Vitamin D as a Biomarker of Ill Health among the Over-50s: A Systematic Review of Cohort Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- -
- Population: healthy people with a mean age over 50 years at study entry, who were untreated or not using pharmacotherapy; we also included populations at risk of biological/behavioral factors or showing abnormal biomarkers (except for hypertension), who were untreated, undiagnosed with specific diseases, and not taking dietary supplements or vitamins. We included articles with results relating to populations showing at baseline less than 25% of subjects with a prevalent diagnosis of a disease, or undergoing any pharmacological treatment, or taking vitamin D supplements. Articles were excluded if data on baseline health status of the cohort were missing;
- -
- Exposure: populations analyzed according to baseline levels of 25(OH)D or 1,25(OH)2D;
- -
- Controls: control groups enrolled from the same population of exposed people (for cohort studies) or as cases (for nested case-control in cohort studies);
- -
- -
- Studies: the analysis included cohort studies, panel studies, and nested case-control in cohorts reporting analyses of associations between vitamin D measured at baseline and at least one outcome measured at follow-up, or analyses of validity/reliability of biomarker/s for the prediction of healthy ageing outcomes at follow-up by means of Receiver operating characteristic (ROC) curve and Area under the curve (AUC), optimal cut-off, test-retest, split-half and parallel form, internal concordance;
- -
- Setting: any type of setting except for public and private clinics for specialist care;
- -
- Time: papers published from January 2001 to March 2019;
- -
- Language: English, Italian, Spanish.
2.2. Search Strategy
2.3. Study Records
2.4. Data Items
2.5. Outcomes Prioritization
2.6. Risk of Bias in Individual Studies
2.7. Data Synthesis
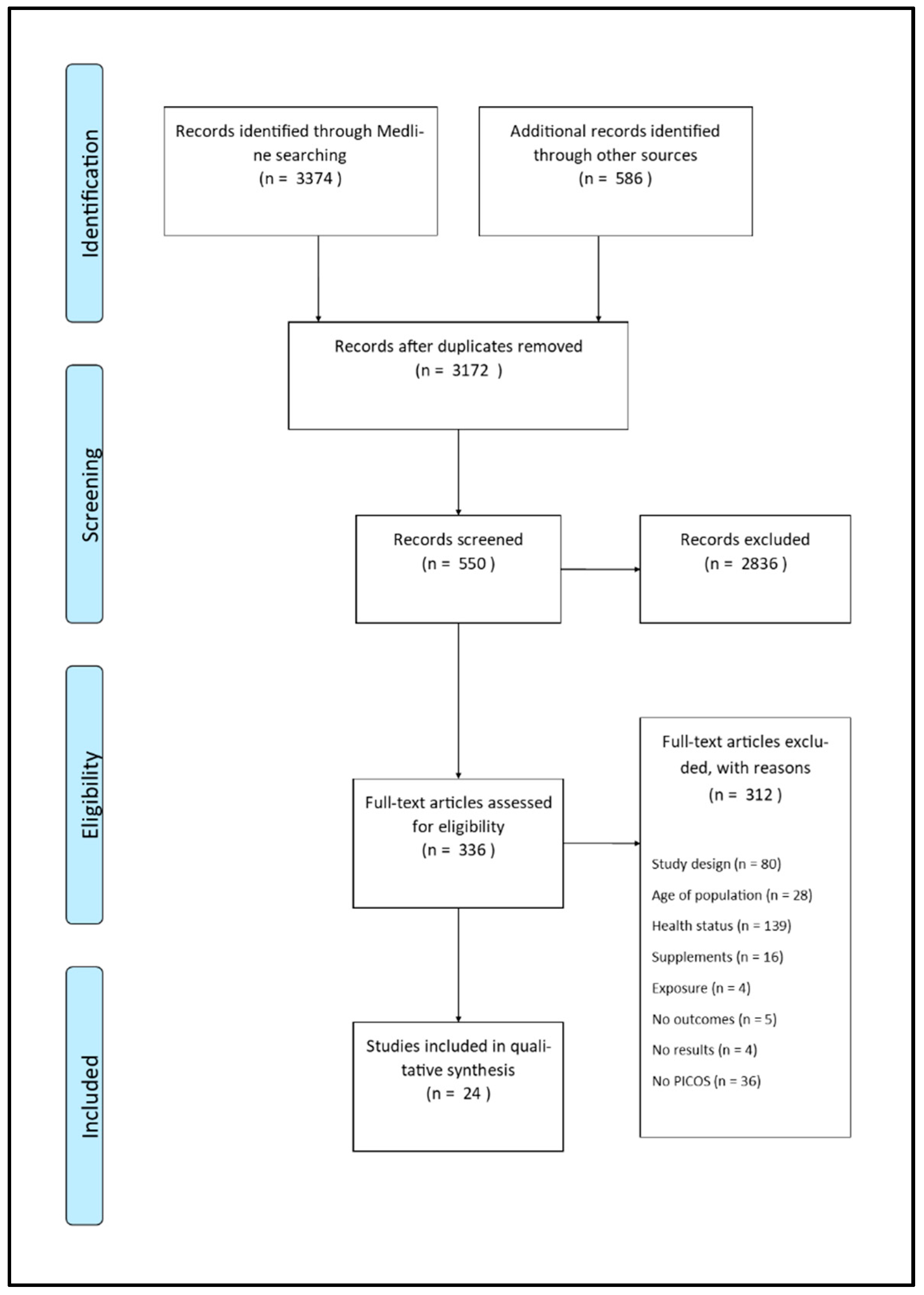
3. Results
3.1. Risk of Bias within Studies
3.2. Predictivity of 25(OH)D Levels
3.2.1. All-Cause Mortality
3.2.2. Cardiovascular, Coronary, and Cardiometabolic Events
3.2.3. Impaired Bone Health
3.2.4. Respiratory Events
3.2.5. Cancer
3.2.6. Sarcopenia
3.2.7. Neurological Diseases and Cognitive Functionality
3.2.8. Secondary Outcome
3.3. Predictivity of 1,25(OH)2D Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-H.; Cameron-Smith, D.; Wessner, B.; Franzke, B. Biomarkers of Aging: From Function to Molecular Biology. Nutrients 2016, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Pareja-Galeano, H.; Alis, R.; Sanchis-Gomar, F.; Lucia, A.; Emanuele, E. Vitamin D, precocious acute myocardial infarction, and exceptional longevity. Int. J. Cardiol. 2015, 199, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Gysemans, C.; Giulietti, A.; Bouillon, R. Vitamin D and diabetes. Diabetologia 2005, 48, 1247–1257. [Google Scholar] [CrossRef]
- Wasson, L.T.; Shimbo, D.; Rubin, M.R.; Shaffer, J.A.; Schwartz, J.E.; Davidson, K.W. Is vitamin D deficiency a risk factor for ischemic heart disease in patients with established cardiovascular disease? 10-year follow-up of the Nova Scotia Health Survey. Int. J. Cardiol. 2011, 148, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Deeg, D.J.H.; Lips, P. Low Vitamin D and High Parathyroid Hormone Levels as Determinants of Loss of Muscle Strength and Muscle Mass (Sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef]
- Grant, W.B. An estimate of the global reduction in mortality rates through doubling vitamin D levels. Eur. J. Clin. Nutr. 2011, 65, 1016–1026. [Google Scholar] [CrossRef]
- Schöttker, B.; Haug, U.; Schomburg, L.; Köhrle, J.; Perna, L.; Müller, H.; Holleczek, B.; Brenner, H. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am. J. Clin. Nutr. 2013, 97, 782–793. [Google Scholar] [CrossRef]
- Gaksch, M.; Jorde, R.; Grimnes, G.; Joakimsen, R.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B.; Njølstad, I.; Løchen, M.L.; März, W.; et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS ONE 2017, 12, e0170791. [Google Scholar] [CrossRef]
- Zhang, R.; Li, B.; Gao, X.; Tian, R.; Pan, Y.; Jiang, Y.; Gu, H.; Wang, Y.; Wang, Y.; Liu, G. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: Dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017, 105, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Marzullo, P. New Insights on Low Vitamin D Plasma Concentration as a Potential Cardiovascular Risk Factor. Open Rheumatol. J. 2018, 12 (Suppl-1, M6), 261–278. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D Deficiency and Secondary Hyperparathyroidism in the Elderly: Consequences for Bone Loss and Fractures and Therapeutic Implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Wicherts, I.S.; van Schoor, N.M.; Boeke, A.J.; Visser, M.; Deeg, D.J.; Smit, J.; Knol, D.L.; Lips, P. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007, 92, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Huang, P.; Liu, P.; Hao, Q.; Chen, S.; Dong, B.; Wang, J. Maturitas Association of vitamin D deficiency and frailty: A systematic review and meta-analysis. Maturitas 2016, 94, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Buta, B.; Choudhury, P.P.; Xue, Q.L.; Chaves, P.; Bandeen-Roche, K.; Shardell, M.; Semba, R.D.; Walston, J.; Michos, E.D.; Appel, L.J.; et al. The Association of Vitamin D Deficiency and Incident Frailty in Older Women: The Role of Cardiometabolic Diseases. J. Am. Geriatr. Soc. 2017, 65, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Groves, N.J.; McGrath, J.J.; Burne, T.H.J. Vitamin D as a Neurosteroid Affecting the Developing and Adult Brain. Annu. Rev. Nutr. 2014, 34, 117–141. [Google Scholar] [CrossRef]
- Buell, J.S.; Scott, T.M.; Dawson-Hughes, B.; Dallal, G.E.; Rosenberg, I.H.; Folstein, M.F.; Tucker, K.L. Vitamin D is associated with cognitive function in elders receiving home health services. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 888–895. [Google Scholar] [CrossRef]
- Di Somma, C.; Scarano, E.; Barrea, L.; Zhukouskaya, V.V.; Savastano, S.; Mele, C.; Scacchi, M.; Aimaretti, G.; Colao, A.; Marzullo, P. Vitamin D and Neurological Diseases: An Endocrine View. Int. J. Mol. Sci. 2017, 18, 2482. [Google Scholar] [CrossRef]
- Rosen, C.J.; Manson, J.E. Frailty: A D-ficiency syndrome of aging? J. Clin. Endocrinol. Metab. 2010, 95, 5210–5212. [Google Scholar] [CrossRef][Green Version]
- Vitamin D and Longevity (VIDAL) Trial: Randomised Feasibility Study. Available online: http://www.isrctn.com/ISRCTN46328341 (accessed on 23 September 2019).
- Meehan, M.; Penckofer, S. The Role of Vitamin D in the Aging Adult. J. Aging Gerontol. 2014, 2, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Mark, K.A.; Dumas, K.J.; Bhaumik, D.; Schilling, B.; Davis, S.; Oron, T.R.; Sorensen, D.J.; Lucanic, M.; Brem, R.B.; Melov, S.; et al. Vitamin D Promotes Protein Homeostasis and Longevity via the Stress Response Pathway Genes skn-1, ire-1, and xbp-1. Cell Rep. 2016, 17, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Iliffe, S.; Tanabe, M. Vitamin D supplementation as a potential cause of U-shaped associations between vitamin D levels and negative health outcomes: A decision tree analysis for risk of frailty. BMC Geriatr. 2017, 17, 236. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, J. The world health report 2002—Reducing risks, promoting healthy life. Educ. Health 2003, 16, 230. [Google Scholar]
- Friedman, S.M.; Mulhausen, P.; Cleveland, M.L.; Coll, P.P.; Daniel, K.M.; Hayward, A.D.; Shah, K.; Skudlarska, B.; White, H.K. Healthy Aging: American Geriatrics Society White Paper Executive Summary. J. Am. Geriatr. Soc. 2015, 67, 17–20. [Google Scholar] [CrossRef] [PubMed]
- OECD and European Union. Health at a Glance: Europe 2016. State of Health in the EU Cycle; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Knobloch, K.; Yoon, U.; Vogt, P.M. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J. Cranio-Maxillofac. Surg. 2011, 39, 91–92. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.M.E.E.G.; Mahanani, W.R.; et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef]
- Depp, C.A.; Jeste, D.V. Definitions and predictors of successful aging: A comprehensive review of larger quantitative studies. Am. J. Geriatr. Psychiatry 2006, 14, 6–20. [Google Scholar] [CrossRef]
- Kirch, W. Functional ability. In Encyclopedia of Public Healht; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Tsai, H.-J.; Chang, F.-K. Associations between body mass index, mid-arm circumference, calf circumference, and functional ability over time in an elderly Taiwanese population. PLoS ONE 2017, 12, e0175062. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.; Palese, A.; Zuttion, R.; Ferrario, B.; Ponta, S.; Hayter, M. Identifying longitudinal sustainable hierarchies in activities of daily living. Arch. Gerontol. Geriatr. 2017, 12, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Seitsamo, J.; Tuomi, K.; Martikainen, R. Activity, functional capacity and well-being in ageing Finnish workers. Occup. Med. 2007, 57, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Kuh, D.; Hardy, R.; Mortality Review Group. FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: Systematic review and meta-analysis. BMJ 2010, 341, c4467. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Goodman, S.H.; Sewell, D.R.; Cooley, E.L.; Leavitt, N. Assessing levels of adaptive functioning: The Role Functioning Scale. Community Ment. Health J. 1993, 29, 119–131. [Google Scholar] [CrossRef]
- Stanford School of Medicine. The Short Portable Mental Status Questionnaire (SPMSQ). Available online: https://geriatrics.stanford.edu/culturemed/overview/assessment/assessment_toolkit/spmsq.html (accessed on 23 September 2019).
- McFall, S. Understanding Society: UK Household Longitudinal Study: Cognitive Ability Measures; Institute for Social and Economic Research University of Essex: Colchester, Essex, UK, 2013. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Craik, F.I.M.; Bialystok, E. Cognition through the lifespan: Mechanisms of change. Trends Cogn. Sci. 2006, 10, 131–138. [Google Scholar] [CrossRef]
- OECD. Guidelines on Measuring Subjective Well-Being; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Diener, E. (Ed.) Assessing Well-Being: The Collected Works of Ed Diener; Springer: New York, NY, USA, 2009. [Google Scholar]
- Huppert, F.A.; Marks, N.; Clark, A.; Siegrist, J.; Stutzer, A.; Vittersø, J.; Wahrendorf, M. Measuring well-being across Europe: Description of the ESS Well-being Module and preliminary findings. Soc. Indic. Res. 2019, 91, 301. [Google Scholar] [CrossRef]
- Neugarten, B.L.; Havighurst, R.J.; Tobin, S.S. The measurement of life satisfaction. J. Gerontol. 1961, 16, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.J.; Murk, P.J. Life Satisfaction Index for the Third Age (LSITA): A Measurement of Successful Aging. In 2006 Midwest Research-to-Practice Conference in Adult, Continuing, and Community Education; Isaac, E.P., Ed.; University of Missouri-St. Louis: St. Louis, MO, USA, 2006; pp. 7–12. [Google Scholar]
- Radloff, L.S. The CES-D Scale A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Huppert, F.; So, T. Flourishing across Europe: Application of a new conceptual framework for defining well-being. Soc. Indic. Res. 2013, 110, 837–861. [Google Scholar] [CrossRef] [PubMed]
- Conradsson, M.; Rosendahl, E.; Littbrand, H.; Gustafson, Y.; Olofsson, B.; Lövheim, H. Usefulness of the Geriatric Depression Scale 15-item version among very old people with and without cognitive impairment. Aging Ment. Health 2013, 17, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Grundy, E.; Bowling, A. Enhancing the quality of extended life years. Identification of the oldest old with a very good and very poor quality of life. Aging Ment. Health 1999, 3, 199–212. [Google Scholar] [CrossRef]
- Baltes, M.M.; Lang, F.R. Everyday functioning and successful aging: The impact of resources. Psychol. Aging 1997, 12, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.A.; Leitzmann, M.F.; Hollis, B.W.; Willett, W.C.; Giovannucci, E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004, 15, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, S.; Rignell-Hydbom, A.; Lindh, C.H.; Jonsson, B.A.G.; Thelin, A.; Rylander, L. High levels of vitamin D associated with less ischemic heart disease—A nested case-control study among rural men in Sweden. Ann. Agric. Environ. Med. 2017, 24, 288–293. [Google Scholar] [CrossRef]
- Heath, A.K.; Williamson, E.J.; Kvaskoff, D.; Hodge, A.M.; Ebeling, P.R.; Baglietto, L.; Neale, R.E.; Giles, G.G.; Eyles, D.W.; English, D.R. 25-Hydroxyvitamin D concentration and all-cause mortality: The Melbourne Collaborative Cohort Study. Public Health Nutr. 2017, 20, 1775–1784. [Google Scholar] [CrossRef]
- Cauley, J.A.; Parimi, N.; Ensrud, K.E.; Bauer, D.C.; Cawthon, P.M.; Cummings, S.R.; Hoffman, A.R.; Shikany, J.M.; Barrett-Connor, E.; Orwoll, E. Serum 25-hydroxyvitamin D and the risk of hip and nonspine fractures in older men. J. Bone Miner. Res. 2010, 25, 545–553. [Google Scholar] [CrossRef]
- Vázquez-Oliva, G.; Zamora, A.; Ramos, R.; Subirana, I.; Grau, M.; Dégano, I.R.; Muñoz, D.; Fitó, M.; Elosua, R.; Marrugat, J. Analysis of Plasma Albumin, Vitamin D, and Apolipoproteins A and B as Predictive Coronary Risk Biomarkers in the REGICOR Study. Rev. Esp. Cardiol. 2018, 71, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Mursu, J.; Nurmi, T.; Voutilainen, S.; Tuomainen, T.-P.; Virtanen, J.K. The association between serum 25-hydroxyvitamin D3 concentration and risk of disease death in men: Modification by magnesium intake. Eur. J. Epidemiol. 2015, 30, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Kim, H.; Yoshida, H.; Shimada, H.; Suzuki, T. Serum 25-hydroxyvitamin D level and risk of falls in Japanese community-dwelling elderly women: A 1-year follow-up study. Osteoporos. Int. 2015, 26, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Hirani, V.; Cumming, R.G.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Hsu, B.; Handelsman, D.J.; Waite, L.M.; Seibel, M.J. Longitudinal Associations Between Vitamin D Metabolites and Sarcopenia in Older Australian men: The Concord Health and Aging in Men Project. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Jassal, S.K.; Chonchol, M.; von Mühlen, D.; Smits, G.; Barrett-Connor, E. Vitamin d, parathyroid hormone, and cardiovascular mortality in older adults: The Rancho Bernardo study. Am. J. Med. 2010, 123, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.M.; Srikanth, P.; Lee, C.G.; Cummings, S.R.; Jans, I.; Cauley, J.A.; Bouillon, R.; Vanderschueren, D.; Orwoll, E.S.; Nielson, C.M. Associations of 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D with Bone Mineral Density, Bone Mineral Density Change, and Incident Nonvertebral Fracture. J. Bone Miner. Res. 2015, 30, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Umehara, K.; Mukai, N.; Hata, J.; Hirakawa, Y.; Ohara, T.; Yoshida, D.; Kishimoto, H.; Kitazono, T.; Hoka, S.; Kiyohara, Y.; et al. Association Between Serum Vitamin D and All-Cause and Cause-Specific Death in a General Japanese Population—The Hisayama Study. Circ. J. 2017, 81, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Looker, A.C. Serum 25-hydroxyvitamin D and risk of major osteoporotic fractures in older U.S. adults. J. Bone Miner. Res. 2013, 28, 997–1006. [Google Scholar] [CrossRef]
- Arabi, A.; Baddoura, R.; El-Rassi, R.; Fuleihan, G.E.-H. PTH level but not 25 (OH) vitamin D level predicts bone loss rates in the elderly. Osteoporos. Int. 2012, 23, 971–980. [Google Scholar] [CrossRef]
- Khaw, K.-T.; Lubetz, R.; Wareham, N. Serum 25-hydroxyvitamin D, mortality, and incident cardiovascular disease, respiratory disease, cancers, and fractures: A 13-y prospective population study. Am. J. Clin. Nutr. 2014, 100, 1361–1370. [Google Scholar] [CrossRef]
- Liu, L.; Chen, M.; Hankins, S.R.; Nùñez, A.E.; Watson, R.A.; Weinstock, P.J.; Newschaffer, C.J.; Eisen, H.J. Drexel Cardiovascular Health Collaborative Education, Research, and Evaluation Group. Serum 25-hydroxyvitamin D concentration and mortality from heart failure and cardiovascular disease, and premature mortality from all-cause in United States adults. Am. J. Cardiol. 2012, 110, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vidal, P.; Vollenweider, P.; Guessous, I.; Henry, H.; Boulat, O.; Waeber, G.; Jornayvaz, F.R. Serum Vitamin D Concentrations Are Not Associated with Insulin Resistance in Swiss Adults. J. Nutr. 2015, 145, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Laughlin, G.A.; Li, H.; Nielson, C.M.; Wang, P.Y.; Dam, T.T.; Cauley, J.A.; Ensrud, K.E.; Stefanick, M.L.; Lau, E.; et al. The association of concurrent vitamin D and sex hormone deficiency with bone loss and fracture risk in older men: The osteoporotic fractures in men (MrOS) study. J. Bone Miner. Res. 2012, 27, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A.; Greendale, G.A.; Ruppert, K.; Lian, Y.; Randolph, J.F., Jr.; Lo, J.C.; Burnett-Bowie, S.A.; Finkelstein, J.S. Serum 25 Hydroxyvitamin D, Bone Mineral Density and Fracture Risk Across the Menopause. J. Clin. Endocrinol. Metab. 2015, 100, 2046–2054. [Google Scholar] [CrossRef][Green Version]
- Afzal, S.; Lange, P.; Bojesen, S.E.; Freiberg, J.J.; Nordestgaard, B.G. Plasma 25-hydroxyvitamin D, lung function and risk of chronic obstructive pulmonary disease. Thorax 2014, 69, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Brondum-Jacobsen, P.; Benn, M.; Jensen, G.B.; Nordestgaard, B.G. 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: Population-based study and meta-analyses of 18 and 17 studies. Arter. Thromb. Vasc. Biol. 2012, 32, 2794–2802. [Google Scholar] [CrossRef] [PubMed]
- Brondum-Jacobsen, P.; Benn, M.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. 25-Hydroxyvitamin D concentrations and risk of venous thromboembolism in the general population with 18,791 participants. J. Thromb. Haemost. 2013, 11, 423–431. [Google Scholar] [CrossRef]
- Lu, L.; Wu, Y.; Qi, Q.; Liu, C.; Gan, W.; Zhu, J.; Li, H.; Lin, X. Associations of Type 2 Diabetes with Common Variants in PPARD and the Modifying Effect of Vitamin D among Middle-Aged and Elderly Chinese. PLoS ONE 2012, 7, e34895. [Google Scholar] [CrossRef]
- Al-Khalidi, B.; Kuk, J.L.; Ardern, C.I. Lifetime risk of cardiometabolic mortality according to vitamin D status of middle and older-aged adults: NHANES III mortality follow-up. J. Steroid Biochem. Mol. Biol. 2019, 186, 34–41. [Google Scholar] [CrossRef]
- Aregbesola, A.; Voutilainen, S.; Nurmi, T.; Virtanen, J.K.; Ronkainen, K.; Tuomainen, T. Serum 25-hydroxyvitamin D3 and the risk of pneumonia in an ageing general population. J. Epidemiol. Community Health 2013, 67, 533–536. [Google Scholar] [CrossRef]
- Afzal, S.; Nordestgaard, B.G.; Bojesen, S.E. Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: A prospective cohort study. J. Investig. Dermatol. 2013, 133, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Byberg, L.; Karlström, B.; Cederholm, T.; Melhus, H.; Sjögren, P.; Kilander, L. Vitamin D is not associated with incident dementia or cognitive impairment: An 18-y follow-up study in community-living old men. Am. J. Clin. Nutr. 2017, 105, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Licher, S.; de Bruijn, R.F.A.G.; Wolters, F.J.; Zillikens, M.C.; Ikram, M.A.K.; Ikram, M.A.K. Vitamin D and the Risk of Dementia: The Rotterdam Study. J. Alzheimers Dis. 2017, 60, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Rush, L.; McCartney, G.; Walsh, D.; MacKay, D. Vitamin D and subsequent all-age and premature mortality: A systematic review. BMC Public Health 2013, 13, 679. [Google Scholar] [CrossRef] [PubMed]
- Schottker, B.; Ball, D.; Gellert, C.; Brenner, H. Serum 25-hydroxyvitamin D levels and overall mortality. A systematic review and meta-analysis of prospective cohort studies. Ageing Res. Rev. 2013, 12, 708–718. [Google Scholar] [CrossRef]
- Grandi, N.C.; Breitling, L.P.; Brenner, H. Vitamin D and cardiovascular disease: Systematic review and meta-analysis of prospective studies. Prev. Med. 2010, 51, 228–233. [Google Scholar] [CrossRef]
- Pittas, A.G.; Sun, Q.; Manson, J.E.; Dawson-Hughes, B.; Hu, F.B. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care 2010, 33, 2021–2023. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; Hashmi, O.; Dutton, D.; Mavrodaris, A.; Stranges, S.; Kandala, N.B.; Clarke, A.; Franco, O.H. Levels of vitamin D and cardiometabolic disorders: Systematic review and meta-analysis. Maturitas 2010, 65, 225–236. [Google Scholar] [CrossRef]
- Yin, L.; Grandi, N.; Raum, E.; Haug, U.; Arnd, V.T.; Brenne, H.R. Meta-analysis: Longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment. Pharmacol. Ther. 2009, 30, 113–125. [Google Scholar] [CrossRef]
- Yin, L.; Ordóñez-mena, J.M.; Chen, T.; Schöttker, B.; Arndt, V.; Brenner, H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: A systematic review and meta-analysis. Prev. Med. 2013, 57, 753–764. [Google Scholar] [CrossRef]
- Gilbert, R.; Metcalfe, C.; Oliver, S.E.; Whiteman, D.C.; Bain, C.; Ness, A.; Donovan, J.; Hamdy, F.; Neal, D.E.; Lane, J.A.; et al. Life course sun exposure and risk of prostate cancer: Population-based nested case-control study and meta-analysis. Int. J. Cancer 2009, 125, 1414–1423. [Google Scholar] [CrossRef]
- Ju, S.Y.; Lee, J.Y.; Kim, D.H. Low 25-hydroxyvitamin D levels and the risk of frailty syndrome: A systematic review and dose-response meta-analysis. BMC Geriatr. 2018, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Lee, Y.; Jeong, S. Serum 25-hydroxyvitamin D levels and the risk of depression: A systematic review and meta-analysis. J. Nutr. Health Aging 2013, 17, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Van der Schaft, J.; Koek, H.L.; Dijkstra, E.; Verhaar, H.J.J. The association between vitamin D and cognition: A systematic review. Ageing Res. Rev. 2013, 12, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Allali, G.; Allain, P.; Bridenbaugh, S.; Schott, A.M.; Kressig, R.W.; Beauchet, O. Vitamin D and cognitive performance in adults: A systematic review. Eur. J. Neurol. 2009, 16, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Bojesen, S.E.; Nordestgaard, B.G. Low 25-hydroxyvitamin D and risk of type 2 diabetes: A prospective cohort study and metaanalysis. Clin. Chem. 2013, 59, 381–391. [Google Scholar] [CrossRef]
- Barouki, R.; Gluckman, P.D.; Grandjean, P.; Hanson, M.; Heindel, J.J. Developmental origins of non-communicable disease: Implications for research and public health. Env. Health 2012, 11, 1–9. [Google Scholar] [CrossRef]
- WHO. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Ferrer, M.; Esquinas, A.; Arancibia, F.; Bauer, T.T.; Gonzalez, G.; Carrillo, A.; Rodriguez-Roisin, R.; Torres, A. Noninvasive ventilation during persistent weaning failure: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2003, 168, 70–76. [Google Scholar] [CrossRef]
- Bousquet, J.; Malva, J.; Nogues, M.; Mañas, L.R.; Vellas, B.; Farrell, J.; MACVIA Research Group. Operational Definition of Active and Healthy Aging (AHA): The European Innovation Partnership (EIP) on AHA Reference Site Questionnaire: Montpellier 20–21 October 2014, Lisbon 2 July 2015. J. Am. Med. Dir. Assoc. 2015, 16, 1020–1026. [Google Scholar] [CrossRef]
| Dimension of Healthy Ageing | Definition | Scale, Questionnaire, Instruments |
|---|---|---|
| Longevity | Physiological ability to survive more than the mean expectancy life | All-causes death Mortality for specific causes Survival |
| Lack of diseases | Lack of diagnosed pathologies | Incidence of diseases such as cardiovascular and cerebrovascular events, diabetes, dementia, and Alzheimer’s, respiratory diseases, etc. or the Healthy Life Years |
| Physical functionality | Ability and autonomy in daily functional tasks [33] | Activities of Daily Living (ADL)/Instrumental Activities of Daily Living (IADL) scales [32], [34,35,36], falls and fear of fall, or physical performance test (i.e., Time Up & Go Test,) [32,37], SF-36 Physical Function/Role Function Scales [38,39,40] |
| Cognitive functionality | Complex ability related to speech, understanding, memory, learning, attention and concentration, reading and writing abilities, calculation ability, opinion, planning ability, problem-solving, etc. | Cognitive screening test (MMSE), SPMSQ score [41], cognitive abilities measures [42], (Mini) Mental Status Test [43], executive function [44] |
| Social functionality | Ability to preserve rules and responsibilities in the different social (formal and informal, productive and unproductive) environments | Modality to pass leisure time, monthly contacts with friends and familiars, participation in social aspects of community, visit friends and familiars, social support, payed work and care of children/partner [32] |
| Well-being and quality of life | Mental status related to all positive and negative evaluation and emotive reactions to lived experiences | Cantril’s Ladder of Life Scale [45], Scale of Positive and Negative Experience (SPANE) [46], the European Social Survey well-being [47], the Life Satisfaction scale/Life Satisfaction Index (LSI) [48,49], the CES-D [50], Psychological Well-Being Scale (PWB) [46], the Flourishing index [51] or the Geriatric Depression Scale [52] |
| Perceived health status | Perception of own health status | General Health Questionnaire 0–5 [53] or SF-36 Quality of Life questionnaire |
| Personality | Structured modality of thought, feeling, and behavior resulting from interaction between environments, genetic makeup, and cultural heritage | Test of perceived control [32] or with the Extraversion (6-item) and goal strength [54] |
| Resources and environment | Security sense comes from financial and social environment | Salary and financial security [32] |
| Ageing status perceived | Perception of own ageing status or sense given to ageing | Likert scales [32] |
| Author | Cohort/Study | Total Score (NOS) |
|---|---|---|
| Afzal 2014 | Copenhagen City Heart Study & Copenhagen General Population Study | 8 |
| Afzal 2013 | Copenhagen City Heart Study | 9 |
| Al-khalidi 2019 | Third National Health and Nutrition Examination Survey (NHANES III) | 9 |
| Arabi 2012 | Lebanese cohort | 7 |
| Aregbesola 2013 | Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD) | 8 |
| Barrett-Connor 2012 | Osteoporotic Fractures in Men Study (MrOS) | 8 |
| Brøndum-Jacobsen 2012 | Copenhagen City Heart Study | 9 |
| Cauley 2010 | Osteoporotic Fractures in Men Study (MrOS) | 8 |
| Heath 2017 | Melbourne Collaborative Cohort Study (MCCS) | 9 |
| Hirani 2018 | Concord Health and Ageing in Men Project (CHAMP) | 7 |
| Holmberg 2017 | Swedish farmers study | 8 |
| Jassal 2010 | The Rancho Bernardo Study | 6 |
| Khaw 2014 | EPIC cohort | 8 |
| Licher 2017 | The Rotterdam Study | 9 |
| Liu 2012 | Third National Health and Nutrition Examination Survey (NHANES III) | 8 |
| Looker 2013 | NHANES III & NHANES 2000–2004 | 8 |
| Marques-Vidal 2015 | Cohorte Lausannoise (CoLaus) study | 7 |
| Mursu 2015 | Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD) | 9 |
| Olsson 2017 | Uppsala Longitudinal Study of Adult men | 9 |
| Platz 2004 | Health Professionals Follow-up Study | 8 |
| Shimizu 2015 | Otasha–Kenshin study | 5 |
| Swanson 2015 | Osteoporotic Fractures in Men Study (MrOS) | 7 |
| Umehara 2017 | Hisayama study | 9 |
| Vàzquez-Oliva 2018 | REGICOR (Registre Gironı del COR) population cohort study | 8 |
| Outcomes | Positive Association | Negative Association | No Statistically Significant Association | Total |
|---|---|---|---|---|
| All-cause mortality | 0 | 5 | 0 | 5 |
| Pulmonary and respiratory events | 2 | 5 | 0 | 7 |
| Cancer events | 2 | 0 | 3 | 5 |
| Cardiovascular and coronary events | 0 | 9 | 3 | 12 |
| Cardiometabolic events | 0 | 1 | 1 | 2 |
| Impaired bone health | 0 | 7 | 10 | 17 |
| Sarcopenia | 0 | 1 | 0 | 1 |
| Dementia and Alzheimer’s | 0 | 2 | 1 | 3 |
| Physical functionality (falls) | 0 | 1 | 0 | 1 |
| Cognitive functionality | 0 | 0 | 1 | 1 |
| Total | 3 | 31 | 19 | 54 |
| Outcomes | Negative Association | No Statistically Significant Association | Total |
|---|---|---|---|
| All-cause mortality | 1 | 0 | 1 |
| Pulmonary and respiratory events | 1 | 0 | 1 |
| Cancer events | 0 | 2 | 2 |
| Cardiovascular and coronary events | 2 | 0 | 2 |
| Bone health | 1 | 3 | 4 |
| Sarcopenia | 1 | 0 | 1 |
| Total | 6 | 5 | 11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caristia, S.; Filigheddu, N.; Barone-Adesi, F.; Sarro, A.; Testa, T.; Magnani, C.; Aimaretti, G.; Faggiano, F.; Marzullo, P. Vitamin D as a Biomarker of Ill Health among the Over-50s: A Systematic Review of Cohort Studies. Nutrients 2019, 11, 2384. https://doi.org/10.3390/nu11102384
Caristia S, Filigheddu N, Barone-Adesi F, Sarro A, Testa T, Magnani C, Aimaretti G, Faggiano F, Marzullo P. Vitamin D as a Biomarker of Ill Health among the Over-50s: A Systematic Review of Cohort Studies. Nutrients. 2019; 11(10):2384. https://doi.org/10.3390/nu11102384
Chicago/Turabian StyleCaristia, Silvia, Nicoletta Filigheddu, Francesco Barone-Adesi, Andrea Sarro, Tommaso Testa, Corrado Magnani, Gianluca Aimaretti, Fabrizio Faggiano, and Paolo Marzullo. 2019. "Vitamin D as a Biomarker of Ill Health among the Over-50s: A Systematic Review of Cohort Studies" Nutrients 11, no. 10: 2384. https://doi.org/10.3390/nu11102384
APA StyleCaristia, S., Filigheddu, N., Barone-Adesi, F., Sarro, A., Testa, T., Magnani, C., Aimaretti, G., Faggiano, F., & Marzullo, P. (2019). Vitamin D as a Biomarker of Ill Health among the Over-50s: A Systematic Review of Cohort Studies. Nutrients, 11(10), 2384. https://doi.org/10.3390/nu11102384







