 | 0.08 |
| Work | 3.2 ± 3.3 | 3.0 ± 3.2 | 3.6 ± 3.4 | 0.133 |
| Relationships with others | 2.0 ± 2.7 | 1.8 ± 2.5 | 2.3 ± 3.0 | 0.063 |
| Vitality | 2.5 ± 2.9 | 2.3 ± 2.7 | 2.9 ± 3.3 | 0.054 |
| Parameter | OR | 95 % CI | p |
|---|---|---|---|
| Mild fatigue | |||
| Weight loss in the last 3 months (%) | 1.109 | 0.975;1.262 | 0.115 |
| Moderate fatigue | |||
| Weight loss in the last 3 months (%) | 1.172 | 1.026;1.338 | 0.019 |
| Severe fatigue | |||
| Number of medications (drugs/day) | 1.22 | 1.023;1.455 | 0.027 |
| Weight loss in the last 3 months (%) | 1.209 | 1.047;1.395 | 0.01 |
| Parameter | OR | 95 % CI | p |
|---|---|---|---|
| Age (years) | 1.027 | 0.994;1.060 | 0.111 |
| Male sex * | 1.190 | 0.771;1.837 | 0.433 |
| Number of comorbidities | 0.978 | 0.920;1.040 | 0.480 |
| Number of medications (drugs/day) | 1.057 | 0.993;1.125 | 0.082 |
| BMI (kg/m2) | 1.036 | 0.995;1.080 | 0.086 |
| Self-reported severe weight loss ** | 1.651 | 1.052;2.590 | 0.029 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franz, K.; Otten, L.; Müller-Werdan, U.; Doehner, W.; Norman, K. Severe Weight Loss and Its Association with Fatigue in Old Patients at Discharge from a Geriatric Hospital. Nutrients 2019, 11, 2415. https://doi.org/10.3390/nu11102415
Franz K, Otten L, Müller-Werdan U, Doehner W, Norman K. Severe Weight Loss and Its Association with Fatigue in Old Patients at Discharge from a Geriatric Hospital. Nutrients. 2019; 11(10):2415. https://doi.org/10.3390/nu11102415
Chicago/Turabian StyleFranz, Kristina, Lindsey Otten, Ursula Müller-Werdan, Wolfram Doehner, and Kristina Norman. 2019. "Severe Weight Loss and Its Association with Fatigue in Old Patients at Discharge from a Geriatric Hospital" Nutrients 11, no. 10: 2415. https://doi.org/10.3390/nu11102415
APA StyleFranz, K., Otten, L., Müller-Werdan, U., Doehner, W., & Norman, K. (2019). Severe Weight Loss and Its Association with Fatigue in Old Patients at Discharge from a Geriatric Hospital. Nutrients, 11(10), 2415. https://doi.org/10.3390/nu11102415





