Ketogenic Diets and Exercise Performance
Abstract
1. Introduction
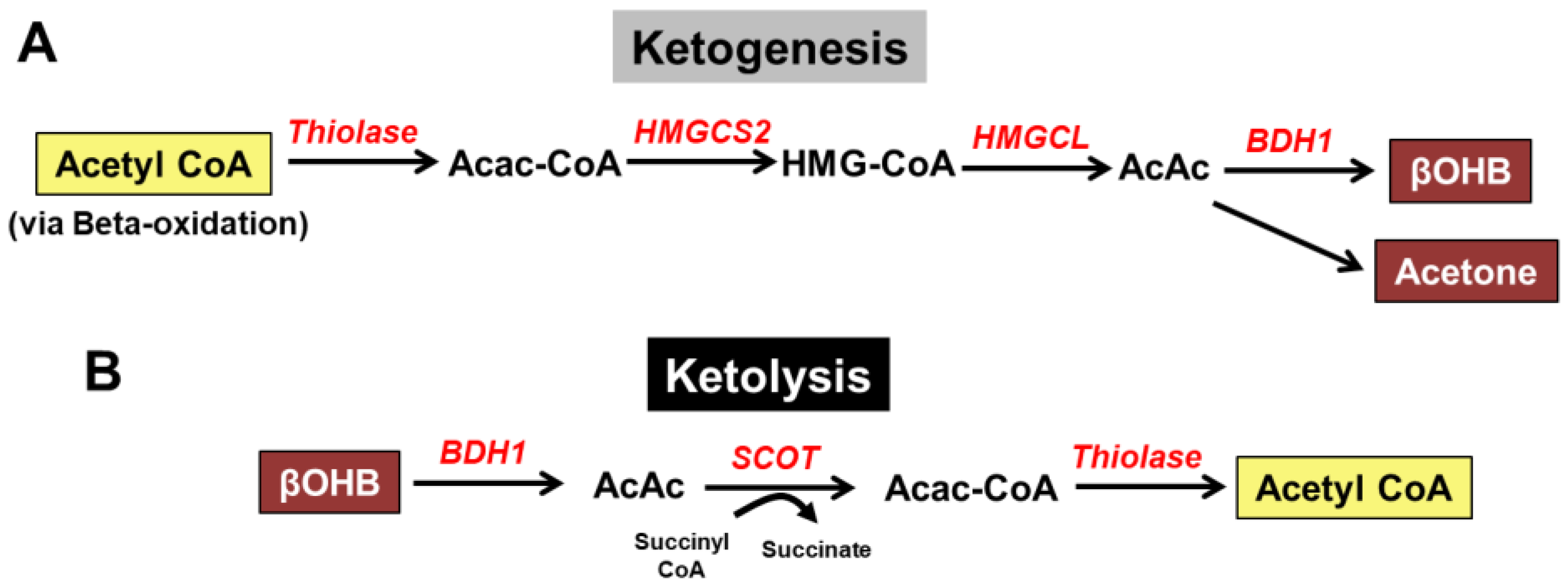
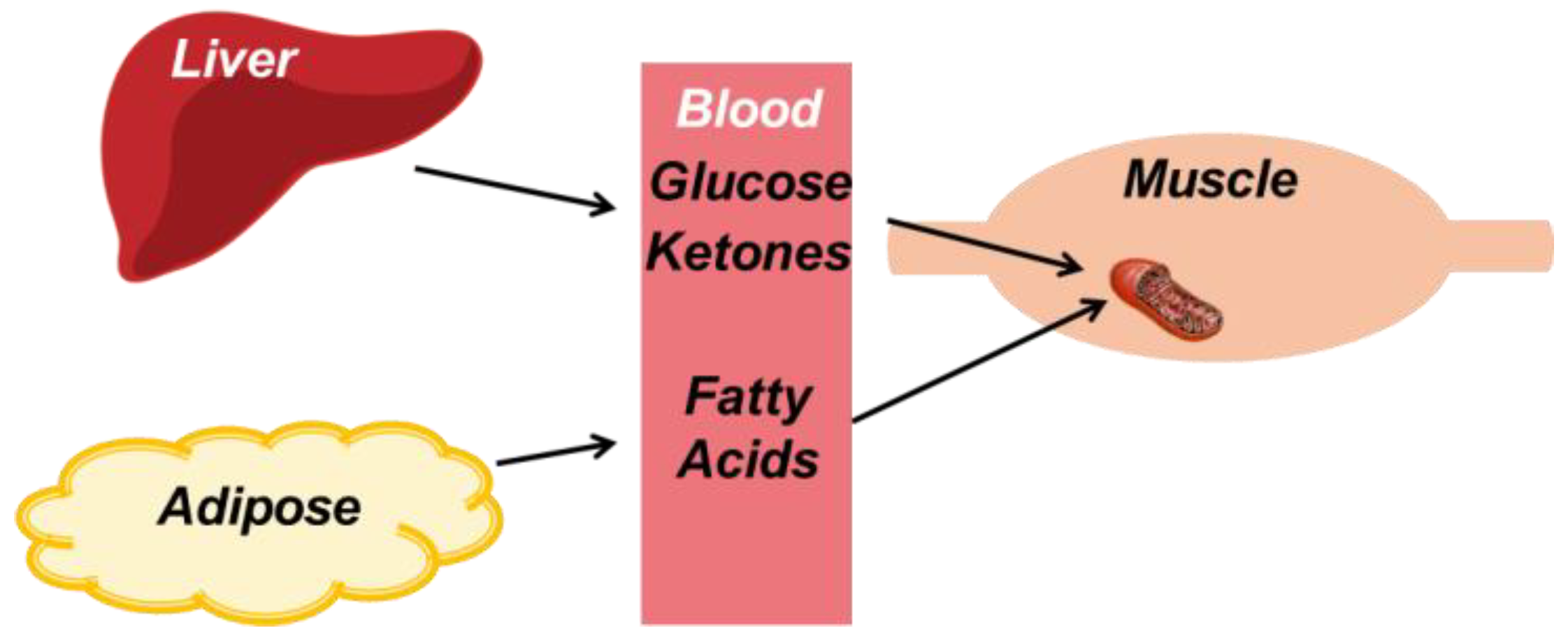
2. Overview of Ketone Body Metabolism
3. Ketogenic Diets and Weight Loss
4. Ketogenic Diets and Exercise Performance
4.1. Overview of Metabolism During Exercise
4.2. The Effects on Aerobic Endurance Exercise
4.3. Its Effects on Anaerobic Exercise
5. Ketone Body Supplementation
6. Ketone Body Metabolism and the Heart
7. Potential Side Effects of Ketogenic Diets
8. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Joshi, S.; Ostfeld, R.J.; McMacken, M. The Ketogenic Diet for Obesity and Diabetes-Enthusiasm Outpaces Evidence. JAMA Intern. Med. 2019, 179, 1163–1164. [Google Scholar] [CrossRef]
- Cox, P.J.; Kirk, T.; Ashmore, T.; Willerton, K.; Evans, R.; Smith, A.; Murray, A.J.; Stubbs, B.; West, J.; McLure, S.W.; et al. Nutritional Ketosis Alters Fuel Preference and Thereby Endurance Performance in Athletes. Cell Metab. 2016, 24, 256–268. [Google Scholar] [CrossRef]
- Murray, A.J.; Knight, N.S.; Cole, M.A.; Cochlin, L.E.; Carter, E.; Tchabanenko, K.; Pichulik, T.; Gulston, M.K.; Atherton, H.J.; Schroeder, M.A.; et al. Novel Ketone Diet Enhances Physical and Cognitive Performance. FASEB J. 2016, 30, 4021–4032. [Google Scholar] [CrossRef]
- Paoli, A. Ketogenic Diet for Obesity: Friend or Foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef]
- Veech, R.L. The Therapeutic Implications of Ketone Bodies: The Effects of Ketone Bodies in Pathological Conditions: Ketosis, Ketogenic Diet, Redox States, Insulin Resistance, and Mitochondrial Metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Kosinski, C.; Jornayvaz, F.R. Effects of Ketogenic Diets on Cardiovascular Risk Factors: Evidence from Animal and Human Studies. Nutrients 2017, 9, 517. [Google Scholar] [CrossRef]
- Shilpa, J.; Mohan, V. Ketogenic Diets: Boon or Bane? Indian J. Med. Res. 2018, 148, 251–253. [Google Scholar] [CrossRef]
- Krebs, H.A. The Regulation of the Release of Ketone Bodies by the Liver. Adv. Enzyme Regul. 1966, 4, 339–354. [Google Scholar] [CrossRef]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Kruger, M.; Hoppel, C.L.; et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef]
- Bedi, K.C., Jr.; Snyder, N.W.; Brandimarto, J.; Aziz, M.; Mesaros, C.; Worth, A.J.; Wang, L.L.; Javaheri, A.; Blair, I.A.; Margulies, K.B.; et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation 2016, 133, 706–716. [Google Scholar] [CrossRef]
- Horton, J.L.; Davidson, M.T.; Kurishima, C.; Vega, R.B.; Powers, J.C.; Matsuura, T.R.; Petucci, C.; Lewandowski, E.D.; Crawford, P.A.; Muoio, D.M.; et al. The Failing Heart Utilizes 3-Hydroxybutyrate as a Metabolic Stress Defense. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Peters, J.P.; Van Slyke, D. Quantitative Clinical Chemistry, 2nd ed.; Williams & Wilkins: Baltimore, MD, USA, 1946. [Google Scholar]
- Salway, J.G. Metabolism at a Glance, 3rd ed.; Blackwell Pub.: Malden, MA, USA, 2004. [Google Scholar]
- Wentz, A.E.; d’Avignon, D.A.; Weber, M.L.; Cotter, D.G.; Doherty, J.M.; Kerns, R.; Nagarajan, R.; Reddy, N.; Sambandam, N.; Crawford, P.A. Adaptation of Myocardial Substrate Metabolism to a Ketogenic Nutrient Environment. J. Biol. Chem. 2010, 285, 24447–24456. [Google Scholar] [CrossRef]
- Rowe, P.; O’Neill, C.; DeWitt, E.; Kolwicz, S.C., Jr. Endurance Exercise Capacity and Substrate Metabolism in Male and Female Mice. FASEB J. 2019, 33, 698.1. [Google Scholar]
- Avogaro, A.; Crepaldi, C.; Miola, M.; Maran, A.; Pengo, V.; Tiengo, A.; del Prato, S. High Blood Ketone Body Concentration in Type 2 Non-Insulin Dependent Diabetic Patients. J. Endocrinol. Investig. 1996, 19, 99–105. [Google Scholar] [CrossRef]
- Guerci, B.; Benichou, M.; Floriot, M.; Bohme, P.; Fougnot, S.; Franck, P.; Drouin, P. Accuracy of an Electrochemical Sensor for Measuring Capillary Blood Ketones by Fingerstick Samples During Metabolic Deterioration after Continuous Subcutaneous Insulin Infusion Interruption in Type 1 Diabetic Patients. Diabetes Care 2003, 26, 1137–1141. [Google Scholar] [CrossRef]
- Reichard, G.A., Jr.; Owen, O.E.; Haff, A.C.; Paul, P.; Bortz, W.M. Ketone-Body Production and Oxidation in Fasting Obese Humans. J. Clin. Investig. 1974, 53, 508–515. [Google Scholar] [CrossRef]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of Ketone Bodies During Exercise and Training: Physiological Basis for Exogenous Supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [CrossRef]
- Volek, J.S.; Freidenreich, D.J.; Saenz, C.; Kunces, L.J.; Creighton, B.C.; Bartley, J.M.; Davitt, P.M.; Munoz, C.X.; Anderson, J.M.; Maresh, C.M.; et al. Metabolic Characteristics of Keto-Adapted Ultra-Endurance Runners. Metabolism 2016, 65, 100–110. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Pissios, P.; Otu, H.; Roberson, R.; Xue, B.; Asakura, K.; Furukawa, N.; Marino, F.E.; Liu, F.F.; Kahn, B.B.; et al. A High-Fat, Ketogenic Diet Induces a Unique Metabolic State in Mice. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1724–E1739. [Google Scholar] [CrossRef]
- Kolwicz, S.C., Jr.; Olson, D.P.; Marney, L.C.; Garcia-Menendez, L.; Synovec, R.E.; Tian, R. Cardiac-Specific Deletion of Acetyl Coa Carboxylase 2 Prevents Metabolic Remodeling during Pressure-Overload Hypertrophy. Circ. Res. 2012, 111, 728–738. [Google Scholar] [CrossRef]
- Yan, J.; Young, M.E.; Cui, L.; Lopaschuk, G.D.; Liao, R.; Tian, R. Increased Glucose Uptake and Oxidation in Mouse Hearts Prevent High Fatty Acid Oxidation but Cause Cardiac Dysfunction in Diet-Induced Obesity. Circulation 2009, 119, 2818–2828. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Wilson, M.C. The Monocarboxylate Transporter Family—Role and Regulation. IUBMB Life 2012, 64, 109–119. [Google Scholar] [CrossRef]
- Cotter, D.G.; Schugar, R.C.; Crawford, P.A. Ketone Body Metabolism and Cardiovascular Disease. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1060–H1076. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Uddin, G.M.; Ho, K.L.; Lopaschuk, G.D. Loss of Metabolic Flexibility in the Failing Heart. Front. Cardiovasc. Med. 2018, 5, 68. [Google Scholar] [CrossRef]
- Opie, L.H. Heart Physiology: From Cell to Circulation, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Kashiwaya, Y.; King, M.T.; Veech, R.L. Substrate Signaling by Insulin: A Ketone Bodies Ratio Mimics Insulin Action in Heart. Am. J. Cardiol. 1997, 80, 50A–64A. [Google Scholar] [CrossRef]
- Sato, K.; Kashiwaya, Y.; Keon, C.A.; Tsuchiya, N.; King, M.T.; Radda, G.K.; Chance, B.; Clarke, K.; Veech, R.L. Insulin, Ketone Bodies, and Mitochondrial Energy Transduction. FASEB J. 1995, 9, 651–658. [Google Scholar] [CrossRef]
- Ho, K.L.; Zhang, L.; Wagg, C.; al Batran, R.; Gopal, K.; Levasseur, J.; Leone, T.; Dyck, J.R.B.; Ussher, J.R.; Muoio, D.M.; et al. Increased Ketone Body Oxidation Provides Additional Energy for the Failing Heart without Improving Cardiac Efficiency. Cardiovasc. Res. 2019, 115, 1606–1616. [Google Scholar] [CrossRef]
- Gosmanov, A.R.; Gosmanova, E.O.; Dillard-Cannon, E. Management of Adult Diabetic Ketoacidosis. Diabetes Metab. Syndr. Obes. 2014, 7, 255–264. [Google Scholar] [CrossRef]
- Kanikarla-Marie, P.; Jain, S.K. Hyperketonemia and Ketosis Increase the Risk of Complications in Type 1 Diabetes. Free Radic. Biol. Med. 2016, 95, 268–277. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Diabetic Ketoacidosis. Lancet 1995, 345, 767–772. [Google Scholar] [CrossRef]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef]
- Ryu, S.; Frith, E.; Pedisic, Z.; Kang, M.; Loprinzi, P.D. Secular Trends in the Association between Obesity and Hypertension among Adults in the United States, 1999-2014. Eur. J. Intern. Med. 2019, 62, 37–42. [Google Scholar] [CrossRef]
- American Diabetes, Association. 6. Obesity Management for the Treatment of Type 2 Diabetes. Diabetes Care 2016, 39 (Suppl. 1), S47–S51. [Google Scholar] [CrossRef]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef]
- Kuchkuntla, A.R.; Limketkai, B.; Nanda, S.; Hurt, R.T.; Mundi, M.S. Fad Diets: Hype or Hope? Curr. Nutr. Rep. 2018, 7, 310–323. [Google Scholar] [CrossRef]
- Wilder, R.M. The Effect of Ketonemia on the Course of Epilepsy. Mayo Clin. Bull. 1921, 2, 307–308. [Google Scholar]
- Woodyatt, R.T. Objects and Methods of Diet Adjustment in Diabetics. Arch. Intern. Med. 1921, 28, 125–141. [Google Scholar] [CrossRef]
- Peterman, M.G. The Ketogenic Diet in Epilepsy. JAMA 1925, 84, 1979–1983. [Google Scholar] [CrossRef]
- Wheless, J.W. History of the Ketogenic Diet. Epilepsia 2008, 49 (Suppl. 8), 3–5. [Google Scholar] [CrossRef]
- Scholl-Burgi, S.; Holler, A.; Pichler, K.; Michel, M.; Haberlandt, E.; Karall, D. Ketogenic Diets in Patients with Inherited Metabolic Disorders. J. Inherit. Metab. Dis. 2015, 38, 765–773. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Group Dietary Intervention Randomized Controlled Trial. Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-Fat Diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef]
- Sondike, S.B.; Copperman, N.; Jacobson, M.S. Effects of a Low-Carbohydrate Diet on Weight Loss and Cardiovascular Risk Factor in Overweight Adolescents. J. Pediatr. 2003, 142, 253–258. [Google Scholar] [CrossRef]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone Diets for Weight Loss and Heart Disease Risk Reduction: A Randomized Trial. JAMA 2005, 293, 43–53. [Google Scholar] [CrossRef]
- McAuley, K.A.; Hopkins, C.M.; Smith, K.J.; McLay, R.T.; Williams, S.M.; Taylor, R.W.; Mann, J.I. Comparison of High-Fat and High-Protein Diets with a High-Carbohydrate Diet in Insulin-Resistant Obese Women. Diabetologia 2005, 48, 8–16. [Google Scholar] [CrossRef]
- De Souza, R.J.; Bray, G.A.; Carey, V.J.; Hall, K.D.; LeBoff, M.S.; Loria, C.M.; Laranjo, N.M.; Sacks, F.M.; Smith, S.R. Effects of 4 Weight-Loss Diets Differing in Fat, Protein, and Carbohydrate on Fat Mass, Lean Mass, Visceral Adipose Tissue, and Hepatic Fat: Results from the Pounds Lost Trial. Am. J. Clin. Nutr. 2012, 95, 614–625. [Google Scholar] [CrossRef]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of Weight-Loss Diets with Different Compositions of Fat, Protein, and Carbohydrates. N. Engl. J. Med. 2009, 360, 859–873. [Google Scholar] [CrossRef]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; Makris, A.P.; Rosenbaum, D.L.; Brill, C.; Stein, R.I.; Mohammed, B.S.; Miller, B.; Rader, D.J.; et al. Weight and Metabolic Outcomes after 2 Years on a Low-Carbohydrate Versus Low-Fat Diet: A Randomized Trial. Ann. Intern. Med. 2010, 153, 147–157. [Google Scholar] [CrossRef]
- Bueno, N.B.; de Melo, I.S.; de Oliveira, S.L.; Ataide, T.D. Very-Low-Carbohydrate Ketogenic Diet V. Low-Fat Diet for Long-Term Weight Loss: A Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef]
- Badman, M.K.; Kennedy, A.R.; Adams, A.C.; Pissios, P.; Maratos-Flier, E. A Very Low Carbohydrate Ketogenic Diet Improves Glucose Tolerance in Ob/Ob Mice Independently of Weight Loss. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1197–E1204. [Google Scholar] [CrossRef]
- Ellenbroek, J.H.; van Dijck, L.; Tons, H.A.; Rabelink, T.J.; Carlotti, F.; Ballieux, B.E.; de Koning, E.J. Long-Term Ketogenic Diet Causes Glucose Intolerance and Reduced Beta- and Alpha-Cell Mass but No Weight Loss in Mice. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E552–E558. [Google Scholar] [CrossRef]
- McArdle, W.D.; Katch, F.I.; Katch, V.L. Essentials of Exercise Physiology, 5th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2016. [Google Scholar]
- Greenberg, C.C.; Jurczak, M.J.; Danos, A.M.; Brady, M.J. Glycogen Branches Out: New Perspectives on the Role of Glycogen Metabolism in the Integration of Metabolic Pathways. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1–E8. [Google Scholar] [CrossRef]
- Stainsby, W.N.; Barclay, J.K. Exercise Metabolism: O 2 Deficit, Steady Level O 2 Uptake and O 2 Uptake for Recovery. Med. Sci. Sports 1970, 2, 177–181. [Google Scholar]
- Arner, P.; Kriegholm, E.; Engfeldt, P.; Bolinder, J. Adrenergic Regulation of Lipolysis in Situ at Rest and During Exercise. J. Clin. Investig. 1990, 85, 893–898. [Google Scholar] [CrossRef]
- Stanley, W.C. Myocardial Lactate Metabolism During Exercise. Med. Sci. Sports Exerc. 1991, 23, 920–924. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low Carbohydrate, High Fat Diet Impairs Exercise Economy and Negates the Performance Benefit from Intensified Training in Elite Race Walkers. J. Physiol. 2017, 595, 2785–2807. [Google Scholar] [CrossRef]
- Carr, A.J.; Sharma, A.P.; Ross, M.L.; Welvaert, M.; Slater, G.J.; Burke, L.M. Chronic Ketogenic Low Carbohydrate High Fat Diet Has Minimal Effects on Acid-Base Status in Elite Athletes. Nutrients 2018, 10, 236. [Google Scholar] [CrossRef]
- Cipryan, L.; Plews, D.J.; Ferretti, A.; Maffetone, P.B.; Laursen, P.B. Effects of a 4-Week Very Low-Carbohydrate Diet on High-Intensity Interval Training Responses. J. Sports Sci. Med. 2018, 17, 259–268. [Google Scholar]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Siedzik, K. Effect of a Four-Week Ketogenic Diet on Exercise Metabolism in Crossfit-Trained Athletes. J. Int. Soc. Sports Nutr. 2019, 16, 16. [Google Scholar] [CrossRef]
- Kephart, W.C.; Pledge, C.D.; Roberson, P.A.; Mumford, P.W.; Romero, M.A.; Mobley, C.B.; Martin, J.S.; Young, K.C.; Lowery, R.P.; Wilson, J.M.; et al. The Three-Month Effects of a Ketogenic Diet on Body Composition, Blood Parameters, and Performance Metrics in Crossfit Trainees: A Pilot Study. Sports (Basel) 2018, 6, 1. [Google Scholar] [CrossRef]
- McSwiney, F.T.; Wardrop, B.; Hyde, P.N.; Lafountain, R.A.; Volek, J.S.; Doyle, L. Keto-Adaptation Enhances Exercise Performance and Body Composition Responses to Training in Endurance Athletes. Metabolism 2018, 81, 25–34. [Google Scholar] [CrossRef]
- O’Neal, E.K.; Smith, A.F.; Heatherly, A.J.; Killen, L.G.; Waldman, H.S.; Hollingsworth, A.; Koh, Y. Effects of a 3-Week High-Fat-Low-Carbohydrate Diet on Lipid and Glucose Profiles in Experienced, Middle-Age Male Runners. Int. J. Exerc. Sci. 2019, 12, 786–799. [Google Scholar]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Maunder, E.; Dulson, D.K. Effect of a Ketogenic Diet on Submaximal Exercise Capacity and Efficiency in Runners. Med. Sci. Sports Exerc. 2019. [Google Scholar] [CrossRef]
- Zajac, A.; Poprzecki, S.; Maszczyk, A.; Czuba, M.; Michalczyk, M.; Zydek, G. The Effects of a Ketogenic Diet on Exercise Metabolism and Physical Performance in Off-Road Cyclists. Nutrients 2014, 6, 2493–2508. [Google Scholar] [CrossRef]
- Zinn, C.; Wood, M.; Williden, M.; Chatterton, S.; Maunder, E. Ketogenic Diet Benefits Body Composition and Well-Being but Not Performance in a Pilot Case Study of New Zealand Endurance Athletes. J. Int. Soc. Sports Nutr. 2017, 14, 22. [Google Scholar] [CrossRef]
- Brinkworth, G.D.; Noakes, M.; Clifton, P.M.; Buckley, J.D. Effects of a Low Carbohydrate Weight Loss Diet on Exercise Capacity and Tolerance in Obese Subjects. Obesity (Silver Spring) 2009, 17, 1916–1923. [Google Scholar] [CrossRef]
- Walberg, J.L.; Ruiz, V.K.; Tarlton, S.L.; Hinkle, D.E.; Thye, F.W. Exercise Capacity and Nitrogen Loss During a High or Low Carbohydrate Diet. Med. Sci. Sports Exerc. 1988, 20, 34–43. [Google Scholar] [CrossRef]
- Phinney, S.D.; Horton, E.S.; Sims, E.A.; Hanson, J.S.; Danforth, E., Jr.; LaGrange, B.M. Capacity for Moderate Exercise in Obese Subjects after Adaptation to a Hypocaloric, Ketogenic Diet. J. Clin. Investig. 1980, 66, 1152–1161. [Google Scholar] [CrossRef]
- White, A.M.; Johnston, C.S.; Swan, P.D.; Tjonn, S.L.; Sears, B. Blood Ketones Are Directly Related to Fatigue and Perceived Effort During Exercise in Overweight Adults Adhering to Low-Carbohydrate Diets for Weight Loss: A Pilot Study. J. Am. Diet. Assoc. 2007, 107, 1792–1796. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Buckley, J.D.; Noakes, M.; Clifton, P.M.; Brinkworth, G.D. Long-Term Effects of a Very Low-Carbohydrate Weight Loss Diet on Exercise Capacity and Tolerance in Overweight and Obese Adults. J. Am. Coll. Nutr. 2014, 33, 267–273. [Google Scholar] [CrossRef]
- Phinney, S.D. Ketogenic Diets and Physical Performance. Nutr. Metab. (Lond.) 2004, 1, 2. [Google Scholar] [CrossRef]
- Holland, A.M.; Kephart, W.C.; Mumford, P.W.; Mobley, C.B.; Lowery, R.P.; Shake, J.J.; Patel, R.K.; Healy, J.C.; McCullough, D.J.; Kluess, H.A.; et al. Effects of a Ketogenic Diet on Adipose Tissue, Liver, and Serum Biomarkers in Sedentary Rats and Rats That Exercised Via Resisted Voluntary Wheel Running. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R337–R351. [Google Scholar] [CrossRef]
- Huang, Q.; Ma, S.; Tominaga, T.; Suzuki, K.; Liu, C. An 8-Week, Low Carbohydrate, High Fat, Ketogenic Diet Enhanced Exhaustive Exercise Capacity in Mice Part 2: Effect on Fatigue Recovery, Post-Exercise Biomarkers and Anti-Oxidation Capacity. Nutrients 2018, 10, 1339. [Google Scholar] [CrossRef]
- Ma, S.; Huang, Q.; Yada, K.; Liu, C.; Suzuki, K. An 8-Week Ketogenic Low Carbohydrate, High Fat Diet Enhanced Exhaustive Exercise Capacity in Mice. Nutrients 2018, 10, 673. [Google Scholar] [CrossRef]
- Miller, W.C.; Bryce, G.R.; Conlee, R.K. Adaptations to a High-Fat Diet That Increase Exercise Endurance in Male Rats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 56, 78–83. [Google Scholar] [CrossRef]
- Nilsson, J.; Ericsson, M.; Joibari, M.M.; Anderson, F.; Carlsson, L.; Nilsson, S.K.; Sjodin, A.; Buren, J. A Low-Carbohydrate High-Fat Diet Decreases Lean Mass and Impairs Cardiac Function in Pair-Fed Female C57bl/6j Mice. Nutr. Metab. (Lond.) 2016, 13, 79. [Google Scholar] [CrossRef]
- Newman, J.C.; Covarrubias, A.J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017, 26, 547–557. [Google Scholar] [CrossRef]
- Parry, H.A.; Kephart, W.C.; Mumford, P.W.; Romero, M.A.; Mobley, C.B.; Zhang, Y.; Roberts, M.D.; Kavazis, A.N. Ketogenic Diet Increases Mitochondria Volume in the Liver and Skeletal Muscle without Altering Oxidative Stress Markers in Rats. Heliyon 2018, 4, e00975. [Google Scholar] [CrossRef]
- Gregory, R.M.; Hamdan, H.; Torisky, D.M.; Akers, J.D. A Low-Carbohydrate Ketogenic Diet Combined with 6-Weeks of Crossfit Training Improves Body Composition and Performance. Int. J. Sports Exerc. Med. 2017, 3, 54. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lowery, R.P.; Roberts, M.D.; Sharp, M.H.; Joy, J.M.; Shields, K.A.; Partl, J.; Volek, J.S.; D’Agostino, D. The Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Males. J. Strength Cond. Res. 2017. [Google Scholar] [CrossRef]
- Paoli, A.; Grimaldi, K.; D’Agostino, D.; Cenci, L.; Moro, T.; Bianco, A.; Palma, A. Ketogenic Diet Does Not Affect Strength Performance in Elite Artistic Gymnasts. J. Int. Soc. Sports Nutr. 2012, 9, 34. [Google Scholar] [CrossRef]
- Greene, D.A.; Varley, B.J.; Hartwig, T.B.; Chapman, P.; Rigney, M. A Low-Carbohydrate Ketogenic Diet Reduces Body Mass without Compromising Performance in Powerlifting and Olympic Weightlifting Athletes. J. Strength Cond. Res. 2018, 32, 3373–3382. [Google Scholar] [CrossRef]
- Vargas, S.; Romance, R.; Petro, J.L.; Bonilla, D.A.; Galancho, I.; Espinar, S.; Kreider, R.B.; Benítez-Porres, J. Efficacy of Ketogenic Diet on Body Composition During Resistance Training in Trained Men: A Randomized Controlled Trial. J. Int. Soc. Sports Nutr. 2018, 15, 31. [Google Scholar] [CrossRef]
- Scott, B.E.; Laursen, P.B.; James, L.J.; Boxer, B.; Chandler, Z.; Lam, E.; Gascoyne, T.; Messenger, J.; Mears, S.A. The Effect of 1,3-Butanediol and Carbohydrate Supplementation on Running Performance. J. Sci. Med. Sport 2019, 22, 702–706. [Google Scholar] [CrossRef]
- Harvey, C.J.d.C.; Schofield, G.M.; Williden, M.; McQuillan, J.A. The Effect of Medium Chain Triglycerides on Time to Nutritional Ketosis and Symptoms of Keto-Induction in Healthy Adults: A Randomised Controlled Clinical Trial. J. Nutr. Metab. 2018, 2018, 2630565. [Google Scholar] [CrossRef]
- Dearlove, D.J.; Faull, O.K.; Rolls, E.; Clarke, K.; Cox, P.J. Nutritional Ketoacidosis During Incremental Exercise in Healthy Athletes. Front. Physiol. 2019, 10, 290. [Google Scholar] [CrossRef]
- Evans, M.; Egan, B. Intermittent Running and Cognitive Performance after Ketone Ester Ingestion. Med. Sci. Sports Exerc. 2018, 50, 2330–2338. [Google Scholar] [CrossRef]
- Leckey, J.J.; Ross, M.L.; Quod, M.; Hawley, J.A.; Burke, L.M. Ketone Diester Ingestion Impairs Time-Trial Performance in Professional Cyclists. Front. Physiol. 2017, 8, 806. [Google Scholar] [CrossRef]
- Kesl, S.L.; Poff, A.M.; Ward, N.P.; Fiorelli, T.N.; Ari, C.; van Putten, A.J.; Sherwood, J.W.; Arnold, P.; D’Agostino, D.P. Effects of Exogenous Ketone Supplementation on Blood Ketone, Glucose, Triglyceride, and Lipoprotein Levels in Sprague-Dawley Rats. Nutr. Metab. (Lond.) 2016, 13, 9. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Santer, P.; Miller, J.J.; Faull, O.K.; Magor-Elliott, S.; Hiyama, S.; Stirling, M.; Clarke, K. On the Metabolism of Exogenous Ketones in Humans. Front. Physiol. 2017, 8, 848. [Google Scholar] [CrossRef]
- Tate, R.L.; Mehlman, M.A.; Tobin, R.B. Metabolic Fate of 1,3-Butanediol in the Rat: Conversion to β-Hydroxybutyrate. J. Nutr. 1971, 101, 1719–1726. [Google Scholar] [CrossRef]
- Clarke, K.; Tchabanenko, K.; Pawlosky, R.; Carter, E.; King, M.T.; Musa-Veloso, K.; Ho, M.; Roberts, A.; Robertson, J.; Vanitallie, T.B.; et al. Kinetics, Safety and Tolerability of (R)-3-Hydroxybutyl (R)-3-Hydroxybutyrate in Healthy Adult Subjects. Regul. Toxicol. Pharmacol. 2012, 63, 401–408. [Google Scholar] [CrossRef]
- Kolwicz, S.C., Jr.; Purohit, S.; Tian, R. Cardiac Metabolism and Its Interactions with Contraction, Growth, and Survival of Cardiomyocytes. Circ. Res. 2013, 113, 603–616. [Google Scholar] [CrossRef]
- Kolwicz, S.C., Jr. An “Exercise” in Cardiac Metabolism. Front. Cardiovasc. Med. 2018, 5, 66. [Google Scholar] [CrossRef]
- Kolwicz, S.C., Jr.; Tian, R. Glucose Metabolism and Cardiac Hypertrophy. Cardiovasc. Res. 2011, 90, 194–201. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Ussher, J.R. Evolving Concepts of Myocardial Energy Metabolism: More Than Just Fats and Carbohydrates. Circ. Res. 2016, 119, 1173–1176. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 Acc/Aha Guideline on the Primary Prevention of Cardiovascular Disease. Circulation 2019. [Google Scholar] [CrossRef]
- Fillmore, N.; Levasseur, J.L.; Fukushima, A.; Wagg, C.S.; Wang, W.; Dyck, J.R.B.; Lopaschuk, G.D. Uncoupling of Glycolysis from Glucose Oxidation Accompanies the Development of Heart Failure with Preserved Ejection Fraction. Mol. Med. 2018, 24, 3. [Google Scholar] [CrossRef]
- Abdurrachim, D.; Teo, X.Q.; Woo, C.C.; Chan, W.X.; Lalic, J.; Lam, C.S.P.; Lee, P.T.H. Empagliflozin Reduces Myocardial Ketone Utilization While Preserving Glucose Utilization in Diabetic Hypertensive Heart Disease: A Hyperpolarized (13) C Magnetic Resonance Spectroscopy Study. Diabetes Obes. Metab. 2019, 21, 357–365. [Google Scholar] [CrossRef]
- Kolwicz, S.C., Jr.; Airhart, S.; Tian, R. Ketones Step to the Plate: A Game Changer for Metabolic Remodeling in Heart Failure? Circulation 2016, 133, 689–691. [Google Scholar] [CrossRef]
- Al-Zaid, N.S.; Dashti, H.M.; Mathew, T.C.; Juggi, J.S. Low Carbohydrate Ketogenic Diet Enhances Cardiac Tolerance to Global Ischaemia. Acta Cardiol. 2007, 62, 381–389. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Douglas, S.L.; Tate, J.M.; Sham, S.; Lloyd, S.G. Impact of High-Fat, Low-Carbohydrate Diet on Myocardial Substrate Oxidation, Insulin Sensitivity, and Cardiac Function after Ischemia-Reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1–H10. [Google Scholar] [CrossRef]
- Wang, P.; Tate, J.M.; Lloyd, S.G. Low Carbohydrate Diet Decreases Myocardial Insulin Signaling and Increases Susceptibility to Myocardial Ischemia. Life Sci. 2008, 83, 836–844. [Google Scholar] [CrossRef]
- Partsalaki, I.; Karvela, A.; Spiliotis, B.E. Metabolic Impact of a Ketogenic Diet Compared to a Hypocaloric Diet in Obese Children and Adolescents. J. Pediatr. Endocrinol. Metab. 2012, 25, 697–704. [Google Scholar] [CrossRef]
- Samaha, F.F.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, T.; Williams, M.; Gracely, E.J.; Stern, L. A Low-Carbohydrate as Compared with a Low-Fat Diet in Severe Obesity. N. Engl. J. Med. 2003, 348, 2074–2081. [Google Scholar] [CrossRef]
- Van der Auwera, I.; Wera, S.; van Leuven, F.; Henderson, S.T. A Ketogenic Diet Reduces Amyloid Beta 40 and 42 in a Mouse Model of Alzheimer’s Disease. Nutr. Metab. (Lond.) 2005, 2, 28. [Google Scholar] [CrossRef]
- Vanitallie, T.B.; Nonas, C.; di Rocco, A.; Boyar, K.; Hyams, K.; Heymsfield, S.B. Treatment of Parkinson Disease with Diet-Induced Hyperketonemia: A Feasibility Study. Neurology 2005, 64, 728–730. [Google Scholar] [CrossRef]
- Villeneuve, N.; Pinton, F.; Buisson, B.; Dulac, O.; Chiron, C.; Nabbout, R. The Ketogenic Diet Improves Recently Worsened Focal Epilepsy. Dev. Med. Child Neurol. 2009, 51, 276–281. [Google Scholar] [CrossRef]
- Gibas, M.K.; Gibas, K.J. Induced and Controlled Dietary Ketosis as a Regulator of Obesity and Metabolic Syndrome Pathologies. Diabetes Metab. Syndr. 2017, 11 (Suppl. 1), S385–S390. [Google Scholar] [CrossRef]
- Goday, A.; Bellido, D.; Sajoux, I.; Crujeiras, A.B.; Burguera, B.; Garcia-Luna, P.P.; Oleaga, A.; Moreno, B.; Casanueva, F.F. Short-Term Safety, Tolerability and Efficacy of a Very Low-Calorie-Ketogenic Diet Interventional Weight Loss Program Versus Hypocaloric Diet in Patients with Type 2 Diabetes Mellitus. Nutr. Diabetes 2016, 6, e230. [Google Scholar] [CrossRef]
- Brehm, B.J.; Seeley, R.J.; Daniels, S.R.; D’Alessio, D.A. A Randomized Trial Comparing a Very Low Carbohydrate Diet and a Calorie-Restricted Low Fat Diet on Body Weight and Cardiovascular Risk Factors in Healthy Women. J. Clin. Endocrinol. Metab. 2003, 88, 1617–1623. [Google Scholar] [CrossRef]
- Nordmann, A.J.; Nordmann, A.; Briel, M.; Keller, U.; Yancy, W.S., Jr.; Brehm, B.J.; Bucher, H.C. Effects of Low-Carbohydrate Vs Low-Fat Diets on Weight Loss and Cardiovascular Risk Factors: A Meta-Analysis of Randomized Controlled Trials. Arch. Intern. Med. 2006, 166, 285–293. [Google Scholar] [CrossRef]
- Ho, V.W.; Leung, K.; Hsu, A.; Luk, B.; Lai, J.; Shen, S.Y.; Minchinton, A.I.; Waterhouse, D.; Bally, M.B.; Lin, W.; et al. A Low Carbohydrate, High Protein Diet Slows Tumor Growth and Prevents Cancer Initiation. Cancer Res. 2011, 71, 4484–4493. [Google Scholar] [CrossRef]
- Otto, C.; Kaemmerer, U.; Illert, B.; Muehling, B.; Pfetzer, N.; Wittig, R.; Voelker, H.U.; Thiede, A.; Coy, J.F. Growth of Human Gastric Cancer Cells in Nude Mice Is Delayed by a Ketogenic Diet Supplemented with Omega-3 Fatty Acids and Medium-Chain Triglycerides. BMC Cancer 2008, 8, 122. [Google Scholar] [CrossRef]
- Roberts, M.N.; Wallace, M.A.; Tomilov, A.A.; Zhou, Z.; Marcotte, G.R.; Tran, D.; Perez, G.; Gutierrez-Casado, E.; Koike, S.; Knotts, T.A.; et al. A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab. 2017, 26, 539–546. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Dangelo, K.; Couch, S.C.; Benoit, S.C.; Clegg, D.J. Dietary Ketosis Enhances Memory in Mild Cognitive Impairment. Neurobiol. Aging 2012, 33, 425.e19–425.e27. [Google Scholar] [CrossRef]
- Ahola-Erkkila, S.; Carroll, C.J.; Peltola-Mjosund, K.; Tulkki, V.; Mattila, I.; Seppanen-Laakso, T.; Oresic, M.; Tyynismaa, H.; Suomalainen, A. Ketogenic Diet Slows Down Mitochondrial Myopathy Progression in Mice. Hum. Mol. Genet. 2010, 19, 1974–1984. [Google Scholar] [CrossRef]
- Bough, K.J.; Wetherington, J.; Hassel, B.; Pare, J.F.; Gawryluk, J.W.; Greene, J.G.; Shaw, R.; Smith, Y.; Geiger, J.D.; Dingledine, R.J. Mitochondrial Biogenesis in the Anticonvulsant Mechanism of the Ketogenic Diet. Ann. Neurol. 2006, 60, 223–235. [Google Scholar] [CrossRef]
- Garcia-Roves, P.; Huss, J.M.; Han, D.H.; Hancock, C.R.; Iglesias-Gutierrez, E.; Chen, M.; Holloszy, J.O. Raising Plasma Fatty Acid Concentration Induces Increased Biogenesis of Mitochondria in Skeletal Muscle. Proc. Natl. Acad. Sci. USA 2007, 104, 10709–10713. [Google Scholar] [CrossRef]
- Santra, S.; Gilkerson, R.W.; Davidson, M.; Schon, E.A. Ketogenic Treatment Reduces Deleted Mitochondrial Dnas in Cultured Human Cells. Ann. Neurol. 2004, 56, 662–669. [Google Scholar] [CrossRef]
- Browning, J.D.; Baker, J.A.; Rogers, T.; Davis, J.; Satapati, S.; Burgess, S.C. Short-Term Weight Loss and Hepatic Triglyceride Reduction: Evidence of a Metabolic Advantage with Dietary Carbohydrate Restriction. Am. J. Clin. Nutr. 2011, 93, 1048–1052. [Google Scholar] [CrossRef]
- Garbow, J.R.; Doherty, J.M.; Schugar, R.C.; Travers, S.; Weber, M.L.; Wentz, A.E.; Ezenwajiaku, N.; Cotter, D.G.; Brunt, E.M.; Crawford, P.A. Hepatic Steatosis, Inflammation, and Er Stress in Mice Maintained Long Term on a Very Low-Carbohydrate Ketogenic Diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G956–G967. [Google Scholar] [CrossRef]
- De Koning, L.; Fung, T.T.; Liao, X.; Chiuve, S.E.; Rimm, E.B.; Willett, W.C.; Spiegelman, D.; Hu, F.B. Low-Carbohydrate Diet Scores and Risk of Type 2 Diabetes in Men. Am. J. Clin. Nutr. 2011, 93, 844–850. [Google Scholar] [CrossRef]
- Dashti, H.M.; Al-Zaid, N.S.; Mathew, T.C.; Al-Mousawi, M.; Talib, H.; Asfar, S.K.; Behbahani, A.I. Long Term Effects of Ketogenic Diet in Obese Subjects with High Cholesterol Level. Mol. Cell. Biochem. 2006, 286, 1–9. [Google Scholar] [CrossRef]
- Ding, J.; Xu, X.; Wu, X.; Huang, Z.; Kong, G.; Liu, J.; Huang, Z.; Liu, Q.; Li, R.; Yang, Z.; et al. Bone Loss and Biomechanical Reduction of Appendicular and Axial Bones under Ketogenic Diet in Rats. Exp. Ther. Med. 2019, 17, 2503–2510. [Google Scholar] [CrossRef]
- Xu, X.; Ding, J.; Wu, X.; Huang, Z.; Kong, G.; Liu, Q.; Yang, Z.; Huang, Z.; Zhu, Q. Bone Microstructure and Metabolism Changes under the Combined Intervention of Ketogenic Diet with Intermittent Fasting: An in Vivo Study of Rats. Exp. Anim. 2019, 68, 371–380. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Z.; Wang, X.; Fu, Z.; Liu, J.; Huang, Z.; Kong, G.; Xu, X.; Ding, J.; Zhu, Q. Ketogenic Diet Compromises Both Cancellous and Cortical Bone Mass in Mice. Calcif. Tissue Int. 2017, 101, 412–421. [Google Scholar] [CrossRef]
- Lauritzen, K.H.; Hasan-Olive, M.M.; Regnell, C.E.; Kleppa, L.; Scheibye-Knudsen, M.; Gjedde, A.; Klungland, A.; Bohr, V.A.; Storm-Mathisen, J.; Bergersen, L.H. A Ketogenic Diet Accelerates Neurodegeneration in Mice with Induced Mitochondrial DNA Toxicity in the Forebrain. Neurobiol. Aging 2016, 48, 34–47. [Google Scholar] [CrossRef]
- Iacovides, S.; Goble, D.; Paterson, B.; Meiring, R.M. Three Consecutive Weeks of Nutritional Ketosis Has No Effect on Cognitive Function, Sleep, and Mood Compared with a High-Carbohydrate, Low-Fat Diet in Healthy Individuals: A Randomized, Crossover, Controlled Trial. Am. J. Clin. Nutr. 2019, 110, 349–357. [Google Scholar] [CrossRef]
- Calton, J.B. Prevalence of Micronutrient Deficiency in Popular Diet Plans. J. Int. Soc. Sports Nutr. 2010, 7, 24. [Google Scholar] [CrossRef]
- Simm, P.J.; Bicknell-Royle, J.; Lawrie, J.; Nation, J.; Draffin, K.; Stewart, K.G.; Cameron, F.J.; Scheffer, I.E.; Mackay, M.T. The Effect of the Ketogenic Diet on the Developing Skeleton. Epilepsy Res. 2017, 136, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, A.G.; Schall, J.I.; Stallings, V.A.; Zemel, B.S. Progressive Bone Mineral Content Loss in Children with Intractable Epilepsy Treated with the Ketogenic Diet. Am. J. Clin. Nutr. 2008, 88, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.A.; Pyzik, P.L.; Rubenstein, J.E.; Hamdy, R.F.; Kossoff, E.H. Empiric Use of Potassium Citrate Reduces Kidney-Stone Incidence with the Ketogenic Diet. Pediatrics 2009, 124, e300–e304. [Google Scholar] [CrossRef] [PubMed]
- Sampath, A.; Kossoff, E.H.; Furth, S.L.; Pyzik, P.L.; Vining, E.P. Kidney Stones and the Ketogenic Diet: Risk Factors and Prevention. J. Child Neurol. 2007, 22, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Marchio, M.; Roli, L.; Giordano, C.; Trenti, T.; Guerra, A.; Biagini, G. Decreased Ghrelin and Des-Acyl Ghrelin Plasma Levels in Patients Affected by Pharmacoresistant Epilepsy and Maintained on the Ketogenic Diet. Clin. Nutr. 2019, 38, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Marchio, M.; Roli, L.; Lucchi, C.; Costa, A.M.; Borghi, M.; Iughetti, L.; Trenti, T.; Guerra, A.; Biagini, G. Ghrelin Plasma Levels after 1 Year of Ketogenic Diet in Children with Refractory Epilepsy. Front Nutr. 2019, 6, 112. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harvey, K.L.; Holcomb, L.E.; Kolwicz, S.C., Jr. Ketogenic Diets and Exercise Performance. Nutrients 2019, 11, 2296. https://doi.org/10.3390/nu11102296
Harvey KL, Holcomb LE, Kolwicz SC Jr. Ketogenic Diets and Exercise Performance. Nutrients. 2019; 11(10):2296. https://doi.org/10.3390/nu11102296
Chicago/Turabian StyleHarvey, Kristin L., Lola E. Holcomb, and Stephen C. Kolwicz, Jr. 2019. "Ketogenic Diets and Exercise Performance" Nutrients 11, no. 10: 2296. https://doi.org/10.3390/nu11102296
APA StyleHarvey, K. L., Holcomb, L. E., & Kolwicz, S. C., Jr. (2019). Ketogenic Diets and Exercise Performance. Nutrients, 11(10), 2296. https://doi.org/10.3390/nu11102296




