Micronutrient Inadequacy in Short Sleep: Analysis of the NHANES 2005–2016
Abstract
1. Introduction
2. Materials and Methods
2.1. Database and Sample
2.2. Sleep Variable—Short Sleep
2.3. Usual Nutrient Intakes
2.4. Statistics
3. Results
3.1. Demographics of the Participants
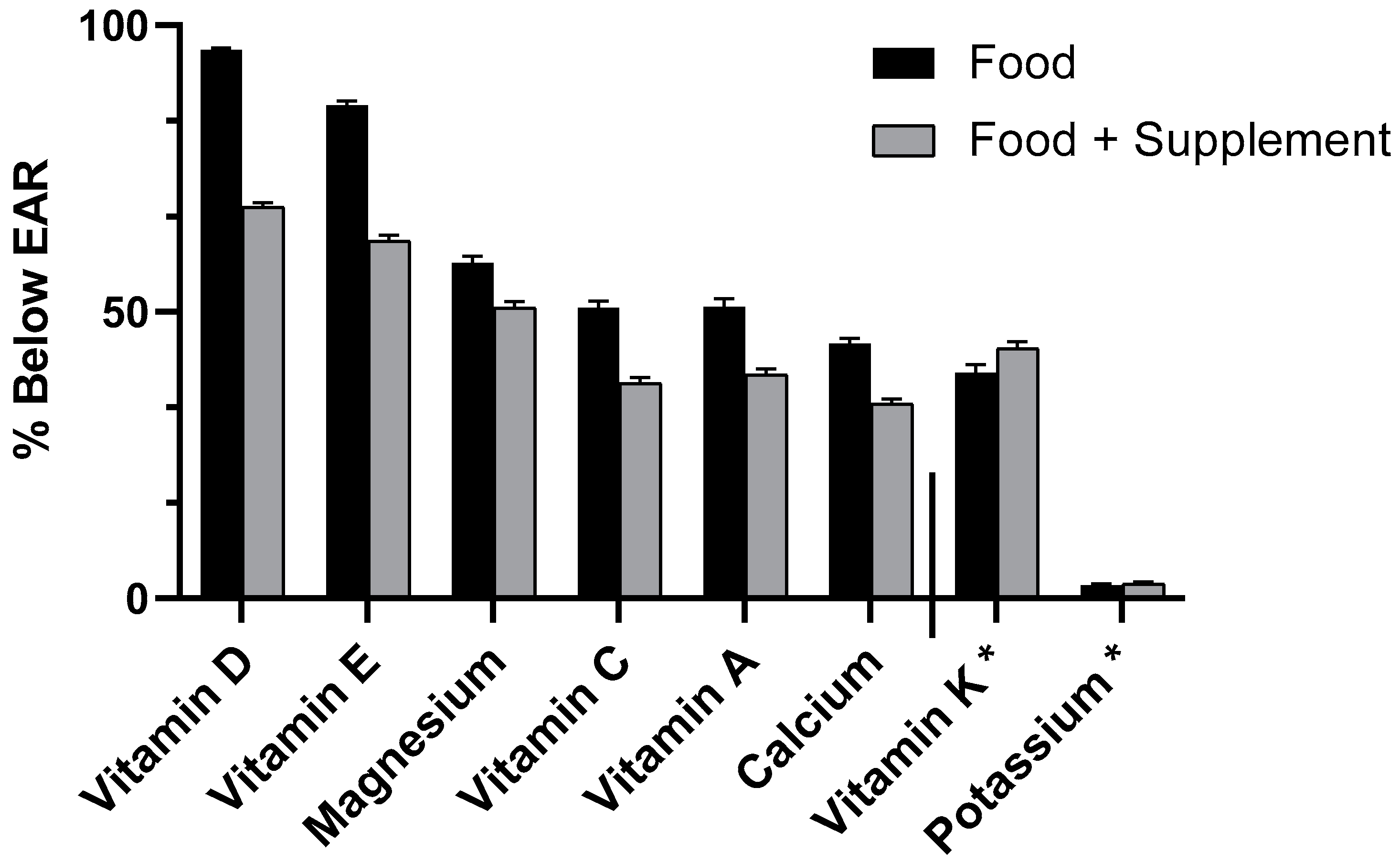
3.2. Micronutrient Usual Intake (UI) (Food Only and Food + Spp) and Inadequacy (% Below EAR) With and Without Short Sleep in All Adults Aged 19+ years
3.3. Micronutrient Usual Intake (UI) (Food Only and Food + Spp) and Inadequacy (% Below EAR) With and Without Short Sleep in Females Aged 19+ years
3.4. Micronutrient Usual Intake (UI) (Food Only and Food + Spp) and Inadequacy (% Below EAR) With and Without Short Sleep Duration in Males Aged 19+ years
4. Discussion
4.1. Vitamin D
4.2. Magnesium
4.3. Vitamins C and E
4.4. Vitamin A
4.5. Calcium
4.6. Other Micronutrients
4.7. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hafner, M.; Stepanek, M.; Taylor, J.; Troxel, W.M.; van Stolk, C. Why Sleep Matters-The Economic Costs of Insufficient Sleep: A Cross-Country Comparative Analysis. Rand Health Q. 2017, 6, 11. [Google Scholar] [PubMed]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. J. Clin. Sleep Med. 2015, 11, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. NHANES-About the National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 15 November 2018).
- Cappuccio, F.P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011, 32, 1484–1492. [Google Scholar] [CrossRef]
- Knutson, K.L.; Van Cauter, E.; Rathouz, P.J.; Yan, L.L.; Hulley, S.B.; Liu, K.; Lauderdale, D.S. Association between sleep and blood pressure in midlife: The CARDIA sleep study. Arch. Intern. Med. 2009, 169, 1055–1061. [Google Scholar] [CrossRef]
- Mullington, J.M.; Simpson, N.S.; Meier-Ewert, H.K.; Haack, M. Sleep loss and inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 775–784. [Google Scholar] [CrossRef]
- Chaput, J.P.; Despres, J.P.; Bouchard, C.; Tremblay, A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia 2007, 50, 2298–2304. [Google Scholar] [CrossRef]
- Petrov, M.E.; Kim, Y.; Lauderdale, D.; Lewis, C.E.; Reis, J.P.; Carnethon, M.R.; Knutson, K.; Glasser, S.J. Longitudinal associations between objective sleep and lipids: The CARDIA study. Sleep 2013, 36, 1587–1595. [Google Scholar] [CrossRef]
- Stranges, S.; Dorn, J.M.; Cappuccio, F.P.; Donahue, R.P.; Rafalson, L.B.; Hovey, K.M.; Freudenheim, J.L.; Kandala, N.B.; Miller, M.A.; Trevisan, M. A population-based study of reduced sleep duration and hypertension: The strongest association may be in premenopausal women. J. Hypertens. 2010, 28, 896–902. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Taggart, F.M.; Kandala, N.B.; Currie, A.; Peile, E.; Stranges, S.; Miller, M.A. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008, 31, 619–626. [Google Scholar] [CrossRef]
- Patel, S.R.; Hu, F.B. Short sleep duration and weight gain: A systematic review. Obesity 2008, 16, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.S.; Glozier, N.; Grunstein, R.R. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med. Rev. 2008, 12, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 2010, 33, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite 2013, 64, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.B.; Frei, B.B.; Fulgoni, V.L.; Weaver, C.M.; Zeisel, S.H. Impact of Frequency of Multi-Vitamin/Multi-Mineral Supplement Intake on Nutritional Adequacy and Nutrient Deficiencies in U.S. Adults. Nutrients 2017, 9, 849. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. 2015–2020 Dietary Guidelines for Americans. Available online: https://health.gov/dietaryguidelines/2015/resources/2015-2020_Dietary_Guidelines.pdf (accessed on 4 March 2019).
- Montville, J.B.; Ahuja, J.K.C.; Martin, C.L.; Heendeniya, K.Y.; Omolewa-Tomobi, G.; Steinfeldt, L.C.; Anand, J.; Adler, M.E.; LaComb, R.P.; Moshfegh, A. USDA Food and Nutrient Database for Dietary Studies (FNDDS), 5.0. Procedia Food Sci. 2013, 2, 99–112. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Agricultural Research Service. USDA Food and Nutrient Database for Dietary Studies 2011–2012. 2014; Food Surveys Research Group Home Page. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/ (accessed on 18 November 2018).
- United States Department of Agriculture. US Department of Agriculture National Agriculture Library: National Nutrient Database for Standard Reference. Available online: http://ndb.nal.usda.gov/ (accessed on 18 November 2018).
- Centers for Disease Control and Prevention; National Center for Health Statistics. Questionnaires, Datasets and Related Documentation, NHANES. Available online: https://wwwn.cdc.gov/nchs/nhanes/ (accessed on 18 November 2018).
- Tooze, J.A.; Kipnis, V.; Buckman, D.W.; Carroll, R.J.; Freedman, L.S.; Guenther, P.M.; Krebs-Smith, S.M.; Subar, A.F.; Dodd, K.W. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: The NCI method. Stat. Med. 2010, 29, 2857–2868. [Google Scholar] [CrossRef]
- Bird, J.K.; Murphy, R.A.; Ciappio, E.D.; McBurney, M.I. Risk of Deficiency in Multiple Concurrent Micronutrients in Children and Adults in the United States. Nutrients 2017, 9, 655. [Google Scholar] [CrossRef]
- Fulgoni, V.L., 3rd; Keast, D.R.; Bailey, R.L.; Dwyer, J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J. Nutr. 2011, 141, 1847–1854. [Google Scholar] [CrossRef]
- Massa, J.; Stone, K.L.; Wei, E.K.; Harrison, S.L.; Barrett-Connor, E.; Lane, N.E.; Paudel, M.; Redline, S.; Ancoli-Israel, S.; Orwoll, E.; et al. Vitamin D and actigraphic sleep outcomes in older community-dwelling men: The MrOS sleep study. Sleep 2015, 38, 251–257. [Google Scholar] [CrossRef]
- Piovezan, R.D.; Hirotsu, C.; Feres, M.C.; Cintra, F.D.; Andersen, M.L.; Tufik, S.; Poyares, D. Obstructive sleep apnea and objective short sleep duration are independently associated with the risk of serum vitamin D deficiency. PLoS ONE 2017, 12, e0180901. [Google Scholar] [CrossRef] [PubMed]
- Bertisch, S.M.; Sillau, S.; de Boer, I.H.; Szklo, M.; Redline, S. 25-Hydroxyvitamin D Concentration and Sleep Duration and Continuity: Multi-Ethnic Study of Atherosclerosis. Sleep 2015, 38, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Kou, T.; Zhuang, B.; Ren, Y.; Dong, X.; Wang, Q. The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1395. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, W.E.; Sar, M.; Clark, S.A.; DeLuca, H.F. Brain target sites for 1,25-dihydroxyvitamin D3. Science 1982, 215, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Gutierrez-Monreal, M.A.; Cuevas-Diaz Duran, R.; Moreno-Cuevas, J.E.; Scott, S.-P. A Role for 1α,25-Dihydroxyvitamin D3 in the Expression of Circadian Genes. J. Biol. Rhythm. 2014, 29, 384–388. [Google Scholar] [CrossRef]
- McCarty, D.E.; Chesson, A.L., Jr.; Jain, S.K.; Marino, A.A. The link between vitamin D metabolism and sleep medicine. Sleep Med. Rev. 2014, 18, 311–319. [Google Scholar] [CrossRef]
- Depoortere, H.; Francon, D.; Llopis, J. Effects of a magnesium-deficient diet on sleep organization in rats. Neuropsychobiology 1993, 27, 237–245. [Google Scholar] [CrossRef]
- Cao, Y.; Zhen, S.; Taylor, A.W.; Appleton, S.; Atlantis, E.; Shi, Z. Magnesium Intake and Sleep Disorder Symptoms: Findings from the Jiangsu Nutrition Study of Chinese Adults at Five-Year Follow-Up. Nutrients 2018, 10, 1354. [Google Scholar] [CrossRef]
- Abbasi, B.; Kimiagar, M.; Sadeghniiat, K.; Shirazi, M.M.; Hedayati, M.; Rashidkhani, B. The effect of magnesium supplementation on primary insomnia in elderly: A double-blind placebo-controlled clinical trial. J. Res. Med. Sci. 2012, 17, 1161–1169. [Google Scholar] [PubMed]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yanez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; van Ooijen, G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.M.; Dias, C.; Diekman, C.; Brochon, H.; Kim, P.; Kaur, M.; Kim, Y.S.; Jang, H.I.; Kim, Y.I. Magnesium Regulates the Circadian Oscillator in Cyanobacteria. J. Biol. Rhythm. 2019, 34, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Dietary factors and fluctuating levels of melatonin. Food Nutr. Res. 2012, 56. [Google Scholar] [CrossRef]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Sleep symptoms associated with intake of specific dietary nutrients. J. Sleep Res. 2014, 23, 22–34. [Google Scholar] [CrossRef]
- Block, G.; Norkus, E.; Hudes, M.; Mandel, S.; Helzlsouer, K. Which plasma antioxidants are most related to fruit and vegetable consumption? Am. J. Epidemiol. 2001, 154, 1113–1118. [Google Scholar] [CrossRef]
- Stamatakis, K.A.; Brownson, R.C. Sleep duration and obesity-related risk factors in the rural Midwest. Prev. Med. 2008, 46, 439–444. [Google Scholar] [CrossRef]
- Noorwali, E.A.; Cade, J.E.; Burley, V.J.; Hardie, L.J. The relationship between sleep duration and fruit/vegetable intakes in UK adults: A cross-sectional study from the National Diet and Nutrition Survey. BMJ Open 2018, 8, e020810. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Khabour, O.F.; Rashid, B.A.; Damaj, I.M.; Salah, H.A. The neuroprotective effect of vitamin E on chronic sleep deprivation-induced memory impairment: The role of oxidative stress. Behav. Brain Res. 2012, 226, 205–210. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Gamaldo, A.A.; Canas, J.A.; Beydoun, H.A.; Shah, M.T.; McNeely, J.M.; Zonderman, A.B. Serum nutritional biomarkers and their associations with sleep among US adults in recent national surveys. PLoS ONE 2014, 9, e103490. [Google Scholar] [CrossRef]
- Sonoda, T.; Lee, S.K. A novel role for the visual retinoid cycle in melanopsin chromophore regeneration. J. Neurosci. 2016, 36, 9016–9018. [Google Scholar] [CrossRef] [PubMed]
- Ransom, J.; Morgan, P.J.; McCaffery, P.J.; Stoney, P.N. The rhythm of retinoids in the brain. J. Neurochem. 2014, 129, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Liebman, M.; Chopin, L.F.; Carter, E.; Clark, A.J.; Disney, G.W.; Hegsted, M.; Kenney, M.A.; Kirmani, Z.A.; Koonce, K.L.; Korslund, M.K.; et al. Factors related to blood pressure in a biracial adolescent female population. Hypertension 1986, 8, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.; Scialfa, J.H.; Terra, I.M.; Cipolla-Neto, J.; Simonneaux, V.; Afeche, S.C. Tryptophan hydroxylase is modulated by L-type calcium channels in the rat pineal gland. Life Sci. 2008, 82, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; O’Callaghan, J.P.; Juskevich, J.; Lovenberg, W. Activation of brain tryptophan hydroxylase by ATP-MG2+: Dependence on calmodulin. Proc. Natl. Acad. Sci. USA 1980, 77, 4688–4691. [Google Scholar] [CrossRef]
- Cunningham, T.D.; Di Pace, B.S. Is Self-Reported Sleep Duration Associated with Osteoporosis? Data from a 4-Year Aggregated Analysis from the National Health and Nutrition Examination Survey. J. Am. Geriatr. Soc. 2015, 63, 1401–1406. [Google Scholar] [CrossRef]
- Xu, X.; Wang, L.; Chen, L.; Su, T.; Zhang, Y.; Wang, T.; Ma, W.; Yang, F.; Zhai, W.; Xie, Y.; et al. Effects of chronic sleep deprivation on bone mass and bone metabolism in rats. J. Orthop. Surg. Res. 2016, 11, 87. [Google Scholar] [CrossRef]
- Hale, L.; Do, D.P. Racial differences in self-reports of sleep duration in a population-based study. Sleep 2007, 30, 1096–1103. [Google Scholar] [CrossRef]
- Whinnery, J.; Jackson, N.; Rattanaumpawan, P.; Grandner, M.A. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep 2014, 37, 601–611. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Stang, P.; Blacketer, C.; Kent, J.M.; Wittenberg, G.M. Clinical Relevance of Sleep Duration: Results from a Cross-Sectional Analysis Using NHANES. J. Clin. Sleep Med. 2016, 12, 813–819. [Google Scholar] [CrossRef]
- Zhang, L.; Samet, J.; Caffo, B.; Punjabi, N.M. Cigarette smoking and nocturnal sleep architecture. Am. J. Epidemiol. 2006, 164, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; McNeil, J.; Despres, J.P.; Bouchard, C.; Tremblay, A. Short sleep duration is associated with greater alcohol consumption in adults. Appetite 2012, 59, 650–655. [Google Scholar] [CrossRef] [PubMed]
| All | Male | Female | ||||
|---|---|---|---|---|---|---|
| Characteristic | n | mean ± SE | n | mean ± SE | n | mean ± SE |
| Age (years) | 26,287 | 47.12 ± 0.29 * | 13,047 | 46.04 ± 0.32 | 13,240 | 48.17 ± 0.31 |
| Female (%) | 26,287 | 50.87 ± 0.36 * | 13,047 | 100.00 ± 0.00 | 13,240 | 0.00 ± 0.00 |
| Ethnicity | ||||||
| Mexican American (%) | 26,287 | 8.64 ± 0.85 | 13,047 | 9.32 ± 0.90 | 13,240 | 7.99 ± 0.82 |
| Other Hispanic (%) | 26,287 | 5.61 ± 0.54 * | 13,047 | 5.48 ± 0.52 | 13,240 | 5.75 ± 0.57 |
| Non-Hispanic White (%) | 26,287 | 66.93 ± 1.58 * | 13,047 | 67.07 ± 1.58 * | 13,240 | 66.80 ± 1.65 * |
| Non-Hispanic Black (%) | 26,287 | 11.41 ± 0.85 * | 13,047 | 10.64 ± 0.77 * | 13,240 | 12.16 ± 0.94 * |
| Other (%) | 26,287 | 7.40 ± 0.47 | 13,047 | 7.50 ± 0.51 | 13,240 | 7.30 ± 0.49 |
| Poverty-Income Ratio (PIR) | ||||||
| ≤1.35 (%) | 24,028 | 24.28 ± 0.90 * | 11,937 | 22.26 ± 0.94 | 12,091 | 26.24 ± 0.94 * |
| 1.35> to <1.85 (%) | 24,028 | 10.15 ± 0.36 | 11,937 | 9.85 ± 0.47 | 12,091 | 10.45 ± 0.35 |
| ≥1.85 (%) | 24,028 | 65.56 ± 1.09 * | 11,937 | 67.89 ± 1.18 | 12,091 | 63.32 ± 1.09 * |
| Education Level | ||||||
| <High School Graduate (%) | 26,186 | 16.73 ± 0.69 | 12,997 | 17.13 ± 0.78 | 13,189 | 16.34 ± 0.72 * |
| High School Graduate (%) | 26,186 | 22.61 ± 0.60 * | 12,997 | 23.42 ± 0.77 * | 13,189 | 21.83 ± 0.65 |
| >High School Graduate (%) | 26,186 | 60.66 ± 1.05 * | 12,997 | 59.45 ± 1.19 * | 13,189 | 61.83 ± 1.07 * |
| Body Mass Index | 25,929 | 28.89 ± 0.09 * | 12,870 | 28.67 ± 0.10 | 13,059 | 29.10 ± 0.10 * |
| Smoking Current (%) | 26,150 | 19.76 ± 0.60 * | 12,975 | 23.01 ± 0.77 * | 13,175 | 16.62 ± 0.60 * |
| Alcohol (drinks) | 26,287 | 0.78 ± 0.02 | 13,047 | 1.10 ± 0.04 | 13,240 | 0.47 ± 0.02 |
| Sleep Hours | 26,211 | 7.08 ± 0.02 * | 13,006 | 7.01 ± 0.02 * | 13,205 | 7.14 ± 0.02 * |
| Short Sleep (%) | 26,211 | 32.72 ± 0.53 | 13,006 | 34.42 ± 0.72 | 13,205 | 31.08 ± 0.66 |
| Usual Intake (Units) | EAR (% Below) | |||||||
|---|---|---|---|---|---|---|---|---|
| Food Only | Food + Supplement | Food Only | Food + Supplement | |||||
| Short Sleep (Y) | Short Sleep (N) | Short Sleep (Y) | Short Sleep (N) | Short Sleep (Y) | Short Sleep (N) | Short Sleep (Y) | Short Sleep (N) | |
| Calcium (mg) | 953.08 ± 8.87 | 983.88 ± 7.41 * | 1099.47 ± 11.16 | 1166.67 ± 10.74 *# | 44.43 ± 0.96 | 42.10 ± 0.88 | 33.92 ± 0.89 | 29.59 ± 0.74 * |
| Copper (mg) | 1.26 ± 0.01 | 1.32 ± 0.01 * | 1.58 ± 0.02 | 1.68 ± 0.01 * | 7.43 ± 0.47 | 5.23 ± 0.34 * | 5.81 ± 0.39 | 3.82 ± 0.35 * |
| Folate, DFE (mcg) | 528.30 ± 5.59 | 548.50 ± 4.05 * | 726.19 ± 8.14 | 784.66 ± 9.44 * | 14.1 ± 0.77 | 11.03 ± 0.58 * | 10.19 ± 0.65 | 7.01 ± 0.52 * |
| Iron (mg) | 14.80 ± 0.14 | 15.12 ± 0.07 | 17.88 ± 0.19 | 18.74 ± 0.19 * | 5.93 ± 0.34 | 4.86 ± 0.20 * | 4.65 ± 0.26 | 3.52 ± 0.20 * |
| Magnesium (mg) | 296.41 ± 2.80 | 309.93 ± 2.07 * | 321.31 ± 3.66 | 343.68 ± 4.65 *# | 58.56 ± 1.12 | 50.91 ± 0.86 * | 50.74 ± 1.08 | 42.61 ± 0.92 * |
| Niacin (mg) | 26.19 ± 0.26 | 25.95 ± 0.15 | 34.36 ± 0.60 | 37.92 ± 0.84 * | 1.56 ± 0.21 | 1.37 ± 0.15 | 1.06 ± 0.17 | 0.82 ± 0.11 |
| Phosphorus (mg) | 1374.93 ± 11.21 | 1400.31 ± 6.37 | 1384.72 ± 10.44 | 1414.23 ± 7.23 | 1.00 ± 0.15 | 0.66 ± 0.10 | 0.97 ± 0.13 | 0.62 ± 0.09 |
| Riboflavin (mg) | 2.14 ± 0.02 | 2.19 ± 0.01 | 4.40 ± 0.17 | 4.86 ± 0.24 | 3.41 ± 0.24 | 2.59 ± 0.18 * | 2.77 ± 0.25 | 1.98 ± 0.16 * |
| Selenium (mcg) | 113.60 ± 1.18 | 114.71 ± 0.63 | 127.09 ± 1.19 | 131.06 ± 1.03 | 0.65 ± 0.09 | 0.46 ± 0.06 | 0.50 ± 0.12 | 0.34 ± 0.07 |
| Thiamin (mg) | 1.60 ± 0.01 | 1.65 ± 0.01 * | 5.00 ± 0.36 | 6.19 ± 0.53 | 7.72 ± 0.59 | 5.89 ± 0.42 | 5.78 ± 0.44 | 3.89 ± 0.32 * |
| Vitamin A (mcg)1 | 604.08 ± 9.89 | 655.41 ± 7.27 * | 919.60 ± 14.34 | 1009.93 ± 19.09 * | 50.87 ± 1.46 | 42.48 ± 0.99 * | 39.00 ± 1.01 | 31.91 ± 0.98 * |
| Vitamin B12 (mcg) | 5.19 ± 0.08 | 5.20 ± 0.05 | 58.32 ± 5.54 | 55.66 ± 3.63 | 4.36 ± 0.44 | 3.76 ± 0.31 | 3.02 ± 0.30 | 2.32 ± 0.17 |
| Vitamin B6 (mg) | 2.10 ± 0.03 | 2.13 ± 0.02 | 5.28 ± 0.25 | 5.46 ± 0.18 | 11.92 ± 0.84 | 10.53 ± 0.57 | 8.54 ± 0.65 | 6.65 ± 0.38 |
| Vitamin C (mg) | 77.73 ± 1.51 | 85.61 ± 1.21 * | 155.93 ± 5.59 | 175.81 ± 4.28 * | 50.68 ± 1.20 | 43.51 ± 1.00 * | 37.56 ± 1.00 | 30.16 ± 0.78 * |
| Vitamin D (mcg)2 | 4.49 ± 0.07 | 4.81 ± 0.05 * | 12.01 ± 0.39 | 14.93 ± 0.57 *# | 95.70 ± 0.33 | 94.58 ± 0.35 | 68.39 ± 0.72 | 62.58 ± 0.69 * |
| Vitamin E (mg)3 | 8.25 ± 0.10 | 8.84 ± 0.09 * | 25.23 ± 1.03 | 29.22 ± 1.38 | 86.04 ± 0.77 | 82.53 ± 0.81 * | 62.35 ± 0.99 | 57.63 ± 0.84 * |
| Zinc (mg) | 11.44 ± 0.11 | 11.69 ± 0.07 | 15.47 ± 0.20 | 16.20 ± 0.16 * | 17.48 ± 0.94 | 14.42 ± 0.69 * | 12.98 ± 0.71 | 10.10 ± 0.62 * |
| Nutrients with AI, (% Above) | ||||||||
| Potassium (mg) | 2651.10 ± 23.93 | 2725.91 ± 14.89 * | 2673.84 ± 23.09 | 2770.77 ± 15.67 * | 2.28 ± 0.27 | 2.52 ± 0.21 | 2.56 ± 0.31 | 2.93 ± 0.26 |
| Total choline (mg) | 332.67 ± 2.74 | 337.07 ± 1.93 | 333.58 ± 2.55 | 338.07 ± 2.17 | 6.99 ± 0.56 | 7.79 ± 0.50 | 7.17 ± 0.76 | 8.01 ± 0.58 |
| Vitamin K (mcg) | 103.36 ± 2.03 | 114.42 ± 1.87 * | 111.66 ± 3.02 | 119.93 ± 1.75 | 39.39 ± 1.42 | 47.79 ± 1.27 * | 43.63 ± 1.20 | 51.23 ± 1.12 * |
| Usual Intake (Units) | EAR (% Below) | |||||||
|---|---|---|---|---|---|---|---|---|
| Food Only | Food + Supplement | Food Only | Food + Supplement | |||||
| Short Sleep (Y) | Short Sleep (N) | Short Sleep (Y) | Short Sleep (N) | Short Sleep (Y) | Short Sleep (N) | Short Sleep (Y) | Short Sleep (N) | |
| Calcium (mg) | 817.64 ± 10.20 | 872.84 ± 8.62 * | 1013.85 ± 15.08 | 1125.50 ± 12.33 *# | 62.27 ± 1.42 | 56.32 ± 1.12 * | 46.02 ± 1.37 | 37.14 ± 0.96 * |
| Copper (mg) | 1.08 ± 0.01 | 1.17 ± 0.01 * | 1.42 ± 0.03 | 1.54 ± 0.02 * | 12.19 ± 0.93 | 7.84 ± 0.60 * | 9.92 ± 0.77 | 6.01 ± 0.56 * |
| Folate, DFE (mcg) | 441.35 ± 6.17 | 476.66 ± 4.85 * | 672.25 ± 11.01 | 743.91 ± 12.36 * | 22.02 ± 1.43 | 15.88 ± 0.96 * | 15.72 ± 1.06 | 10.01 ± 0.69 * |
| Iron (mg) | 12.31 ± 0.14 | 13.04 ± 0.10 * | 16.66 ± 0.34 | 17.84 ± 0.26 * | 11.84 ± 0.66 | 9.09 ± 0.32 * | 9.33 ± 0.53 | 6.65 ± 0.36 * |
| Magnesium (mg) | 252.32 ± 2.87 | 272.93 ± 2.18 * | 279.89 ± 11.94 | 309.88 ± 6.08 | 59.58 ± 1.48 | 49.4 ± 1.08 * | 51.2 ± 1.41 | 40.48 ± 1.03 * |
| Niacin (mg) | 20.53 ± 0.23 | 21.13 ± 0.14 | 29.00 ± 0.88 | 31.94 ± 0.69 * | 2.79 ± 0.42 | 2.22 ± 0.29 | 1.93 ± 0.34 | 1.36 ± 0.21 |
| Phosphorus (mg) | 1129.11 ± 9.95 | 1192.61 ± 8.95 * | 1136.63 ± 9.44 | 1201.59 ± 8.85 *# | 1.79 ± 0.31 | 1.05 ± 0.19 | 1.72 ± 0.28 | 1.00 ± 0.16 |
| Riboflavin (mg) | 1.76 ± 0.02 | 1.88 ± 0.02 * | 4.32 ± 0.27 | 4.82 ± 0.35 | 4.08 ± 0.40 | 2.58 ± 0.28 * | 3.22 ± 0.35 | 1.96 ± 0.22 * |
| Selenium (mcg) | 91.24 ± 0.90 | 95.51 ± 0.56 * | 102.84 ± 5.06 | 109.38 ± 3.79 | 1.24 ± 0.17 | 0.80 ± 0.11 | 1.01 ± 0.22 | 0.60 ± 0.14 |
| Thiamin (mg) | 1.32 ± 0.01 | 1.40 ± 0.01 * | 5.08 ± 0.49 | 5.87 ± 0.53 | 12.09 ± 1.06 | 8.28 ± 0.72 * | 8.97 ± 0.76 | 5.36 ± 0.56 * |
| Vitamin A (mcg) 1 | 544.15 ± 13.78 | 617.15 ± 8.16 * | 893.63 ± 19.99 | 999.71 ± 22.81 * | 49.3 ± 2.24 | 37.90 ± 1.18 * | 36.84 ± 1.58 | 27.41 ± 1.09 * |
| Vitamin B12 (mcg) | 4.07 ± 0.08 | 4.28 ± 0.05 | 70.47 ± 9.62 | 68.85 ± 4.18 | 7.65 ± 0.90 | 5.96 ± 0.62 | 5.27 ± 0.62 | 3.70 ± 0.37 |
| Vitamin B6 (mg) | 1.66 ± 0.02 | 1.76 ± 0.02 * | 5.30 ± 0.41 | 5.41 ± 0.25 | 19.4 ± 1.28 | 15.35 ± 0.92 | 13.75 ± 1.13 | 9.86 ± 0.68 * |
| Vitamin C (mg) | 70.27 ± 1.73 | 79.53 ± 1.77 * | 157.58 ± 11.91 | 173.88 ± 7.1 | 48.81 ± 1.44 | 40.35 ± 1.26 * | 34.85 ± 1.14 | 26.77 ± 0.94 * |
| Vitamin D (mcg) 2 | 3.82 ± 0.08 | 4.18 ± 0.06 * | 13.47 ± 0.55 | 17.07 ± 0.95 * | 98.52 ± 0.23 | 97.75 ± 0.25 | 66.18 ± 0.97 | 59.51 ± 0.87 * |
| Vitamin E (mg) 3 | 7.06 ± 0.10 | 7.98 ± 0.11 * | 28.02 ± 1.58 | 30.27 ± 1.38 | 93.88 ± 0.67 | 89.17 ± 0.95 * | 64.63 ± 1.1 | 59.60 ± 1.03 * |
| Zinc (mg) | 9.19 ± 0.12 | 9.74 ± 0.08 * | 13.52 ± 0.22 | 14.53 ± 0.15 * | 19.43 ± 1.32 | 14.40 ± 0.83 * | 14.27 ± 1.03 | 9.83 ± 0.62 * |
| Nutrients with AI, (% Above) | ||||||||
| Potassium (mg) | 2241.94 ± 25.24 | 2384.35 ± 17.43 * | 2256.44 ± 21.03 | 2415.34 ± 15.83 * | 0.09 ± 0.03 | 0.20 ± 0.04 | 0.11 ± 0.03 | 0.22 ± 0.04 |
| Total choline (mg) | 263.95 ± 2.51 | 277.38 ± 2.26 * | 264.39 ± 2.23 | 275.29 ± 2.37 * | 2.69 ± 0.40 | 4.09 ± 0.52 | 2.66 ± 0.48 | 3.80 ± 0.61 |
| Vitamin K (mcg) | 99.90 ± 2.62 | 113.42 ± 2.35 * | 110.95 ± 5.91 | 118.93 ± 2.05 | 47.25 ± 1.82 | 56.56 ± 1.50 * | 51.20 ± 1.52 | 59.88 ± 1.25 * |
| Usual Intake (Units) | EAR (% Below) | |||||||
|---|---|---|---|---|---|---|---|---|
| Food Only | Food + Supplement | Food Only | Food + Supplement | |||||
| Short Sleep (Y) | Short Sleep (N) | Short Sleep (Y) | Short Sleep (N) | Short Sleep (Y) | Short Sleep (N) | Short Sleep (Y) | Short Sleep (N) | |
| Calcium (mg) | 1079.42 ± 10.93 | 1105.26 ± 9.76 | 1183.04 ± 13.18 | 1211.53 ± 13.02 | 27.64 ± 0.91 | 26.62 ± 0.91 | 22.07 ± 0.86 | 21.38 ± 0.82 |
| Copper (mg) | 1.42 ± 0.02 | 1.47 ± 0.01 | 1.72 ± 0.02 | 1.82 ± 0.02 * | 2.99 ± 0.32 | 2.51 ± 0.26 | 2.02 ± 0.26 | 1.53 ± 0.22 |
| Folate, DFE (mcg) | 610.05 ± 7.75 | 626.44 ± 5.52 | 777.89 ± 11.55 | 828.41 ± 9.68 * | 6.68 ± 0.55 | 5.79 ± 0.50 | 4.76 ± 0.52 | 3.85 ± 0.42 |
| Iron (mg) | 17.13 ± 0.18 | 17.38 ± 0.12 | 19.06 ± 0.30 | 19.73 ± 0.24 | 0.37 ± 0.08 | 0.27 ± 0.13 | 0.19 ± 0.05 | 0.19 ± 0.03 |
| Magnesium (mg) | 337.99 ± 3.73 | 350.13 ± 2.72 * | 361.63 ± 8.42 | 380.22 ± 12.07 | 57.27 ± 1.31 | 52.61 ± 0.94 * | 50.2 ± 1.32 | 44.89 ± 0.99 * |
| Niacin (mg) | 31.52 ± 0.38 | 31.21 ± 0.25 | 39.53 ± 0.64 | 44.37 ± 1.51 * | 0.34 ± 0.07 | 0.40 ± 0.08 | 0.22 ± 0.06 | 0.24 ± 0.05 |
| Phosphorus (mg) | 1604.52 ± 15.40 | 1627.01 ± 8.67 | 1626.43 ± 13.28 | 1644.8 ± 9.79 | 0.22 ± 0.05 | 0.20 ± 0.05 | 0.23 ± 0.05 | 0.22 ± 0.03 |
| Riboflavin (mg) | 2.50 ± 0.03 | 2.53 ± 0.02 | 4.46 ± 0.21 | 4.92 ± 0.24 | 2.80 ± 0.29 | 2.59 ± 0.23 | 2.22 ± 0.24 | 2.05 ± 0.21 |
| Selenium (mcg) | 134.59 ± 1.70 | 135.59 ± 1.03 | 150.71 ± 4.58 | 154.45 ± 5.23 | 0.08 ± 0.03 | 0.09 ± 0.02 | 0.05 ± 0.02 | 0.05 ± 0.02 |
| Thiamin (mg) | 1.87 ± 0.02 | 1.91 ± 0.01 | 4.93 ± 0.49 | 6.55 ± 0.94 | 3.76 ± 0.37 | 3.22 ± 0.34 | 2.81 ± 0.31 | 2.32 ± 0.29 |
| Vitamin A (mcg) 1 | 659.82 ± 13.35 | 696.2 ± 11.04 | 944.66 ± 20.29 | 1021.05 ± 23.44 | 52.34 ± 1.43 | 47.62 ± 1.11 * | 41.14 ± 1.28 | 36.79 ± 1.22 |
| Vitamin B12 (mcg) | 6.24 ± 0.14 | 6.20 ± 0.09 | 46.55 ± 5.16 | 41.44 ± 5.77 | 1.33 ± 0.30 | 1.38 ± 0.24 | 0.87 ± 0.24 | 0.83 ± 0.20 |
| Vitamin B6 (mg) | 2.51 ± 0.04 | 2.53 ± 0.02 | 5.27 ± 0.29 | 5.52 ± 0.21 | 5.04 ± 0.59 | 5.19 ± 0.55 | 3.50 ± 0.42 | 3.25 ± 0.31 |
| Vitamin C (mg) | 84.85 ± 2.52 | 92.29 ± 1.54 | 154.11 ± 11.53 | 177.78 ± 5.19 | 52.28 ± 1.81 | 46.86 ± 1.11 | 40.26 ± 1.73 | 33.89 ± 0.90 * |
| Vitamin D (mcg) 2 | 5.14 ± 0.10 | 5.51 ± 0.09 * | 10.59 ± 0.51 | 12.63 ± 0.46 * | 93.03 ± 0.56 | 91.12 ± 0.62 | 70.47 ± 0.85 | 65.92 ± 0.96 * |
| Vitamin E (mg) 3 | 9.35 ± 0.15 | 9.78 ± 0.11 | 22.54 ± 1.18 | 28.08 ± 2.11 | 78.75 ± 1.29 | 75.27 ± 1.01 | 60.10 ± 1.37 | 55.54 ± 0.98 * |
| Zinc (mg) | 13.57 ± 0.16 | 13.81 ± 0.13 | 17.34 ± 0.35 | 17.99 ± 0.27 | 15.60 ± 1.08 | 14.40 ± 1.01 | 11.79 ± 0.77 | 10.44 ± 0.89 |
| Nutrients with AI, (% Above) | ||||||||
| Potassium (mg) | 3033.55 ± 34.93 | 3097.19 ± 19.03 | 3078.15 ± 36.37 | 3151.68 ± 22.65 | 4.35 ± 0.50 | 5.06 ± 0.43 | 4.95 ± 0.60 | 5.84 ± 0.55 |
| Total choline (mg) | 396.75 ± 4.20 | 401.98 ± 2.88 | 400.27 ± 3.73 | 405.75 ± 3.00 | 10.98 ± 0.94 | 11.86 ± 0.76 | 11.50 ± 1.18 | 12.54 ± 0.95 |
| Vitamin K (mcg) | 106.78 ± 2.17 | 115.66 ± 2.08 * | 112.36 ± 2.29 | 120.76 ± 2.28 * | 32.17 ± 1.50 | 38.27 ± 1.37 * | 36.25 ± 1.65 | 41.77 ± 1.36 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikonte, C.J.; Mun, J.G.; Reider, C.A.; Grant, R.W.; Mitmesser, S.H. Micronutrient Inadequacy in Short Sleep: Analysis of the NHANES 2005–2016. Nutrients 2019, 11, 2335. https://doi.org/10.3390/nu11102335
Ikonte CJ, Mun JG, Reider CA, Grant RW, Mitmesser SH. Micronutrient Inadequacy in Short Sleep: Analysis of the NHANES 2005–2016. Nutrients. 2019; 11(10):2335. https://doi.org/10.3390/nu11102335
Chicago/Turabian StyleIkonte, Chioma J., Jonathan G. Mun, Carroll A. Reider, Ryan W. Grant, and Susan Hazels Mitmesser. 2019. "Micronutrient Inadequacy in Short Sleep: Analysis of the NHANES 2005–2016" Nutrients 11, no. 10: 2335. https://doi.org/10.3390/nu11102335
APA StyleIkonte, C. J., Mun, J. G., Reider, C. A., Grant, R. W., & Mitmesser, S. H. (2019). Micronutrient Inadequacy in Short Sleep: Analysis of the NHANES 2005–2016. Nutrients, 11(10), 2335. https://doi.org/10.3390/nu11102335




