The Impact of Obesity on the Association between Vitamin D Deficiency and Cardiovascular Disease
Abstract
1. Introduction
2. Links between Vitamin D Status and Cardiovascular Disease
2.1. Early Evidence from Association Studies and Experimental Data
2.2. Prospective Studies and Randomized Clinical Trials
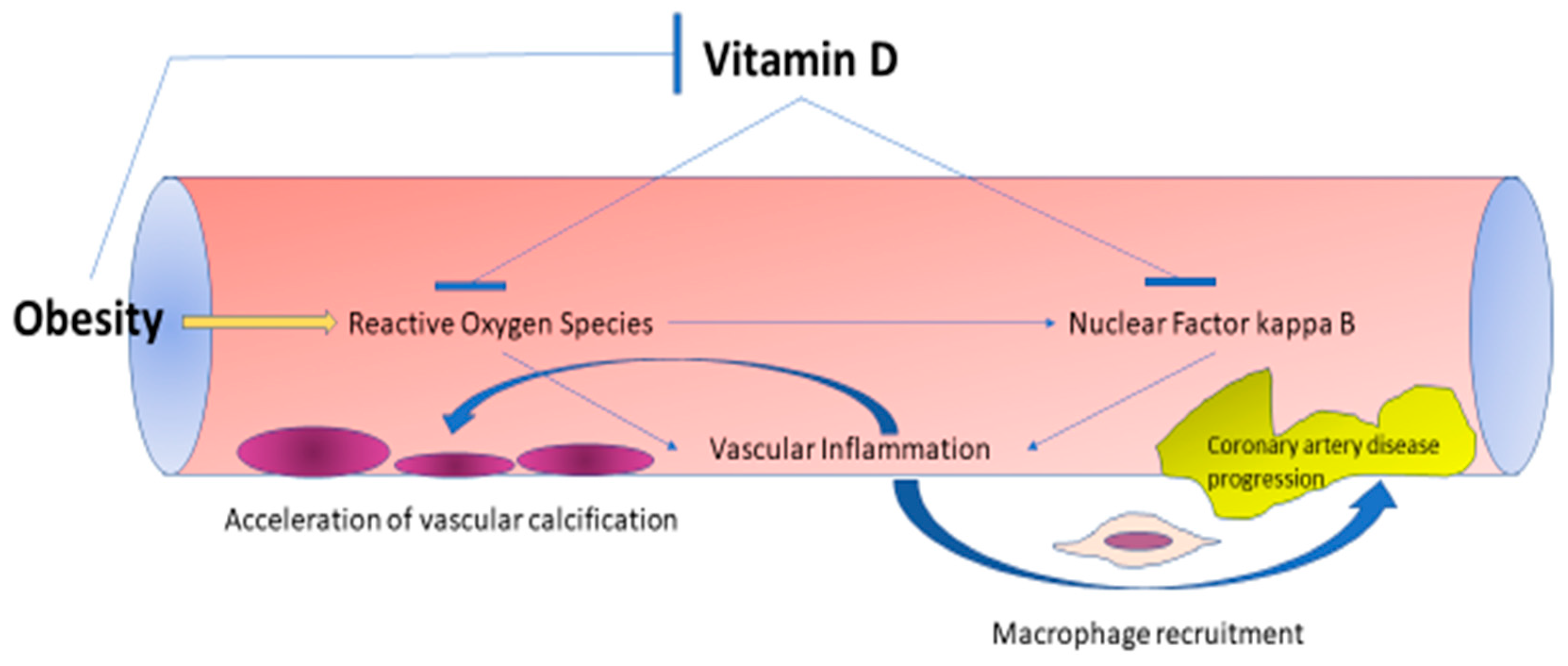
3. The Inter-Relationship between Obesity, Vitamin D, and Cardiovascular Health
3.1. Effects of Obesity on Vitamin D Deficiency and Cardiovascular Disease
3.2. Pathophysiology of Vitamin D Deficiency in Obesity
3.3. Effects of Vitamin D Deficiency on Cardiovascular Health in Obese and Non-Obese Populations
4. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Cano, A.; Chedraui, P.; Goulis, D.G.; Lopes, P.; Mishra, G.; Mueck, A.; Senturk, L.M.; Simoncini, T.; Stevenson, J.C.; Stute, P.; et al. Calcium in the prevention of postmenopausal osteoporosis: EMAS clinical guide. Maturitas 2018, 107, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Apostolakis, M.; Armeni, E.; Bakas, P.; Lambrinoudaki, I. Vitamin D and cardiovascular disease. Maturitas 2018, 115, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Paschou, S.A.; Goulis, D.G. Vitamin D Supplementation and Cardiovascular Disease Risk. JAMA Cardiol. 2017, 2, 1281–1282. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.W.; Shu, X.O.; Cai, Q.; Khankari, N.K.; Steinwandel, M.D.; Jurutka, P.W.; Blot, W.J.; Zheng, W. Total and Free Circulating Vitamin D and Vitamin D-Binding Protein in Relation to Colorectal Cancer Risk in a Prospective Study of African Americans. Cancer Epidemiol Biomarkers Prev. 2017, 26, 1242–1247. [Google Scholar] [CrossRef]
- Miclea, A.; Miclea, M.; Pistor, M.; Hoepner, A.; Chan, A.; Hoepner, R. Vitamin D supplementation differentially affects seasonal multiple sclerosis disease activity. Brain Behav. 2017, 7, e00761. [Google Scholar] [CrossRef]
- Lambrinoudaki, I.; Patikas, E.; Kaparos, G.; Armeni, E.; Rizos, D.; Thoda, P.; Alexandrou, A.; Antoniou, A.; Tsivgoulis, G.; Gatzonis, S.; et al. Vitamin D receptor Bsm1 polymorphism, calcium metabolism and bone mineral density in patients with multiple sclerosis: A pilot study. Neurol. Sci. 2013, 34, 1433–1439. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Heaney, R.P.; Holick, M.F. Why the IOM recommendations for vitamin D are deficient. J. Bone Min. Res. 2011, 26, 455–457. [Google Scholar] [CrossRef]
- Cashman, K.D.; Ritz, C.; Kiely, M.; Collaborators, O. Improved Dietary Guidelines for Vitamin D: Application of Individual Participant Data (IPD)-Level Meta-Regression Analyses. Nutrients 2017, 9, 469. [Google Scholar] [CrossRef]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef]
- Pilz, S.; Dobnig, H.; Tomaschitz, A.; Kienreich, K.; Meinitzer, A.; Friedl, C.; Wagner, D.; Piswanger-Sölkner, C.; März, W.; Fahrleitner-Pammer, A. Low 25-hydroxyvitamin D is associated with increased mortality in female nursing home residents. J. Clin. Endocrinol. Metab. 2012, 97, E653–E657. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.; Paschou, S.A.; Kandaraki, E.; Anagnostis, P.; Annweiler, C.; Tarlatzis, B.C.; Hollis, B.W.; Grant, W.B.; Goulis, D.G. Hypovitaminosis D in pregnancy in the Mediterranean region: A systematic review. Eur. J. Clin. Nutr. 2016, 70, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [PubMed]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Mason, H.; Shoaibi, A.; Ghandour, R.; O’Flaherty, M.; Capewell, S.; Khatib, R.; Jabr, S.; Unal, B.; Sözmen, K.; Arfa, C.; et al. A cost effectiveness analysis of salt reduction policies to reduce coronary heart disease in four Eastern Mediterranean countries. PLoS ONE 2014, 9, e84445. [Google Scholar] [CrossRef]
- O’Keeffe, C.; Kabir, Z.; O’Flaherty, M.; Walton, J.; Capewell, S.; Perry, I.J. Modelling the impact of specific food policy options on coronary heart disease and stroke deaths in Ireland. BMJ Open 2013, 3, e002837. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Oikonomou, E.; Maniatis, K.; Kioufis, S.; Kokkou, E.; Vavuranakis, M.; Zaromitidou, M.; Kassi, E.; Miliou, A.; et al. Vitamin D3, D2 and arterial wall properties in coronary artery disease. Curr. Pharm. Des. 2014, 20, 5914–5918. [Google Scholar] [CrossRef]
- Giovannucci, E.; Liu, Y.; Hollis, B.W.; Rimm, E.B. 25-hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch. Intern. Med. 2008, 168, 1174–1180. [Google Scholar] [CrossRef]
- Welles, C.C.; Whooley, M.A.; Karumanchi, S.A.; Hod, T.; Thadhani, R.; Berg, A.H.; Ix, J.H.; Mukamal, K.J. Vitamin D deficiency and cardiovascular events in patients with coronary heart disease: Data from the Heart and Soul Study. Am. J. Epidemiol. 2014, 179, 1279–1287. [Google Scholar] [CrossRef]
- Zhang, R.; Li, B.; Gao, X.; Tian, R.; Pan, Y.; Jiang, Y.; Gu, H.; Wang, Y.; Wang, Y.; Liu, G. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: Dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017, 105, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Schöttker, B.; Jorde, R.; Peasey, A.; Thorand, B.; Jansen, E.H.; de Groot, L.; Streppel, M.; Gardiner, J.; Ordóñez-Mena, J.M.; Perna, L.; et al. Vitamin D and mortality: Meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ 2014, 348, g3656. [Google Scholar] [CrossRef] [PubMed]
- Faridi, K.F.; Zhao, D.; Martin, S.S.; Lupton, J.R.; Jones, S.R.; Guallar, E.; Ballantyne, C.M.; Lutsey, P.L.; Michos, E.D. Serum vitamin D and change in lipid levels over 5 y: The Atherosclerosis Risk in Communities study. Nutrition 2017, 38, 85–93. [Google Scholar] [CrossRef]
- Yin, K.; You, Y.; Swier, V.; Tang, L.; Radwan, M.M.; Pandya, A.N.; Agrawal, D.K. Vitamin D Protects Against Atherosclerosis via Regulation of Cholesterol Efflux and Macrophage Polarization in Hypercholesterolemic Swine. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2432–2442. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Swier, V.J.; Boosani, C.S.; Radwan, M.M.; Agrawal, D.K. Vitamin D Deficiency Accelerates Coronary Artery Disease Progression in Swine. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef]
- Hadjadj, L.; Monori-Kiss, A.; Horváth, E.M.; Heinzlmann, A.; Magyar, A.; Sziva, R.E.; Miklós, Z.; Pál, É.; Gál, J.; Szabó, I.; et al. Geometric, elastic and contractile-relaxation changes in coronary arterioles induced by Vitamin D deficiency in normal and hyperandrogenic female rats. Microvasc. Res. 2019, 122, 78–84. [Google Scholar] [CrossRef]
- Ellam, T.; Hameed, A.; ul Haque, R.; Muthana, M.; Wilkie, M.; Francis, S.E.; Chico, T.J. Vitamin D deficiency and exogenous vitamin D excess similarly increase diffuse atherosclerotic calcification in apolipoprotein E knockout mice. PLoS ONE 2014, 9, e88767. [Google Scholar] [CrossRef]
- Sunbul, M.; Bozbay, M.; Mammadov, C.; Cincin, A.; Atas, H.; Ozsenel, E.B.; Sari, I.; Basaran, Y. Effect of vitamin D deficiency and supplementation on myocardial deformation parameters and epicardial fat thickness in patients free of cardiovascular risk. Int. J. Cardiovasc. Imaging 2015, 31, 765–772. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Nameni, G.; Hajiluian, G.; Mesgari-Abbasi, M. Cardiac tissue oxidative stress and inflammation after vitamin D administrations in high fat- diet induced obese rats. BMC Cardiovasc. Disord. 2017, 17, 161. [Google Scholar] [CrossRef]
- Salum, E.; Kals, J.; Kampus, P.; Salum, T.; Zilmer, K.; Aunapuu, M.; Arend, A.; Eha, J.; Zilmer, M. Vitamin D reduces deposition of advanced glycation end-products in the aortic wall and systemic oxidative stress in diabetic rats. Diabetes Res. Clin. Pract. 2013, 100, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef] [PubMed]
- Al Mheid, I.; Quyyumi, A.A. Vitamin D and Cardiovascular Disease: Controversy Unresolved. J. Am. Coll. Cardiol. 2017, 70, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Hsia, J.; Heiss, G.; Ren, H.; Allison, M.; Dolan, N.C.; Greenland, P.; Heckbert, S.R.; Johnson, K.C.; Manson, J.E.; Sidney, S.; et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007, 115, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A.; Chlebowski, R.T.; Wactawski-Wende, J.; Robbins, J.A.; Rodabough, R.J.; Chen, Z.; Johnson, K.C.; O’Sullivan, M.J.; Jackson, R.D.; Manson, J.E. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: The Women’s Health Initiative. J. Women’s Health 2013, 22, 915–929. [Google Scholar] [CrossRef]
- Scragg, R.; Stewart, A.W.; Waayer, D.; Lawes, C.M.; Toop, L.; Sluyter, J.; Murphy, J.; Khaw, K.T.; Camargo, C.A. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 608–616. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Menon, R.K.; Sharp, S.J.; Mannan, N.; Timms, P.M.; Martineau, A.R.; Rickard, A.P.; Boucher, B.J.; Chowdhury, T.A.; Griffiths, C.J.; et al. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: Results of a randomized double-blind placebo-controlled trial. Diabetes Obes. Metab. 2016, 18, 392–400. [Google Scholar] [CrossRef]
- Pilz, S.; Gaksch, M.; Kienreich, K.; Grübler, M.; Verheyen, N.; Fahrleitner-Pammer, A.; Treiber, G.; Drechsler, C.; ó Hartaigh, B.; Obermayer-Pietsch, B.; et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: A randomized controlled trial. Hypertension 2015, 65, 1195–1201. [Google Scholar] [CrossRef]
- Schnatz, P.F.; Jiang, X.; Aragaki, A.K.; Nudy, M.; O’Sullivan, D.M.; Williams, M.; LeBlanc, E.S.; Martin, L.W.; Manson, J.E.; Shikany, J.M.; et al. Effects of Calcium, Vitamin D, and Hormone Therapy on Cardiovascular Disease Risk Factors in the Women’s Health Initiative: A Randomized Controlled Trial. Obstet. Gynecol. 2017, 129, 121–129. [Google Scholar] [CrossRef]
- Zittermann, A.; Ernst, J.B.; Prokop, S.; Fuchs, U.; Dreier, J.; Kuhn, J.; Knabbe, C.; Birschmann, I.; Schulz, U.; Berthold, H.K.; et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): A 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur. Heart J. 2017, 38, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Makariou, S.E.; Elisaf, M.; Challa, A.; Tentolouris, N.; Liberopoulos, E.N. No effect of vitamin D supplementation on cardiovascular risk factors in subjects with metabolic syndrome: A pilot randomised study. Arch. Med. Sci. Atheroscler. Dis. 2017, 2, e52–e60. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, P.; Johnsen, S.P.; Gillman, M.W.; Sorensen, H.T. Interpretation of observational studies. Heart 2004, 90, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Plesner, J.L.; Dahl, M.; Fonvig, C.E.; Nielsen, T.R.H.; Kloppenborg, J.T.; Pedersen, O.; Hansen, T.; Holm, J.C. Obesity is associated with vitamin D deficiency in Danish children and adolescents. J. Pediatr. Endocrinol. Metab. 2018, 31, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Dylag, H.; Rowicka, G.; Strucinska, M.; Riahi, A. Assessment of vitamin D status in children aged 1-5 with simple obesity. Rocz. Panstw. Zakl. Hig. 2014, 65, 325–330. [Google Scholar]
- Rontoyanni, V.G.; Avila, J.C.; Kaul, S.; Wong, R.; Veeranki, S.P. Association between Obesity and Serum 25(OH)D Concentrations in Older Mexican Adults. Nutrients 2017, 9, 97. [Google Scholar] [CrossRef]
- Forrest, K.Y.Z.; Stuhldreher, W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 2011, 31, 48–54. [Google Scholar] [CrossRef]
- Bettencourt, A.; Boleixa, D.; Reis, J.; Oliveira, J.C.; Mendonça, D.; Costa, P.P.; da Silva, B.M.; Marinho, A.; da Silva, A.M. Serum 25-hydroxyvitamin D levels in a healthy population from the North of Portugal. J. Steroid Biochem. Mol. Biol. 2018, 175, 97–101. [Google Scholar] [CrossRef]
- Stokić, E.; Kupusinac, A.; Tomić-Naglić, D.; Zavišić, B.K.; Mitrović, M.; Smiljenić, D.; Soskić, S.; Isenović, E. Obesity and vitamin D deficiency: Trends to promote a more proatherogenic cardiometabolic risk profile. Angiology 2015, 66, 237–243. [Google Scholar] [CrossRef]
- Fatima, S.S.; Farooq, S.; Tauni, M.A.; Irfan, O.; Alam, F. Effect of raised body fat on vitamin D, leptin and bone mass. J. Pak. Med. Assoc. 2015, 65, 1315–1319. [Google Scholar]
- Shafinaz, I.S.; Moy, F.M. Vitamin D level and its association with adiposity among multi-ethnic adults in Kuala Lumpur, Malaysia: A cross sectional study. BMC Public Health 2016, 16, 232. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Thun, M.J.; Petrelli, J.M.; Rodriguez, C.; Heath, C.W.J. Body-mass index and mortality in a prospective cohort of U.S. adults. N. Engl. J. Med. 1999, 341, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Caleyachetty, R.; Thomas, G.N.; Toulis, K.A.; Mohammed, N.; Gokhale, K.M.; Balachandran, K.; Nirantharakumar, K. Metabolically Healthy Obese and Incident Cardiovascular Disease Events Among 3.5 Million Men and Women. J. Am. Coll. Cardiol. 2017, 70, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Berrington de Gonzalez, A.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef]
- Sandfort, V.; Lai, S.; Ahlman, M.A.; Mallek, M.; Liu, S.; Sibley, C.T.; Turkbey, E.B.; Lima, J.A.; Bluemke, D.A. Obesity Is Associated with Progression of Atherosclerosis During Statin Treatment. J. Am. Heart Assoc. 2016, 5, e003621. [Google Scholar] [CrossRef]
- Ahmad, F.S.; Ning, H.; Rich, J.D.; Yancy, C.W.; Lloyd-Jones, D.M.; Wilkins, J.T. Hypertension, Obesity, Diabetes, and Heart Failure-Free Survival: The Cardiovascular Disease Lifetime Risk Pooling Project. JACC Heart Fail. 2016, 4, 911–919. [Google Scholar] [CrossRef]
- Kouli, G.M.; Panagiotakos, D.B.; Kyrou, I.; Georgousopoulou, E.N.; Chrysohoou, C.; Tsigos, C.; Tousoulis, D.; Pitsavos, C. Visceral adiposity index and 10-year cardiovascular disease incidence: The ATTICA study. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 881–889. [Google Scholar] [CrossRef]
- Araujo, J.; Severo, M.; Barros, H.; Ramos, E. Duration and degree of adiposity: Effect on cardiovascular risk factors at early adulthood. Int. J. Obes. 2017, 41, 1526–1530. [Google Scholar] [CrossRef]
- Kelly, A.S.; Jacobs, D.R.J.; Sinaiko, A.R.; Moran, A.; Steffen, L.M.; Steinberger, J. Relation of circulating oxidized LDL to obesity and insulin resistance in children. Pediatr. Diabetes 2010, 11, 552–555. [Google Scholar] [CrossRef]
- Geiss, L.S.; Kirtland, K.; Lin, J.; Shrestha, S.; Thompson, T.; Albright, A.; Gregg, E.W. Changes in diagnosed diabetes, obesity, and physical inactivity prevalence in US counties, 2004–2012. PLoS ONE 2017, 12, e0173428. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Mawer, E.B.; Backhouse, J.; Holman, C.A.; Lumb, G.A.; Stanbury, S.W. The distribution and storage of vitamin D and its metabolites in human tissues. Clin. Sci. 1972, 43, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Forbes, G.B.; Welle, S.L. Lean body mass in obesity. Int. J. Obes. 1983, 7, 99–107. [Google Scholar]
- Cipriani, C.; Pepe, J.; Piemonte, S.; Colangelo, L.; Cilli, M.; Minisola, S. Vitamin d and its relationship with obesity and muscle. Int. J. Endocrinol. 2014, 2014, 841248. [Google Scholar] [CrossRef]
- Jung, Y.S.; Wu, D.; Smith, D.; Meydani, S.N.; Han, S.N. Dysregulated 1,25-dihydroxyvitamin D levels in high-fat diet-induced obesity can be restored by changing to a lower-fat diet in mice. Nutr. Res. 2018, 53, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, L.; Hachemi, M.A.; Karkeni, E.; Couturier, C.; Astier, J.; Defoort, C.; Svilar, L.; Martin, J.C.; Tourniaire, F.; Landrier, J.F. Diet induced obesity modifies vitamin D metabolism and adipose tissue storage in mice. J. Steroid Biochem. Mol. Biol. 2019, 185, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Buckendahl, P.; Sharma, K.; Miller, J.W.; Shapses, S.A. Expression of vitamin D hydroxylases and bone quality in obese mice consuming saturated or monounsaturated enriched high-fat diets. Nutr. Res. 2018, 60, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Osmancevic, A.; Gillstedt, M.; Landin-Wilhelmsen, K.; Larkö, A.M.W.; Larkö, O.; Holick, M.F.; Krogstad, A.L. Size of the exposed body surface area, skin erythema and body mass index predict skin production of vitamin D. J. Photochem. Photobiol. B 2015, 149, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Florez, H.; Martinez, R.; Chacra, W.; Strickman-Stein, N.; Levis, S. Outdoor exercise reduces the risk of hypovitaminosis D in the obese. J. Steroid Biochem. Mol. Biol. 2007, 103, 679–681. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Scala, L.; Cigolini, M.; Zenari, L.; Falezza, G.; Arcaro, G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 517–524. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Wan, B.; Zhang, H.; Wu, S.; Zhu, Z.; Lin, Y.; Wang, M.; Zhang, N.; Lin, S.; et al. Association between Vitamin D Status and Non-Alcoholic Fatty Liver Disease: A Population-Based Study. J. Nutr. Sci. Vitam. 2019, 65, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, Y. Vitamin D Insufficiency Exacerbates Adipose Tissue Macrophage Infiltration and Decreases AMPK/SIRT1 Activity in Obese Rats. Nutrients 2017, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Shamardl, H.A.; El-Ashmony, S.M.; Kamel, H.F.; Fatani, S.H. Potential Cardiovascular and Renal Protective Effects of Vitamin D and Coenzyme Q10 in l-NAME-Induced Hypertensive Rats. Am. J. Med. Sci. 2017, 354, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.B.; Przybyl, L.; Haase, N.; von Versen-Höynck, F.; Qadri, F.; Jørgensen, J.S.; Sorensen, G.L.; Fruekilde, P.; Poglitsch, M.; Szijarto, I.; et al. Vitamin D depletion aggravates hypertension and target-organ damage. J. Am. Heart Assoc. 2015, 4, e001417. [Google Scholar] [CrossRef]
- Gupta, G.K.; Agrawal, T.; Rai, V.; Del Core, M.G.; Hunter, W.J., 3rd; Agrawal, D.K. Vitamin D Supplementation Reduces Intimal Hyperplasia and Restenosis following Coronary Intervention in Atherosclerotic Swine. PLoS ONE 2016, 11, e0156857. [Google Scholar] [CrossRef]
- Martins, D.; Meng, Y.X.; Tareen, N.; Artaza, J.; Lee, J.E.; Farodolu, C.; Gibbons, G.; Norris, K. The Effect of Short Term Vitamin D Supplementation on the Inflammatory and Oxidative Mediators of Arterial Stiffness. Health 2014, 6, 1503–1511. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef]
- Codoner-Franch, P.; Tavarez-Alonso, S.; Simo-Jorda, R.; Laporta-Martin, P.; Carratala-Calvo, A.; Alonso-Iglesias, E. Vitamin D status is linked to biomarkers of oxidative stress, inflammation, and endothelial activation in obese children. J. Pediatr. 2012, 161, 848–854. [Google Scholar] [CrossRef]
- Gradinaru, D.; Borsa, C.; Ionescu, C.; Margina, D.; Prada, G.I.; Jansen, E. Vitamin D status and oxidative stress markers in the elderly with impaired fasting glucose and type 2 diabetes mellitus. Aging Clin. Exp. Res. 2012, 24, 595–602. [Google Scholar]
- Wang, E.W.; Siu, P.M.; Pang, M.Y.; Woo, J.; Collins, A.R.; Benzie, I.F.F. Vitamin D deficiency, oxidative stress and antioxidant status: Only weak association seen in the absence of advanced age, obesity or pre-existing disease. Br. J. Nutr. 2017, 118, 11–16. [Google Scholar] [CrossRef]
| First Author, Year | Study Type | Population | Vitamin D Parameter | Outcome | Reported Interaction with Vitamin D |
|---|---|---|---|---|---|
| Studies in animals | |||||
| Salum, 2013 [31] | Experimental | Diabetic rats | Vitamin D supplementation | Carboxymethylycin accumulation | Negative, significant |
| Ellam, 2014 [28] | Experimental | Apolipoprotein E knockout mice | Induced deficiency | Atheroma calcification | Positive, significant |
| Yin, 2015 [24] | Experimental | Hypercholesterolemic swine | Induced deficiency | Macrophage recruitment | Positive, significant |
| Chen, 2016 [25] | Experimental | Hypercholesterolemic swine | Induced deficiency | NFkB activity | Positive, significant |
| Chang, 2017 [72] | Experimental | Obese rats | Induced deficiency | Macrophage recruitment | Positive, significant |
| Farhangi, 2017 [30] | Experimental | Obese rats | Induced deficiency | Superoxide dismutase/Catalase activity | Negative, significant |
| Hadjadj, 2019 [27] | Experimental | Hyperandrogenic female rats | Induced deficiency | LAD relaxation capacity | Negative, significant |
| Studies in humans | |||||
| Hsia, 2007 [34] | Experimental, clinical | Postmenopausal women | Vitamin D supplementation | Major adverse cardiovascular events / Stroke | Non-significant |
| Giovanucci, 2008 [19] | Observational clinical | Healthy adult men | Baseline concentrations | Major adverse cardiovascular events | Inverse, significant |
| Cauley, 2013 [35] | Post-hoc experimental, clinical | Postmenopausal women | Vitamin D supplementation | All-cause mortality | Non-significant |
| Martins, 2014 [76] | Experimental, clinical | Obese Adults | Vitamin D supplementation | Arterial stiffness | Inverse, significant |
| Schöttker, 2014 [22] | Meta-analysis | General population | Baseline concentrations | Cardiovascular mortality | Inverse, significant |
| Welles, 2014 [20] | Observational clinical | Stable cardiovascular disease | Baseline concentrations | Major adverse cardiovascular events | Inverse, significant |
| Sunbul, 2015 [29] | Observational | Healthy adults | Baseline concentrations | Global longitudinal strain | Statistically significant |
| Faridi, 2017 [23] | Observational clinical | General population | Baseline concentrations | Total cholesterol | Inverse, significant |
| Scragg, 2017 [36] | Experimental, clinical | General population | Vitamin D supplementation | Cardiovascular disease incidence | Non-significant |
| Zhang, 2017 [21] | Meta-analysis | General population | Baseline concentrations | Cardiovascular mortality | Inverse, significant |
| Manson, 2019 [37] | Experimental, clinical | General population | Vitamin D supplementation | Major adverse cardiovascular events / Stroke | Non-significant |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paschou, S.A.; Kosmopoulos, M.; Nikas, I.P.; Spartalis, M.; Kassi, E.; Goulis, D.G.; Lambrinoudaki, I.; Siasos, G. The Impact of Obesity on the Association between Vitamin D Deficiency and Cardiovascular Disease. Nutrients 2019, 11, 2458. https://doi.org/10.3390/nu11102458
Paschou SA, Kosmopoulos M, Nikas IP, Spartalis M, Kassi E, Goulis DG, Lambrinoudaki I, Siasos G. The Impact of Obesity on the Association between Vitamin D Deficiency and Cardiovascular Disease. Nutrients. 2019; 11(10):2458. https://doi.org/10.3390/nu11102458
Chicago/Turabian StylePaschou, Stavroula A., Marinos Kosmopoulos, Ilias P. Nikas, Michael Spartalis, Evanthia Kassi, Dimitrios G. Goulis, Irene Lambrinoudaki, and Gerasimos Siasos. 2019. "The Impact of Obesity on the Association between Vitamin D Deficiency and Cardiovascular Disease" Nutrients 11, no. 10: 2458. https://doi.org/10.3390/nu11102458
APA StylePaschou, S. A., Kosmopoulos, M., Nikas, I. P., Spartalis, M., Kassi, E., Goulis, D. G., Lambrinoudaki, I., & Siasos, G. (2019). The Impact of Obesity on the Association between Vitamin D Deficiency and Cardiovascular Disease. Nutrients, 11(10), 2458. https://doi.org/10.3390/nu11102458