Resveratrol Counteracts Insulin Resistance—Potential Role of the Circulation
Abstract
1. Does Resveratrol Improve Insulin Sensitivity? Evidence from Clinical Trials
2. Mechanisms by Which Resveratrol May Improve Insulin Sensitivity in Skeletal Muscle
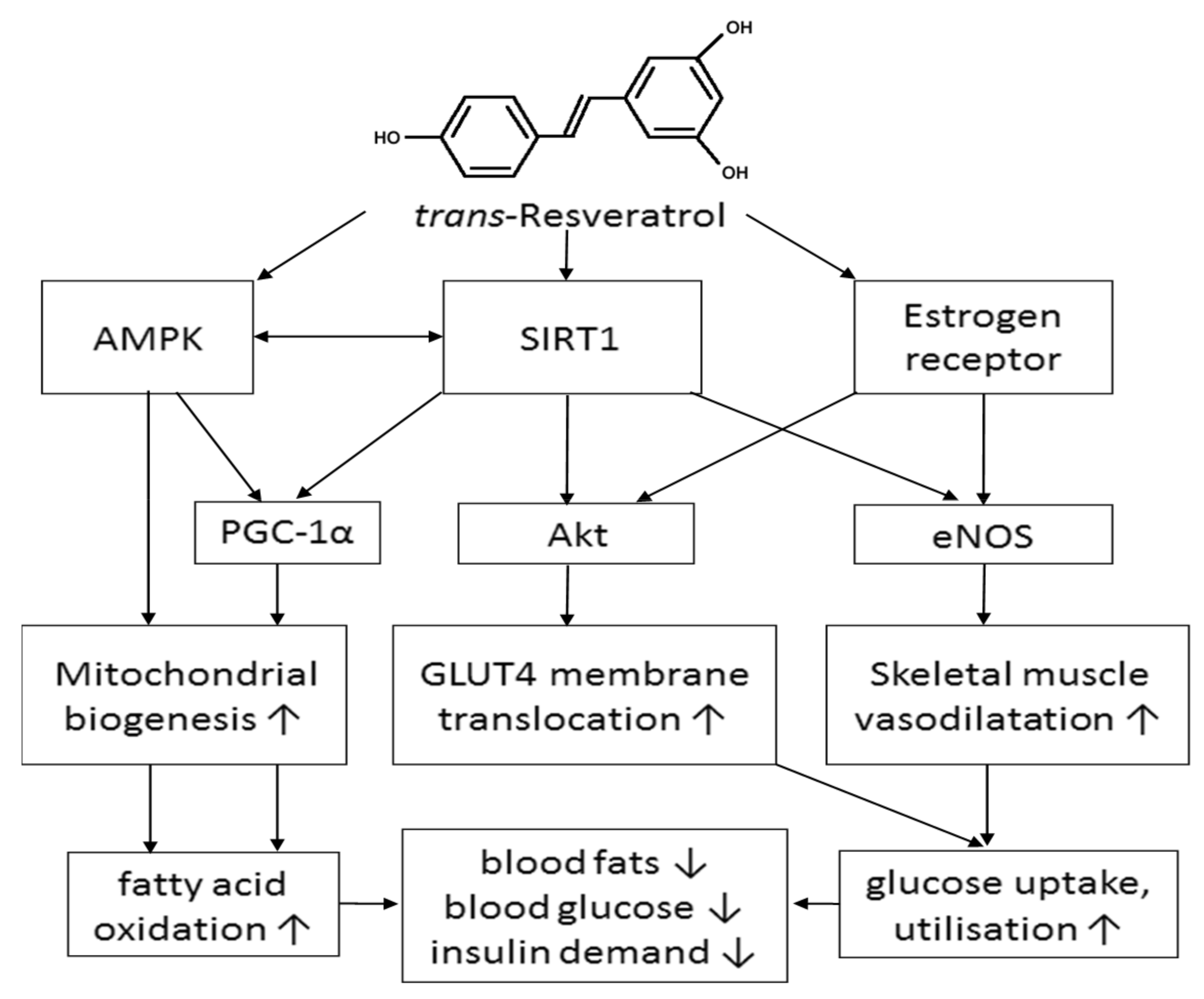
2.1. Human Sirtuin 1 (SIRT1) and AMP-Activated Protein Kinase (AMPK) Activation
2.2. Increased Glucose Uptake via Activation of Estrogen Receptors (ER)
2.3. Improving Glucose Utilisation by Increasing Blood Flow in Skeletal Muscle
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Labinskyy, N.; Csiszar, A.; Veress, G.; Stef, G.; Pacher, P.; Oroszi, G. Vascular dysfunction in aging: Potential effects of resveratrol; An anti-inflammatory phytoestrogen. Curr. Med. Chem. 2006, 13, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-Y.; Hsieh, P.-S.; Huang, J.-P.; Lu, L.-S.; Hung, L.-M. Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes 2008, 57, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N.; Förstermann, U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 2012, 26, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, W.; Zhang, P.; He, S.; Huang, D. Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials. Clin. Nutr. 2015, 34, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. Dissection of the protein phosphorylation cascades involved in insulin and growth factor action. Biochem. Soc. Trans. 1993, 21, 555–567. [Google Scholar]
- Barrett, E.J.; Eggleston, E.M.; Inyard, A.C.; Wang, H.; Li, G.; Chai, W.; Liu, Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 2009, 52, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Manrique, C.; Lastra, G.; Sowers, J.R. New insights into insulin action and resistance in the vasculature. Year Diabetes Obes. 2014, 1311, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Cleland, S.J.; Petrie, J.R.; Small, M.; Elliott, H.L.; Connell, J.M.C. Insulin action is associated with endothelial function in hypertension and type 2 diabetes. Hypertension 2000, 35, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.-T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Nabipour, I.; Lieben, L.X.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid. Based Complement. Altern. Med. 2013, 2013, 851267. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.J.; Razie, H.; Allah, Y.H.; Mehrnoosh, Z.; Hosein, H.M.; Parvin, D. The impact of resveratrol supplementation on blood glucose; insulin; insulin resistance; triglyceride; and periodontal markers in type 2 diabetic patients with chronic periodontitis. Phytother. Res. 2017, 31, 108–114. [Google Scholar]
- Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: Systematic review and meta-analysis. Nutr. Metab. 2017, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Pollack, R.M.; Barzilai, N.; Anghel, V.; Kulkarni, A.S.; Golden, A.; O’Broin, P. Resveratrol improves vascular function and mitochondrial number but not glucose metabolism in older adults. J. Gerontol. 2017, 72, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A. Anti-inflammatory effects of resveratrol: Possible role in prevention of age-related cardiovascular disease. Ann. N. Y. Acad. Sci. 2011, 1215, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, Á.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Koh, A.F.Y. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle sirt1 expression and energy expenditure. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; de Ligt, M.; Phielix, E.; van de Weijer, T.; Hansen, J.; Moonen-Kornips, E. Resveratrol as add-on therapy in subjects with well-controlled type 2 diabetes: A randomized controlled trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- Bashmakov, Y.K.; Assaad-Khalil, S.; Petyaev, I.M. Resveratrol may be beneficial in treatment of diabetic foot syndrome. Méd. Hypotheses 2011, 77, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Floel, A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Van der Made, S.M.; Plat, J.; Mensink, R.P. Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: A randomized, placebo-controlled crossover trial. PLoS ONE 2015, 10, e0118393. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.Y.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. Sirt1 is required for ampk activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Méndez-del Villar, M.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; Lizárraga-Valdez, R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.X.; Coates, A.M.; Buckley, J.D.; Howe, P.R.C. Evidence for circulatory benefits of resveratrol in humans. Resveratrol Health 2013, 1290, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Vang, O.; Ahmad, N.; Baile, C.A.; Baur, J.A.; Brown, K.; Csiszar, A. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS ONE 2011, 6, e19881. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Larrosa, M.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef] [PubMed]
- Kathrin, P.; Gerald, R.; Petra, M.R.; Dawn, C.; Insa, M.A.W. Resveratrol and lifespan in model organisms. Curr. Med. Chem. 2016, 23, 4639–4680. [Google Scholar]
- Abbasi, O.E.; Goodarzi, M.T.; Higgins, V.; Adeli, K. Role of resveratrol in the management of insulin resistance and related conditions: Mechanism of action. Crit. Rev. Clin. Lab. Sci. 2017, 54, 267–293. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.; Prentki, M. Amp kinase and malonyl-coa: Targets for therapy of the metabolic syndrome. Nat. Rev. Drug Discov. 2004, 3, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Thiebaud, D.; Jacot, E.; DeFronzo, R.A.; Maeder, E.; Jequier, E.; Felber, J.P. The effect of graded doses of insulin on total glucose uptake; glucose oxidation; and glucose storage in man. Diabetes 1982, 31, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Um, J.-H.; Park, S.-J.; Kang, H.; Yang, S.; Foretz, M.; McBurney, M.W.; Kim, M.K.; Viollet, B.; Chung, J.H. Amp-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 2009, 59, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small molecule activators of sirtuins extend saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Park, C.E.; Kim, M.-J.; Lee, J.H.; Min, B.-I.; Bae, H.; Choe, W.; Kim, S.-S.; Ha, J. Resveratrol stimulates glucose transport in c2c12 myotubes by activating amp-activated protein kinase. Exp. Mol. Med. 2007, 39, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, J.; Zhang, Z.; Li, W.; Sun, Y.; Zhang, Q. Effects of resveratrol on the amelioration of insulin resistance in KKAy mice. Can. J. Physiol. Pharmacol. 2012, 90, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.A.; Pownall, H.J.; Hamilton, D.J. Estrogen: An Emerging Regulator of Insulin Action and Mitochondrial Function. J. Diabetes Res. 2015, 2015, 916585. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.H.; Witczak, C.A.; Hirshman, M.F.; Goodyear, L.J.; Greenberg, A.S. Estradiol stimulates akt, amp-activated protein kinase (AMPK) and tbc1d1/4, but not glucose uptake in rat soleus. Biochem. Biophys. Res. Commun. 2009, 382, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors α and β1. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.P.A.; Machado, U.F.; Warner, M.; Gustafsson, J.A. Muscle GLUT4 regulation by estrogen receptors ER beta and ER alpha. Proc. Natl. Acad. Sci. USA 2006, 103, 1605–1608. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M.; Wickramasinghe, N.S.; Ivanova, M.M.; Dougherty, S.M. Resveratrol stimulates nitric oxide production by increasing estrogen receptor-src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008, 22, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M.; Blankenship, K.A.; Risinger, K.E.; Bhatnagar, S.; Noisin, E.L.; Sumanasekera, W.K.; Zhao, L.; Brey, D.M.; Keynton, R.S. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors and in endothelial cells. J. Biol. Chem. 2004, 280, 7460–7468. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.; Mark, A. Cardiovascular and sympathetic action of insulin: the insulin hypothesis of hypertension revisited. Cardiovasc. Risk Factors 1993, 3, 159–163. [Google Scholar]
- Bakker, W.; Eringa, E.C.; Sipkema, P.; van Hinsbergh, V.W.M. Endothelial dysfunction and diabetes: Roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2008, 335, 165–189. [Google Scholar] [CrossRef] [PubMed]
- Julius, S.; Gudbrandsson, T.; Jamerson, K.; Tariq Shahab, S.; Andersson, O. The hemodynamic link between insulin resistance and hypertension. J. Hypertens. 1991, 9, 983–986. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Pendergrass, M.; Bertoldo, A.; Bonadonna, R.; Nucci, G.; Mandarino, L.; Cobelli, C.; DeFronzo, R.A. Muscle glucose transport and phosphorylation in type 2 diabetic, obese nondiabetic, and genetically predisposed individuals. AJP Endocrinol. Metab. 2006, 292, E92–E100. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Forstermann, U.; Li, H. Resveratrol and endothelial nitric oxide. Molecules 2014, 19, 16102–16121. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.X.; Howe, P.R.C.; Buckley, J.D.; Coates, A.M.; Kunz, I.; Berry, N.M. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Takase, B.; Uehata, A.; Akima, T.; Nagai, T.; Nishioka, T.; Hamabe, A.; Satomura, K.; Ohsuzu, F.; Kurita, A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am. J. Cardiol. 1998, 82, 1535–1539. [Google Scholar] [CrossRef]
- Davison, K.; Bircher, S.; Hill, A.; Coates, A.M.; Howe, P.R.C.; Buckley, J.D. Relationships between obesity, cardiorespiratory fitness, and cardiovascular function. J. Obes. 2010, 2010, 191253. [Google Scholar] [CrossRef] [PubMed]
- Keymel, S.; Heinen, Y.; Balzer, J.; Rassaf, T.; Kelm, M.; Lauer, T. Characterization of macro-and microvascular function and structure in patients with type 2 diabetes mellitus. Am. J. Card. Dis. 2011, 1, 68–75. [Google Scholar]
- Marques, B.C.A.A.; Trindade, M.; Aquino, J.C.F.; Cunha, A.R.; Gismondi, R.O.; Neves, M.F. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin. Exp. Hypertens. 2018, 40, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Nealon, R.S.; Howe, P.R.C.; Jansen, L.; Garg, M.; Wong, R.H.X. Impaired cerebrovascular responsiveness and cognitive performance in adults with type 2 diabetes. J. Diabetes Complicat. 2017, 31, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.; Deary, I.; Ryan, C. Cognition and diabetes: A lifespan perspective. Lancet Neurol. 2008, 7, 184–190. [Google Scholar] [CrossRef]
- Cigada, M.; Marzorati, S.; Tredici, S.; Iapichino, G. Cerebral CO2 vasoreactivity evaluation by transcranial Doppler ultrasound technique: A standardized methodology. Intensive Care Med. 2000, 26, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Raederstorff, D.; Howe, P. Acute resveratrol consumption improves neurovascular coupling capacity in adults with type 2 diabetes mellitus. Nutrients 2016, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.X.; Nealon, R.S.; Scholey, A.; Howe, P.R.C. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.; Howe, P.; Wong, R. Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients 2017, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.E.; Huxley, R.R.; Woodward, M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014, 383, 1973–1980. [Google Scholar] [CrossRef]
- Chatterjee, S.; Peters, S.A.E.; Woodward, M.; Arango, S.M.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.; et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Tankó, L.B.; Christiansen, C.; Cox, D.A.; Geiger, M.J.; McNabb, M.A.; Cummings, S.R. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J. Bone Miner. Res. 2005, 20, 1912–1920. [Google Scholar]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G. Blood Pressure Is Reduced and Insulin Sensitivity Increased in Glucose-Intolerant, Hypertensive Subjects after 15 Days of Consuming High-Polyphenol Dark Chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, R.H.X.; Howe, P.R.C. Resveratrol Counteracts Insulin Resistance—Potential Role of the Circulation. Nutrients 2018, 10, 1160. https://doi.org/10.3390/nu10091160
Wong RHX, Howe PRC. Resveratrol Counteracts Insulin Resistance—Potential Role of the Circulation. Nutrients. 2018; 10(9):1160. https://doi.org/10.3390/nu10091160
Chicago/Turabian StyleWong, Rachel H. X., and Peter R. C. Howe. 2018. "Resveratrol Counteracts Insulin Resistance—Potential Role of the Circulation" Nutrients 10, no. 9: 1160. https://doi.org/10.3390/nu10091160
APA StyleWong, R. H. X., & Howe, P. R. C. (2018). Resveratrol Counteracts Insulin Resistance—Potential Role of the Circulation. Nutrients, 10(9), 1160. https://doi.org/10.3390/nu10091160





