Oral Supplementation of Specific Collagen Peptides Combined with Calf-Strengthening Exercises Enhances Function and Reduces Pain in Achilles Tendinopathy Patients
Abstract
1. Introduction
2. Methods
2.1. Exercise Intervention
2.2. Outcome Measurements.
2.3. Victorian Institute of Sports Assessment–Achilles (VISA-A) Questionnaire, Patient Satisfaction and Return-to-Running
2.4. Real-Time Harmonic Contrast Enhanced Ultrasound (CEUS) Measurements
2.5. Blood Sampling
2.6. Product Safety and Monitoring of Adverse Events
2.7. Statistical Analyses
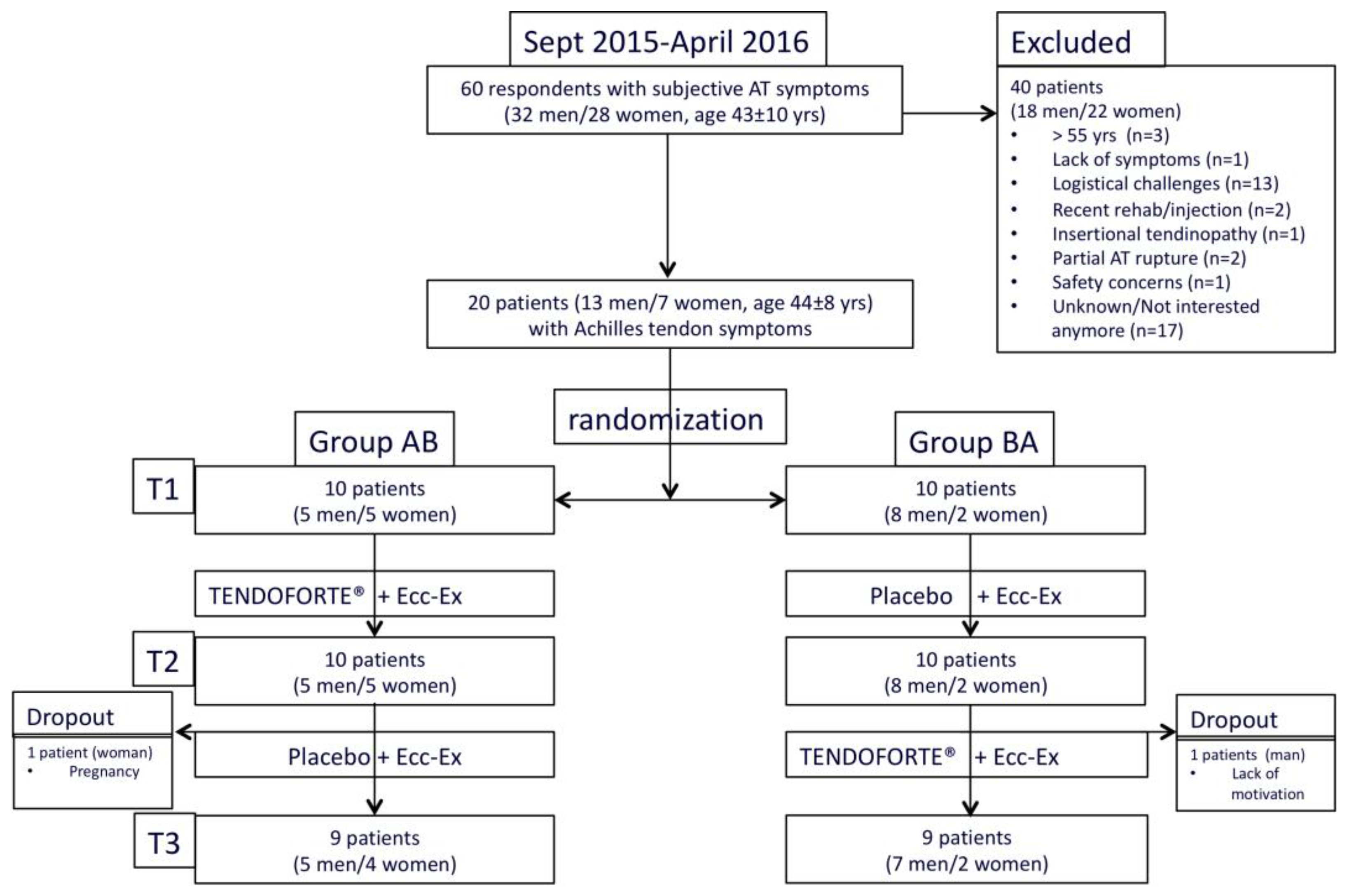
3. Results
3.1. Study Population
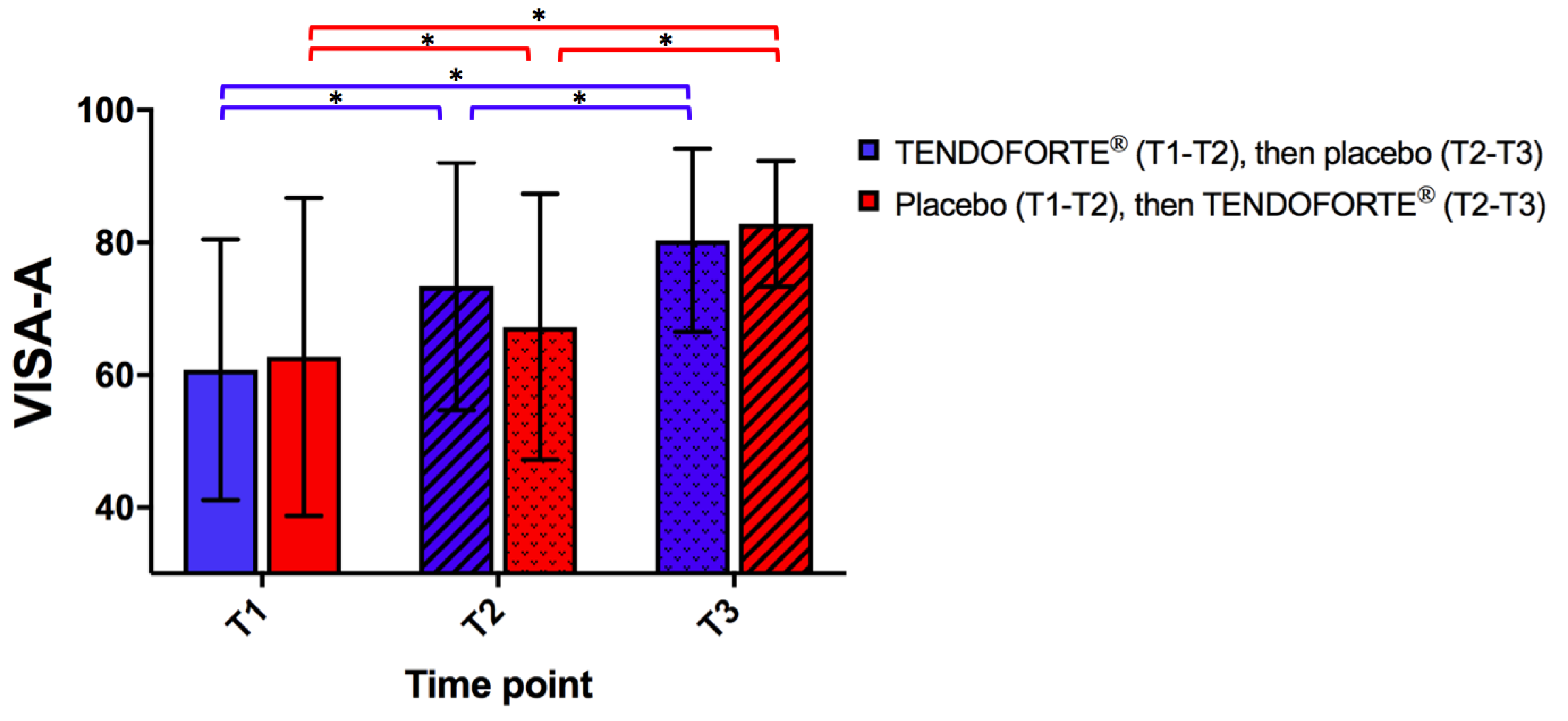
3.2. Change in VISA-A Scores
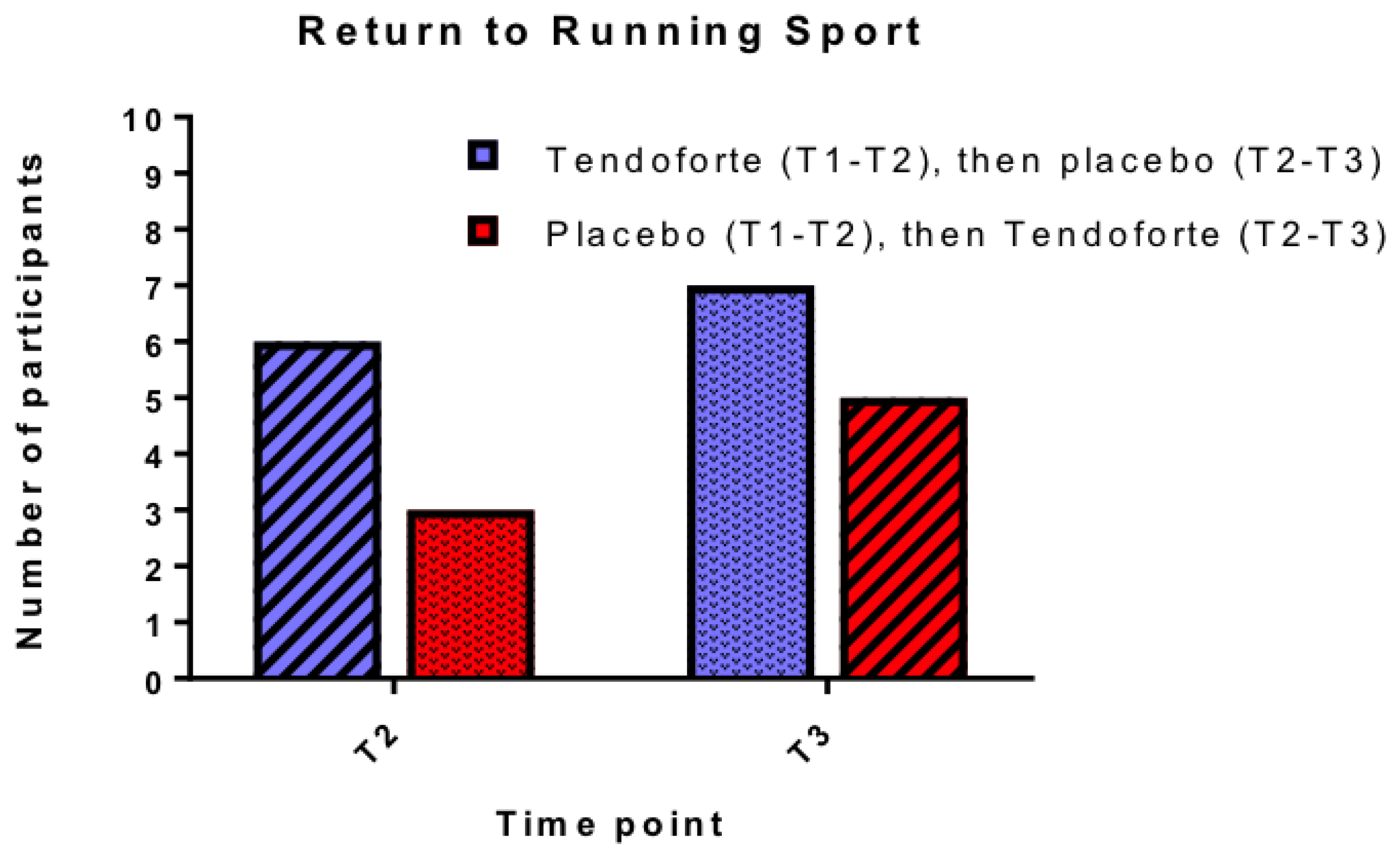
3.3. Patient Satisfaction and Return-to-Running Sports
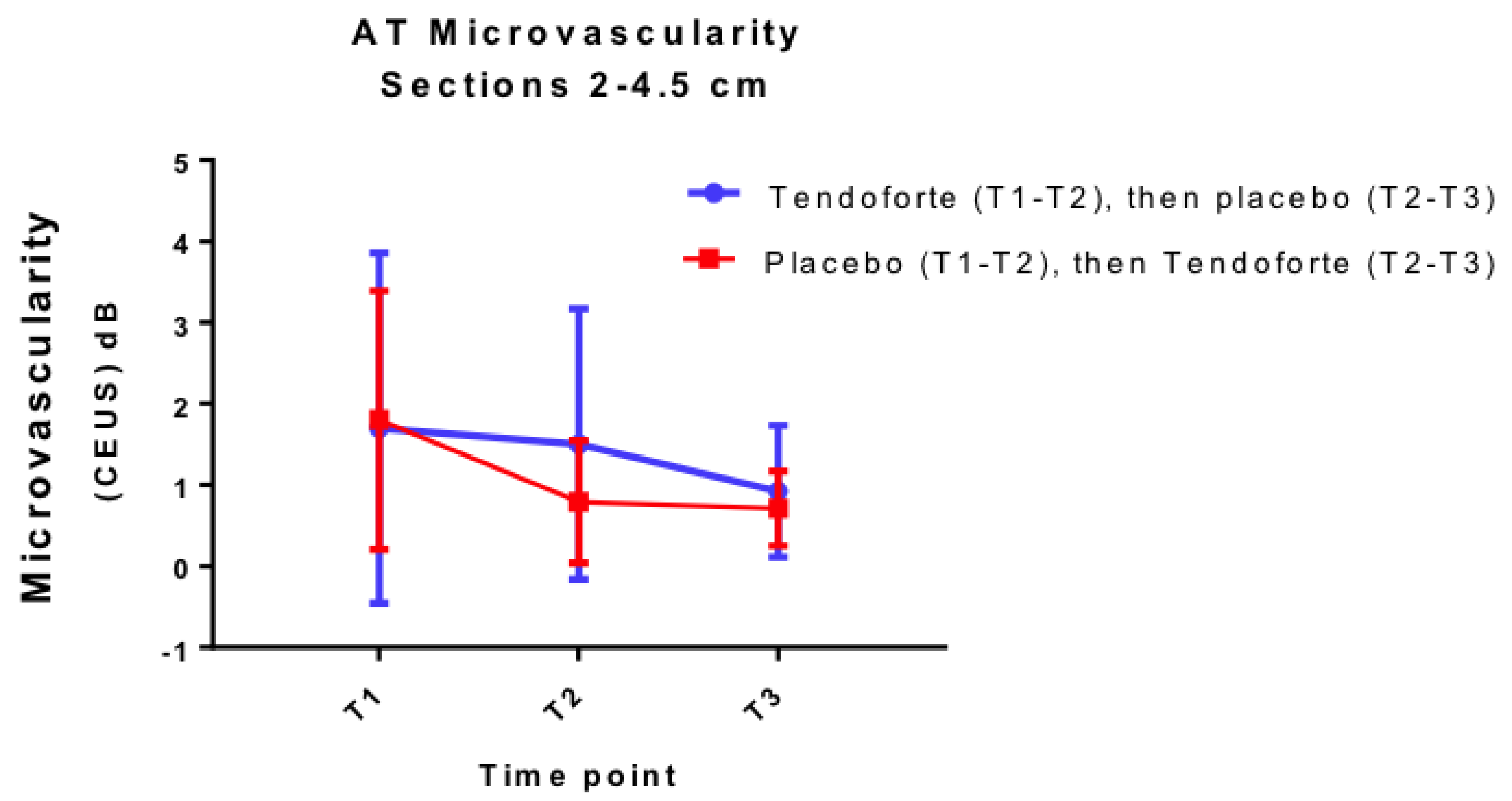
3.4. Change in Achilles Tendon Microvascularity and Blood Markers
3.5. Compliance with Interventions and Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Inclusion criteria |
| Clinical diagnosis of >2 months of uni- or bilateral chronic mid-portion Achilles tendinopathy, including pain on palpation 2–6 cm above the insertion of the Achilles tendon upon physical examination Active participation in sports activities Desire to return to original level of sports Age 18–55 years |
| Exclusion criteria |
| Clinical suspicion of insertional Achilles tendon disorders or (partial) rupture * Clinical suspicion of plantar flexor tenosynovitis Clinical suspicion of n.suralis pathology Clinical suspicion of peroneal subluxation Clinical suspicion of spondylarthropathy, gout, hyperlipidemia, Rheumatoid Arthritis and sarcoidosis. Condition that prevents the patients from executing an active exercise programme Previous eccentric calf strengthening exercises Any type of Achilles tendon injection therapy in the previous 3 months Pregnancy Contraindications for use of DEFINITY® contrast agent Quinolones or statin-related tendinopathy |
| Eccentric calf exercise program |
| Exercise 1: Gastrocnemius Heel Drop Hold onto something stable for balance. Begin with the heel raised and knee straight Slowly lower the heel below the step Push up to the starting position by using the uninjured leg Perform 3 × 15 repetitions, 2 × daily, 7 days/week. |
| Exercise 2: Soleus Heel Drop Hold onto something stable for balance. Begin with heel raised and knee bent Slowly lower the heel below the step Push up to the starting position by using the uninjured leg Perform 3 × 15 repetitions, 2 × daily, 7 days/week What Should I Expect to Feel After the Exercise? When performed properly, this exercise program is going to cause some tendon and muscle soreness, especially during the first one to two weeks. Studies have shown that it is a safe training program with no risk of new injuries. The soreness will become much less as you progress with the exercises over the course of weeks. Please STOP the program and contact your physician or physiotherapist if you experience significant pain or discomfort. When you can perform the exercise without experiencing any pain or discomfort, start to increase the weight. This can be achieved by using a calf raise machine, wearing a weighted back pack or vest. Add 5 kg per week until at 60 kg. |
| Physiotherapy Follow-up Protocol for Return to Running 4 week review All been given Alfredson program Hop pain less than 1–2/10 can commence running 2 times/week Shuffle-Jog warm up 5 mins on grass 3 sets of 5 run throughs 80 m (or equivalent) 50–60% pace on grass Rolling start No more than 20% increase in distance per week 3 sets 6 reps 4 sets 5 reps Should be no pain by 3rd run through Repeat over 3 weeks If pain is increased overall, rest a week, return to exercises and then recommence as above If Ok progress to 3 sessions/week (every second day) Shuffle-Jog warm up 5 mins on grass 4 sets 6 run throughs 80 m ( or equivalent) 55–65% pace on grass 4 sets 7 reps or equivalent distance (one session may be steady state) 4 sets 8 reps or equivalent distance (one session may be steady state) Repeat 3 weeks Review at 6 weeks Tailored approach to return to pre- baseline/ aspirational training loads Educational support regarding realistic goals Weekly increase limited to 7–10% increase Review at 12 weeks |
| Name: Date of Birth: Study ID: Date of filling out questionnaire: ( ) - 3 months after starting treatment ( ) - 6 month after starting treatment |
| Dear participant, Through the following brief questionnaire we would like to assess your opinion on the treatment that you have been given over the past 3 months. Can you please indicate how you would judge the effect of the treatment that you were given? excellent () good () moderate () poor () The groups "excellent" and "good" were regarded as successful and the groups of "moderate" and "poor" as not successful. |
| Could you please indicate underneath whether you have been able to return to sports in the past 3 months? A. I am or have not been active in sports B. I have not been able to return to sports C. I have been able to return to sports but not in my desired sport D. I have been able to return to my desired sport, but not yet at my old level E. I have been able to return to my old level in the desired sport. The groups "D" and "E" were regarded as successful and the groups of "A, B or C" as not successful. |
References
- van der Plas, A.; de Jonge, S.; de Vos, R.J.; van der Heide, H.J.; Verhaar, J.A.; Weir, A.; Tol, J.L. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br. J. Sports Med. 2012, 46, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Gaida, J.E.; Alfredson, H.; Kiss, Z.S.; Bass, S.L.; Cook, J.L. Asymptomatic Achilles tendon pathology is associated with a central fat distribution in men and a peripheral fat distribution in women: A cross sectional study of 298 individuals. BMC Musculoskelet. Disord. 2010, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Gaida, J.E.; Alfredson, L.; Kiss, Z.S.; Wilson, A.M.; Alfredson, H.; Cook, J.L. Dyslipidemia in Achilles tendinopathy is characteristic of insulin resistance. Med. Sci. Sports Exerc. 2009, 41, 1194–1197. [Google Scholar] [CrossRef]
- Barbosa, A.W.; Benevides, G.P.; Alferes, L.M.; Salomao, E.M.; Gomes-Marcondes, M.C.; Gomes, L. A leucine-rich diet and exercise affect the biomechanical characteristics of the digital flexor tendon in rats after nutritional recovery. Amino Acids 2012, 42, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, D.; Svensson, R.B.; Scheijen, J.; Eliasson, P.; Mogensen, P.; Hag, A.M.; Kjaer, M.; Schalkwijk, C.G.; Schjerling, P.; Magnusson, S.P.; et al. An advanced glycation endproduct (AGE)-rich diet promotes accumulation of AGEs in Achilles tendon. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.; Lee-Barthel, A.; Ross, M.L.; Wang, B.; Baar, K. Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am. J. Clin. Nutr. 2017, 105, 136–143. [Google Scholar] [CrossRef]
- Balius, R.; Alvarez, G.; Baro, F.; Jimenez, F.; Pedret, C.; Costa, E.; Martinez-Puig, D. A 3-Arm Randomized Trial for Achilles Tendinopathy: Eccentric Training, Eccentric Training Plus a Dietary Supplement Containing Mucopolysaccharides, or Passive Stretching Plus a Dietary Supplement Containing Mucopolysaccharides. Curr. Ther. Res. Clin. Exp. 2016, 78, 1–7. [Google Scholar] [CrossRef]
- Clark, K.L.; Sebastianelli, W.; Flechsenhar, K.R.; Aukermann, D.F.; Meza, F.; Millard, R.L.; Deitch, J.R.; Sherbondy, P.S.; Albert, A. 24-Week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain. Curr. Med. Res. Opin. 2008, 24, 1485–1496. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; Gollhofer, A.; Konig, D. Improvement of activity-related knee joint discomfort following supplementation of specific collagen peptides. Appl. Physiol. Nutr. Metab. 2017, 42, 588–595. [Google Scholar] [CrossRef]
- Watanabe-Kamiyama, M.; Shimizu, M.; Kamiyama, S.; Taguchi, Y.; Sone, H.; Morimatsu, F.; Shirakawa, H.; Furukawa, Y.; Komai, M. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J. Agric. Food Chem. 2010, 58, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.P.; De Oliveira, L.P.; Da Re Guerra, F.; Dos Santos De Almeida, M.; Marcondes, M.C.; Pimentel, E.R. Glycine improves biochemical and biomechanical properties following inflammation of the achilles tendon. Anat. Rec. 2015, 298, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Minaguchi, J.; Koyama, Y.; Meguri, N.; Hosaka, Y.; Ueda, H.; Kusubata, M.; Hirota, A.; Irie, S.; Mafune, N.; Takehana, K. Effects of ingestion of collagen peptide on collagen fibrils and glycosaminoglycans in Achilles tendon. J. Nutr. Sci. Vitaminol. 2005, 51, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.P.; Viola, M.; Carneiro, G.D.; D’Angelo, M.L.; Vicente, C.P.; Passi, A.; Pimentel, E.R. Glycine improves the remodeling process of tenocytes in vitro. Cell Biol. Int. 2018, 42, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Dressler, P.; Gehring, D.; Zdzieblik, D.; Oesser, S.; Gollhofer, A.; Konig, D. Improvement of Functional Ankle Properties Following Supplementation with Specific Collagen Peptides in Athletes with Chronic Ankle Instability. J. Sports Sci. Med. 2018, 17, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, S.A.; Hazleman, B.L.; Riley, G.P. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002, 4, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Pingel, J.; Harrison, A.; Simonsen, L.; Suetta, C.; Bulow, J.; Langberg, H. The microvascular volume of the Achilles tendon is increased in patients with tendinopathy at rest and after a 1-hour treadmill run. Am. J. Sports Med. 2013, 41, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- Praet, S.F.E.; Ong, J.H.; Purdam, C.; Welvaert, M.; Lovell, G.; Dixon, L.; Gaida, J.E.; Anglim, J.; Manzanero, S.; Vlahovich, N.; et al. Microvascular volume in symptomatic Achilles tendons is associated with VISA-A score. J. Sci. Med. Sport 2018, 21, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Docking, S.I.; Rosengarten, S.D.; Daffy, J.; Cook, J. Structural integrity is decreased in both Achilles tendons in people with unilateral Achilles tendinopathy. J. Sci. Med. Sport 2015, 18, 383–387. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Nuite, M.; Krishnan, N.; Ruthazer, R.; Price, L.L.; Burstein, D.; Griffith, J.; Flechsenhar, K. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: A pilot randomized controlled trial. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2011, 19, 399–405. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; Konig, D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Konig, D.; Oesser, S.; Scharla, S.; Zdzieblik, D.; Gollhofer, A. Specific Collagen Peptides Improve Bone Mineral Density and Bone Markers in Postmenopausal Women-A Randomized Controlled Study. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Mansournia, M.A.; Altman, D.G. Invited commentary: Methodological issues in the design and analysis of randomised trials. Br. J. Sports Med. 2018, 52, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Habets, B.; van Cingel, R.E. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: A systematic review on different protocols. Scand. J. Med. Sci. Sports 2015, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.M.; Cook, J.L.; Purdam, C.; Visentini, P.J.; Ross, J.; Maffulli, N.; Taunton, J.E.; Khan, K.M.; Victorian Institute of Sport Tendon Study, G. The VISA-A questionnaire: A valid and reliable index of the clinical severity of Achilles tendinopathy. Br. J. Sports Med. 2001, 35, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Tilley, B.J.; Cook, J.L.; Docking, S.I.; Gaida, J.E. Is higher serum cholesterol associated with altered tendon structure or tendon pain? A systematic review. Br. J. Sports Med. 2015, 49, 1504–1509. [Google Scholar] [CrossRef]
- Andia, I.; Abate, M. Hyperuricemia in Tendons. Adv. Exp. Med. Biol. 2016, 920, 123–132. [Google Scholar] [CrossRef]
- FDA. Database of Select Committee on GRAS Substances (SCGRAS) Opinion: Gelatin 1975 (updated 09/29/2015). Available online: https://wayback.archive-it.org/7993/20170607025252/https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm261307.htm (accessed on 15 December 2018).
- Baron, G.; Ravaud, P.; Samson, A.; Giraudeau, B. Missing data in randomized controlled trials of rheumatoid arthritis with radiographic outcomes: A simulation study. Arthritis Care Res. 2008, 59, 25–31. [Google Scholar] [CrossRef]
- McCormack, J.; Underwood, F.; Slaven, E.; Cappaert, T. The Minimum Clinically Important Difference on the Visa-a and Lefs for Patients with Insertional Achilles Tendinopathy. Int. J. Sports Phys. Ther. 2015, 10, 639–644. [Google Scholar]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Anti-inflammatory and anti-catabolic effects of TENDOACTIVE(R) on human tenocytes in vitro. Histol. Histopathol. 2011, 26, 1173–1185. [Google Scholar] [PubMed]
- Nadal, F.; Bové, T.; Sanchís, D.; Martinez Puig, D. Effectiveness of treatment of tendinitis and plantar fasciitis by TENDOACTIVE™. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2009, 17, S253. [Google Scholar] [CrossRef]
- Magnusson, S.P.; Hansen, M.; Langberg, H.; Miller, B.; Haraldsson, B.; Westh, E.K.; Koskinen, S.; Aagaard, P.; Kjaer, M. The adaptability of tendon to loading differs in men and women. Int. J. Exp. Pathol. 2007, 88, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Wezenbeek, E.; De Clercq, D.; Mahieu, N.; Willems, T.; Witvrouw, E. Activity-Induced Increase in Achilles Tendon Blood Flow Is Age and Sex Dependent. Am. J. Sports Med. 2018, 46, 2678–2686. [Google Scholar] [CrossRef] [PubMed]
- Wezenbeek, E.; Willems, T.; Mahieu, N.; De Muynck, M.; Vanden Bossche, L.; Steyaert, A.; De Clercq, D.; Witvrouw, E. The Role of the Vascular and Structural Response to Activity in the Development of Achilles Tendinopathy: A Prospective Study. Am. J. Sports Med. 2018, 46, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Morifuji, M.; Ohara, H.; Matsumoto, H.; Takeuchi, Y.; Sato, K. Hydroxyproline-containing dipeptides and tripeptides quantified at high concentration in human blood after oral administration of gelatin hydrolysate. Int. J. Food Sci. Nutr. 2010, 61, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, T.; Suou, T.; Hirayama, C. A simplified gelatin tolerance test to evaluate gastric and pancreatic proteolytic activities. Res. Commun. Chem. Pathol. Pharmacol. 1992, 78, 97–108. [Google Scholar] [PubMed]
- Murphy, M.; Travers, M.; Gibson, W.; Chivers, P.; Debenham, J.; Docking, S.; Rio, E. Rate of Improvement of Pain and Function in Mid-Portion Achilles Tendinopathy with Loading Protocols: A Systematic Review and Longitudinal Meta-Analysis. Sports Med. 2018. [Google Scholar] [CrossRef]
- de Jonge, S.; de Vos, R.J.; Van Schie, H.T.; Verhaar, J.A.; Weir, A.; Tol, J.L. One-year follow-up of a randomised controlled trial on added splinting to eccentric exercises in chronic midportion Achilles tendinopathy. Br. J. Sports Med. 2010, 44, 673–677. [Google Scholar] [CrossRef]
- de Vos, R.J.; Weir, A.; Cobben, L.P.; Tol, J.L. The value of power Doppler ultrasonography in Achilles tendinopathy: A prospective study. Am. J. Sports Med. 2007, 35, 1696–1701. [Google Scholar] [CrossRef]
- Knobloch, K.; Kraemer, R.; Lichtenberg, A.; Jagodzinski, M.; Gossling, T.; Richter, M.; Zeichen, J.; Hufner, T.; Krettek, C. Achilles tendon and paratendon microcirculation in midportion and insertional tendinopathy in athletes. Am. J. Sports Med. 2006, 34, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Peers, K.H.; Brys, P.P.; Lysens, R.J. Correlation between power Doppler ultrasonography and clinical severity in Achilles tendinopathy. Int. Orthop. 2003, 27, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Rio, E.; Docking, S.I. Adaptation of the pathological tendon: You cannot trade in for a new one, but perhaps you don’t need to? Br. J. Sports Med. 2018, 52, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Beyer, R.; Kongsgaard, M.; Hougs Kjaer, B.; Ohlenschlaeger, T.; Kjaer, M.; Magnusson, S.P. Heavy Slow Resistance versus Eccentric Training as Treatment for Achilles Tendinopathy: A Randomized Controlled Trial. Am. J. Sports Med. 2015, 43, 1704–1711. [Google Scholar] [CrossRef]
- Ohberg, L.; Alfredson, H. Effects on neovascularisation behind the good results with eccentric training in chronic mid-portion Achilles tendinosis? Knee Surg. Sports Traumatol. Arthrosc. 2004, 12, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Pufe, T.; Petersen, W.; Kurz, B.; Tsokos, M.; Tillmann, B.; Mentlein, R. Mechanical factors influence the expression of endostatin—An inhibitor of angiogenesis—In tendons. J. Orthop. Res. 2003, 21, 610–616. [Google Scholar] [CrossRef]
- Pufe, T.; Kurz, B.; Petersen, W.; Varoga, D.; Mentlein, R.; Kulow, S.; Lemke, A.; Tillmann, B. The influence of biomechanical parameters on the expression of VEGF and endostatin in the bone and joint system. Ann. Anat. 2005, 187, 461–472. [Google Scholar] [CrossRef]
- Pufe, T.; Petersen, W.J.; Miosge, N.; Goldring, M.B.; Mentlein, R.; Varoga, D.J.; Tillmann, B.N. Endostatin/collagen XVIII—An inhibitor of angiogenesis—Is expressed in cartilage and fibrocartilage. Matrix Biol. 2004, 23, 267–276. [Google Scholar] [CrossRef]
- Schunk, M.; Oesser, S. Specific collagen peptides benefit the biosynthesis of matrix molecules of tendons and ligaments. J. Int. Soc. Sports Nutr. 2013, 10, P23. [Google Scholar] [CrossRef]
- Coombes, B.K.; Tucker, K.; Vicenzino, B.; Vuvan, V.; Mellor, R.; Heales, L.; Nordez, A.; Hug, F. Achilles and patellar tendinopathy display opposite changes in elastic properties: A shear wave elastography study. Scand. J. Med. Sci. Sports 2018, 28, 1201–1208. [Google Scholar] [CrossRef]
| Group AB | Group BA | Total | |
|---|---|---|---|
| Total | 10 | 10 | 20 |
| Male/Female | 5/5 | 8/2 | 13/7 |
| Age (years) * | 45.3 ± 6.4 | 42.0 ± 9.4 | 43.7 ± 8.0 |
| Body mass index (BMI) (kg.m−2) * | 23.4 ± 3.23 | 25.5 ± 3.3 | 24.4 ± 3.3 |
| Total Cholesterol (mmol∙L−1) | 5.6 ± 0.7 | 5.2 ± 0.8 | 5.4 ± 0.8 |
| Triglycerides (mmol∙L−1) | 1.9 ± 2.1 | 1.7 ± 1.1 | 1.8 ± 1.7 |
| Urate (mmol∙L−1) | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| Symptom duration (months) &,# | 24 (0–360) | 32 (0–180) | 18 (0–360) |
| VISA-A symptomatic tendons * | 58.5 ± 19.9 (n = 17) | 55.9 ± 21.9 (n = 15) | 57.6 ± 20.3 (n = 32) |
| VISA-A asymptomatic tendons * | 77.0 ± 18.4 (n = 3) | 88.5 ± 10.3 (n = 4) | 84.7 ± 12.9 (n = 7) |
| Total Chol. (mmol∙L−1) | T1 (n = 20) | T2 (n = 20) | T3 (n = 18) |
| group AB | 5.6 ± 0.7 | 5.6 ± 0.9 | 5.7 ± 1.1 |
| group BA | 5.2 ± 0.8 | 5.2 ± 1.1 | 5.4 ± 0.6 |
| Triglycerides (mmol∙L−1) | T1 (n = 20) | T2 (n = 20) | T3 (n = 18) |
| group AB | 1.9 ± 2.1 | 1.6 ± 0.7 | 1.7 ± 0.6 |
| group BA | 1.7 ± 1.1 | 1.6 ± 0.8 | 1.6 ± 0.6 |
| Urate (mmol∙L−1) | T1 (n = 20) | T2 (n = 20) | T3 (n = 18) |
| group AB | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| group BA | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praet, S.F.E.; Purdam, C.R.; Welvaert, M.; Vlahovich, N.; Lovell, G.; Burke, L.M.; Gaida, J.E.; Manzanero, S.; Hughes, D.; Waddington, G. Oral Supplementation of Specific Collagen Peptides Combined with Calf-Strengthening Exercises Enhances Function and Reduces Pain in Achilles Tendinopathy Patients. Nutrients 2019, 11, 76. https://doi.org/10.3390/nu11010076
Praet SFE, Purdam CR, Welvaert M, Vlahovich N, Lovell G, Burke LM, Gaida JE, Manzanero S, Hughes D, Waddington G. Oral Supplementation of Specific Collagen Peptides Combined with Calf-Strengthening Exercises Enhances Function and Reduces Pain in Achilles Tendinopathy Patients. Nutrients. 2019; 11(1):76. https://doi.org/10.3390/nu11010076
Chicago/Turabian StylePraet, Stephan F.E., Craig R. Purdam, Marijke Welvaert, Nicole Vlahovich, Gregg Lovell, Louise M. Burke, Jamie E. Gaida, Silvia Manzanero, David Hughes, and Gordon Waddington. 2019. "Oral Supplementation of Specific Collagen Peptides Combined with Calf-Strengthening Exercises Enhances Function and Reduces Pain in Achilles Tendinopathy Patients" Nutrients 11, no. 1: 76. https://doi.org/10.3390/nu11010076
APA StylePraet, S. F. E., Purdam, C. R., Welvaert, M., Vlahovich, N., Lovell, G., Burke, L. M., Gaida, J. E., Manzanero, S., Hughes, D., & Waddington, G. (2019). Oral Supplementation of Specific Collagen Peptides Combined with Calf-Strengthening Exercises Enhances Function and Reduces Pain in Achilles Tendinopathy Patients. Nutrients, 11(1), 76. https://doi.org/10.3390/nu11010076





