Influence of Maternal Fish Intake on the Anthropometric Indices of Children in the Western Amazon
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anthropometric Data
2.3. Assessment of Total Hair Hg (HHg) Concentration
2.4. Assessment of Anemia
2.5. Study Variables and Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Stratakis, N.; Roumeliotaki, T.; Oken, E.; Barros, H.; Basterrechea, M.; Charles, M.A.; Eggesbø, M.; Forastiere, F.; Gaillard, R.; Gehring, U.; et al. Fish intake in pregnancy and child growth: A pooled analysis of 15 European and US birth cohorts. JAMA Pediatr. 2016, 170, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Campoy, C.; Escolano-Margarit, M.V.; Anjos, T.; Szajewska, H.; Uauy, R. Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. Br. J. Nutr. 2012, 107, S85–S106. [Google Scholar] [CrossRef] [PubMed]
- Emmett, P.M.; Jones, L.R.; Golding, J. Pregnancy diet and associated outcomes in the Avon longitudinal study of parents and children. Nutr. Rev. 2015, 73, 154–174. [Google Scholar] [CrossRef] [PubMed]
- Leventakou, V.; Roumeliotaki, T.; Martinez, D.; Barros, H.; Brantsaeter, A.L.; Casas, M.; Charles, M.A.; Cordier, S.; Eggesbø, M.; van Eijsden, M.; et al. Fish intake during pregnancy, fetal growth, and gestational length in 19 European birth cohort studies. Am. J. Clin. Nutr. 2014, 99, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, C.; Sasaki, S.; Ikeno, T.; Araki, A.; Ito, S.; Kajiwara, J.; Todaka, T.; Hachiya, N.; Yasutake, A.; Murata, K.; et al. Effects of in utero exposure to polychlorinated biphenyls, methylmercury, and polyunsaturated fatty acids on birth size. Sci. Total Environ. 2015, 533, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Agay-Shay, K.; Martinez, D.; Valvi, D.; Garcia-Esteban, R.; Basagaña, X.; Robinson, O.; Casas, M.; Sunyer, J.; Vrijheid, M. Exposure to endocrine-disrupting chemicals during pregnancy and weight at 7 years of age: A multi-pollutant approach. Environ. Health Perspect. 2015, 123, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G. Fish are central in the diet of Amazonian riparians: Should we worry about their mercury concentrations? Environ. Res. 2003, 92, 232–244. [Google Scholar] [CrossRef]
- Gil, A.; Gil, F. Fish, a Mediterranean source of n-3 PUFA: Benefits do not justify limiting consumption. Br. J. Nutr. 2015, 113, S58–S67. [Google Scholar] [CrossRef] [PubMed]
- Vieira Rocha, A.; Cardoso, B.R.; Cominetti, C.; Bueno, R.B.; de Bortoli, M.C.; Farias, L.A.; Favaro, D.I.; Camargo, L.M.; Cozzolino, S.M. Selenium status and hair mercury levels in riverine children from Rondônia, Amazonia. Nutrition 2014, 30, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Dufour, D.L.; Piperata, B.A.; Murrieta, R.S.; Wilson, W.M.; Williams, D.D. Amazonian foods and implications for human biology. Ann. Hum. Biol. 2016, 43, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G.; Barbosa, A.C.; Ferrari, I.; De Souza, J.R. Fish consumption (hair mercury) and nutritional status of Amazonian Amer—Indian children. Am. J. Hum. Biol. 2005, 17, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G.; Marques, R.C. Mercury levels and human health in the Amazon Basin. Ann. Hum. Biol. 2016, 43, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Garrofe Dórea, J.; Rodrigues Bastos, W.; de Freitas Rebelo, M.; de Freitas Fonseca, M.; Malm, O. Maternal mercury exposure and neuro-motor development in breastfed infants from Porto Velho (Amazon), Brazil. Int. J. Hyg. Environ. Health 2007, 210, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Dórea, J.G.; Bernardi, J.V.; Bastos, W.R.; Malm, O. Maternal fish consumption in the nutrition transition of the Amazon Basin: Growth of exclusively breastfed infants during the first 5 years. Ann. Hum. Biol. 2008, 35, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Bernardi, J.V.; Dórea, J.G.; Brandão, K.G.; Bueno, L.; Leão, R.S.; Malm, O. Fish consumption during pregnancy, mercury transfer, and birth weight along the Madeira river basin in Amazonia. Int. J. Environ. Res. Public Health 2013, 10, 2150–2163. [Google Scholar] [CrossRef] [PubMed]
- Piperata, B.A.; Spence, J.E.; Da-Gloria, P.; Hubbe, M. The nutrition transition in amazonia: Rapid economic change and its impact on growth and development in Ribeirinhos. Am. J. Phys. Anthropol. 2011, 146, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.P.L.; Marques, R.C.; Dórea, J.G. Child nutritional status in the changing socioeconomic region of the Northern Amazon, Brazil. Int. J. Environ. Res. Public Health 2017, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. 2011. Available online: http://www.who.int/vmnis/indicators/haemoglobin/en/ (accessed on 17 April 2012).
- WHO Anthro for Personal Computers, version 3.2.2; Software for Assessing Growth and Development of the World’s Children; WHO: Geneva, Switzerland, 2011.
- Nobre, J.S.; da Motta Singer, J. Residual analysis for linear mixed models. Biom. J. 2007, 49, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Balakrishna, N.; Griffiths, P.L. Modeling physical growth using mixed effects models. Am. J. Phys. Anthropol. 2013, 150, 58–67. [Google Scholar] [CrossRef] [PubMed]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Turunen, A.W.; Männistö, S.; Kiviranta, H.; Marniemi, J.; Jula, A.; Tiittanen, P.; Suominen-Taipale, L.; Vartiainen, T.; Verkasalo, P.K. Dioxins, polychlorinated biphenyls, methyl mercury and omega-3 polyunsaturated fatty acids as biomarkers of fish consumption. Eur. J. Clin. Nutr. 2010, 64, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Bernardi, J.V.E.; Cunha, M.P.L.; Dórea, J.G. Impact of organic mercury exposure and home delivery on neurodevelopment of Amazonian children. Int. J. Hyg. Environ. Health 2016, 219, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.F.; Dórea, J.G.; Bastos, W.R.; Marques, R.C.; Torres, J.P.; Malm, O. Poor psychometric scores of children living in isolated riverine and agrarian communities and fish-methylmercury exposure. Neurotoxicology 2008, 29, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, E.M.; Valente, J.G.; Sichieri, R.; Silva, E.C. Validation and calibration of mercury intake through self-referred fish consumption in riverine populations in Pantanal Mato-Grossense, Brazil. Environ. Res. 2001, 88, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Hacon, S.S.; Dórea, J.G.; Fonseca, M.d.F.; Oliveira, B.A.; Mourão, D.S.; Ruiz, C.M.; Gonçalves, R.A.; Mariani, C.F.; Bastos, W.R. The influence of changes in lifestyle and mercury exposure in riverine populations of the Madeira River (Amazon Basin) near a hydroelectric project. Int. J. Environ. Res. Public Health 2014, 11, 2437–2455. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.M.; de Almeida, R.; Holanda, I.B.; Mussy, M.H.; Galvão, R.C.; Crispim, P.T.; Dórea, J.G.; Bastos, W.R. Total and methyl-mercury in hair and milk of mothers living in the city of Porto Velho and in villages along the Rio Madeira, Amazon, Brazil. Int. J. Hyg. Environ. Health 2013, 216, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Giugliani, E.R.; Horta, B.L.; Loret de Mola, C.; Lisboa, B.O.; Victora, C.G. Effect of breastfeeding promotion interventions on child growth: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Weihe, P.; White, R.F. Milestone development in infants exposed to methylmercury from human milk. Neurotoxicology 1995, 16, 27–33. [Google Scholar] [PubMed]
- Piperata, B.A. Nutritional status of Ribeirinhos in Brazil and the nutrition transition. Am. J. Phys. Anthropol. 2007, 133, 868–878. [Google Scholar] [CrossRef] [PubMed]
- De Marques, R.F.; Taddei, J.A.; Konstantyner, T.; Lopez, F.A.; Marques, A.C.; de Oliveira, C.S.; Braga, J.A. Anthropometric indices and exclusive breastfeeding in the first six months of life: A comparison with reference standards NCHS, 1977 and WHO, 2006. Int. Breastfeed. J. 2015, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Chen, C.; Chen, L.; Wang, H.; Yang, T.; Xie, H.; Tong, Y.; Hu, D.; Zhang, W.; Wang, X. Low-level prenatal mercury exposure in North China: An exploratory study of anthropometric effects. Environ. Sci. Technol. 2015, 49, 6899–6908. [Google Scholar] [CrossRef] [PubMed]
- Benefice, E.; Monrroy, S.J.; Rodriguez, R.W. A nutritional dilemma: Fish consumption, mercury exposure and growth of children in Amazonian Bolivia. Int. J. Environ. Health Res. 2008, 18, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Park, J.S.; Shin, S.; Yang, H.R.; Moon, J.S.; Ko, J.S. Mercury exposure in healthy Korean weaning-age infants: Association with growth, feeding and fish intake. Int. J. Environ. Res. Public Health 2015, 12, 14669–14689. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Chang, J.Y.; Hong, J.; Shin, S.; Park, J.S.; Oh, S. Low-level toxic metal exposure in healthy weaning-age infants: Association with growth, dietary intake, and iron deficiency. Int. J. Environ. Res. Public Health 2017, 14, 388. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.Y.; Li, M.M.; Wang, J.; Yan, J.; Zhou, C.C.; Yan, C.H. Blood mercury concentration, fish consumption and anthropometry in Chinese children: A national study. Environ. Int. 2018, 110, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Aquino, E.M.; do Carmo Leal, M.; Monteiro, C.A.; Barros, F.C.; Szwarcwald, C.L. Maternal and child health in Brazil: Progress and challenges. Lancet 2011, 377, 1863–1876. [Google Scholar] [CrossRef]
- Brazilian Institute of Geography and Statistics. Estatísticas do Registro Civil 2013. 2013. Available online: http://biblioteca.ibge.gov.br/visualizacao/periodicos/135/rc_2013_v40.pdf (accessed on 17 February 2014).
- Lourenço, B.H.; Villamor, E.; Augusto, R.A.; Cardoso, M.A. Determinants of linear growth from infancy to school-aged years: A population-based follow-up study in urban Amazonian children. BMC Public Health 2012, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Huicho, L.; Huayanay-Espinoza, C.A.; Herrera-Perez, E.; Segura, E.R.; Niño de Guzman, J.; Rivera-Ch, M.; Barros, A.J. Factors behind the success story of under-five stunting in Peru: A district ecological multilevel analysis. BMC Pediatr. 2017, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Benício, M.H.; Martins, A.P.; Venancio, S.I.; Barros, A.J. Estimates of the prevalence of child malnutrition in Brazilian municipalities in 2006. Rev. Saúde Pública 2013, 47, 560–570. (In Portuguese) [Google Scholar]
- Horta, B.L.; Santos, R.V.; Welch, J.R.; Cardoso, A.M.; dos Santos, J.V.; Assis, A.M.; Lira, P.C.; Coimbra, C.E., Jr. Nutritional status of indigenous children: Findings from the First National Survey of Indigenous People’s Health and Nutrition in Brazil. Int. J. Equity Health 2013, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Arana, A.; Vargas-Machuca, R.; Antiporta, D. Health and nutrition of indigenous and nonindigenous children in the Peruvian Amazon. Rev. Panam Salud Publica 2015, 38, 49–56. (In Spanish) [Google Scholar]
- Joseph, S.A.; Casapía, M.; Blouin, B.; Maheu-Giroux, M.; Rahme, E.; Gyorkos, T.W. Risk factors associated with malnutrition in one-year-old children living in the Peruvian Amazon. PLoS Negl. Trop. Dis. 2014, 8, e3369. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, A.D.; Urlacher, S.S.; Beheim, B.; von Rueden, C.; Jaeggi, A.; Stieglitz, J.; Trumble, B.C.; Gurven, M.; Kaplan, H. Growth references for Tsimane forager-horticulturalists of the Bolivian Amazon. Am. J. Phys. Anthropol. 2017, 162, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Urlacher, S.S.; Blackwell, A.D.; Liebert, M.A.; Liebert, M.A.; Madimenos, F.C.; Cepon-Robins, T.J.; Gildner, T.E.; Snodgrass, J.J.; Sugiyama, L.S. Physical growth of the Shuar: Height, weight, and BMI references for an indigenous Amazonian Population. Am. J. Hum. Biol. 2016, 28, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, A.A.; Mantovani, S.A.; Delfino, B.M.; Pereira, T.M.; Martins, A.C.; Oliart-Guzmán, H.; Brãna, A.M.; Branco, F.L.; Campos, R.G.; Guimarães, A.S.; et al. Nutritional status of children under 5 years of age in the Brazilian Western Amazon before and after the Interoceanic highway paving: A population-based study. BMC Public Health 2013, 13, 1098. [Google Scholar] [CrossRef] [PubMed]
- Cobayashi, F.; Augusto, R.A.; Lourenço, B.H.; Muniz, P.T.; Cardoso, M.A. Factors associated with stunting and overweight in Amazonian children: A population-based, cross-sectional study. Public Health Nutr. 2014, 17, 551–560. [Google Scholar] [CrossRef] [PubMed]
- FDA. Fish: What Pregnant Women and Parents Should Know: Draft Updated Advice by FDA and EPA. 2014. Available online: https://www.fda.gov/Food/ResourcesForYou/Consumers/ucm393070.htm (accessed on 17 January 2009).
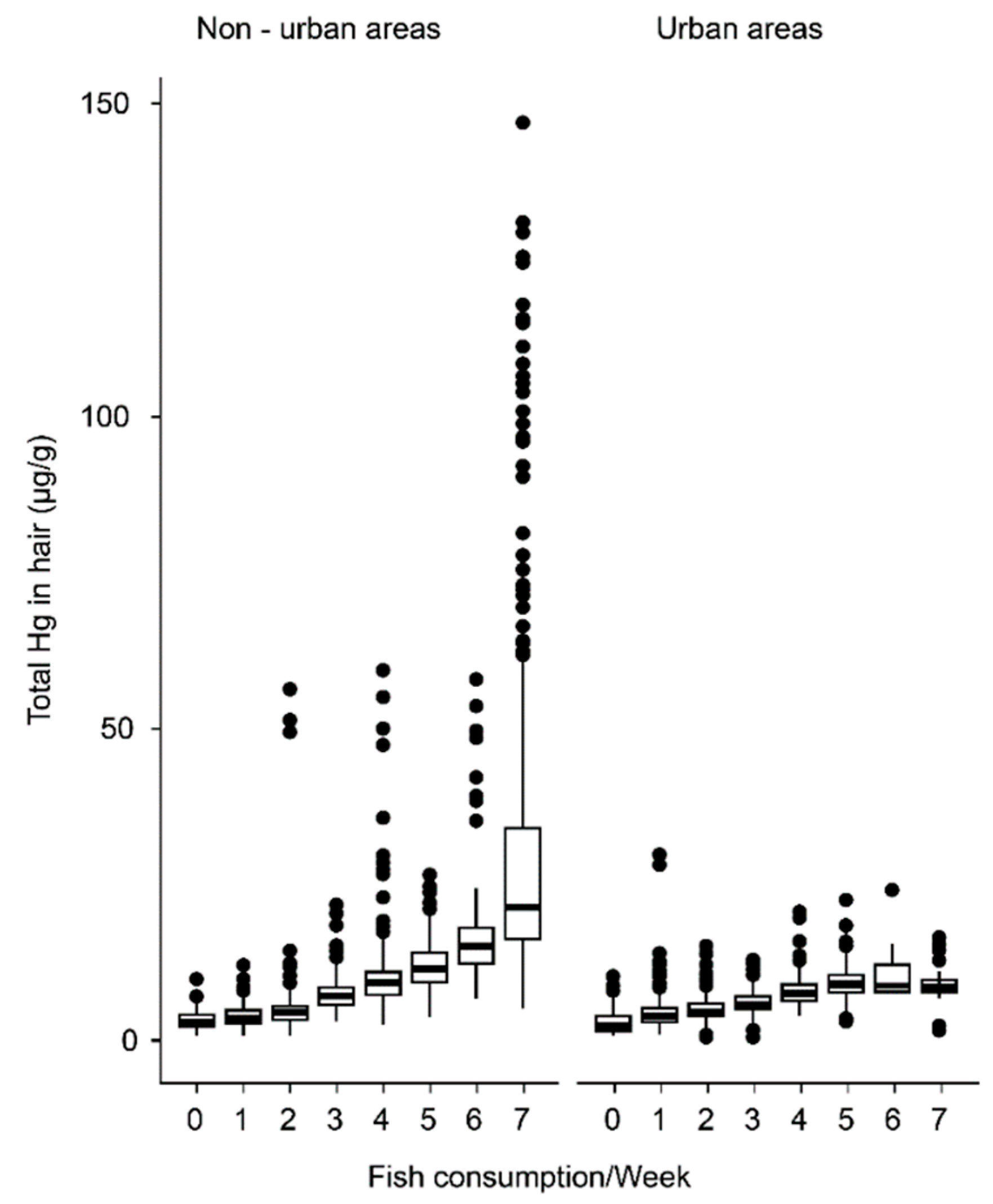
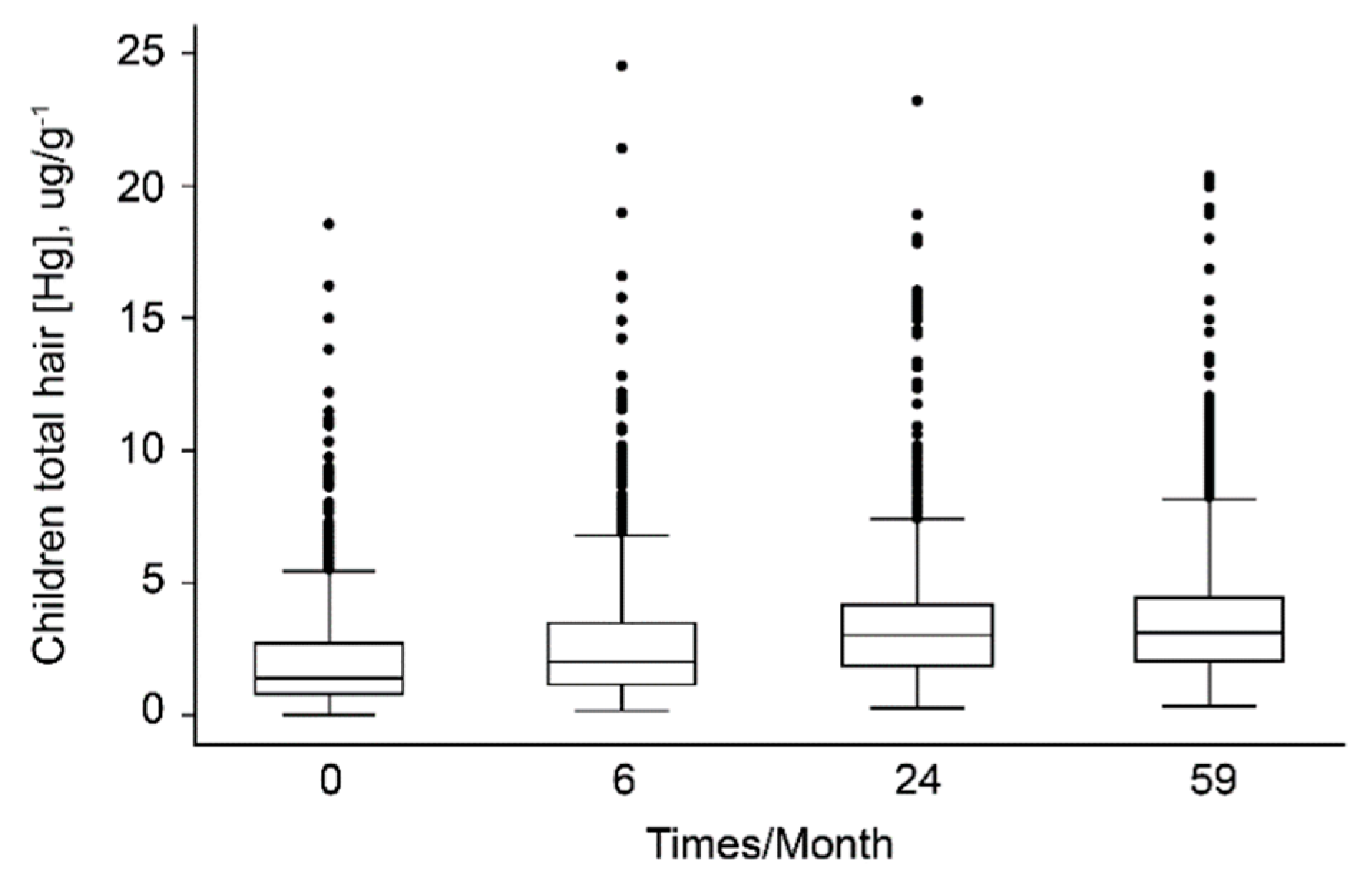


| Urban | Non-Urban | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Min | Mean | Max | (±SD) | Min | Mean | Max | (±SD) |
| Mothers | ||||||||
| Income 1 | 100 | 752.0 | 4500 | 504.49 | 50 | 560.60 | 2500 | 338.95 |
| Maternal schooling in years | 0 | 6.80 | 16 | 3.28 | 0 | 5.22 | 16 | 2.73 |
| Number of children | 0 | 1.96 | 8 | 1.60 | 0 | 2.00 | 12 | 1.80 |
| Maternal age (years) | 13 | 23.05 | 42 | 6.09 | 13 | 22.60 | 43 | 5.87 |
| Gestational age (week) | 35 | 39.33 | 45 | 1.53 | 32 | 38.91 | 43 | 1.62 |
| Fish consumption per week | 0 | 2.27 | 7 | 1.44 | 0 | 3.30 | 7 | 2.32 |
| Breastfeeding (months) 2 | 0 | 5.6 | 36 | 5.58 | 0 | 7.4 | 40 | 5.82 |
| HHgM (µg/g) 3 | ||||||||
| 0 months | 0.73 | 5.56 | 29.32 | 3.09 | 1.02 | 11.61 | 253 | 17.02 |
| 6 months | 0.5 | 4.95 | 19.59 | 2.41 | 0.7 | 10.49 | 125.21 | 13.79 |
| 24 months | 0.49 | 5.66 | 29.72 | 2.83 | 0.87 | 10.76 | 129.15 | 13.35 |
| 59 months | 0.55 | 5.67 | 15.84 | 2.69 | 0.56 | 11.28 | 146.87 | 14.52 |
| Children | ||||||||
| Length at birth (cm) | 43 | 50.99 | 59.5 | 2.66 | 43 | 50.5 | 59 | 2.57 |
| Weight at birth (g) | 2200 | 3281 | 5950 | 498.03 | 2010 | 3150 | 5250 | 422.05 |
| H/A (Z-score) 4 | ||||||||
| 0 months | −2.58 | 0.76 | 5.56 | 1.42 | −3.14 | 0.65 | 2.29 | 1.35 |
| 6 months | −3.13 | 0.24 | 2.29 | 1.06 | −3.13 | 0.22 | 2.48 | 1.06 |
| 24 months | −2.03 | −0.32 | 4.86 | 0.92 | −2.23 | −0.31 | 3.20 | 0.80 |
| 59 months | −1.83 | −0.71 | 1.23 | 0.57 | −1.94 | −0.69 | 1.02 | 0.57 |
| W/A (Z-score) 4 | ||||||||
| 0 months | −2.67 | −0.41 | 506 | 1.02 | −2.53 | −0.18 | 3.81 | 0.88 |
| 6 months | −2.92 | −0.76 | 1.24 | 0.69 | −2.84 | −0.69 | 1.45 | 0.71 |
| 24 months | −2.25 | 0.12 | 3.19 | 0.93 | −2.16 | 0.20 | 3.09 | 0.83 |
| 59 months | −2.42 | −0.54 | 2.29 | 0.93 | −2.42 | −0.47 | 2.15 | 0.93 |
| W/H (Z-score) 4 | ||||||||
| 0 months | −4.19 | −1.03 | 2.75 | 0.88 | −4.34 | −1.08 | 2.38 | 0.93 |
| 6 months | −3.94 | −1.11 | 2.43 | 0.83 | −2.57 | −1.01 | 1.5 | 0.70 |
| 24 months | −3.53 | 0.35 | 3.88 | 1.39 | −4.11 | 0.44 | 4.78 | 1.23 |
| 59 months | −2.38 | −0.21 | 3.48 | 1.01 | −2.45 | −0.13 | 3.05 | 0.95 |
| HHgC (µg/g) 3 | ||||||||
| 0 months | 0.0001 | 2.26 | 24.55 | 1.74 | 0.0001 | 3.53 | 19.99 | 2.70 |
| 6 months | 0.0001 | 2.24 | 15.65 | 1.60 | 0.0001 | 3.41 | 23.24 | 2.72 |
| 24 months | 0.0001 | 2.28 | 21.41 | 1.85 | 0.0001 | 3.49 | 18.53 | 2.56 |
| 59 months | 0.0001 | 2.18 | 12.83 | 1.61 | 0.0001 | 3.42 | 16.58 | 2.34 |
| Hemoglobin (g/dL) | ||||||||
| 24 months | 8.2 | 10.96 | 13.0 | 1.03 | 8.1 | 11.02 | 13.4 | 1.01 |
| 59 months | 8.3 | 10.80 | 13.1 | 1.02 | 8.1 | 10.87 | 12.8 | 0.96 |
| Fixed Effect | β | SE | 95% CI | p-Value | Random Effect | Variance | SD | Model Adjustment | |
|---|---|---|---|---|---|---|---|---|---|
| H/A | |||||||||
| Intercept | −2.182 | 0.3081 | −2.786, −1.578 | <0.0001 | Intercept | 0.5757 | 0.7587 | −2 Log-Similarity | −7604.6 |
| Child age | −0.0200 | 0.0006 | −0.0212, −0.0187 | <0.0001 | Child age | 0.0779 | 0.2791 | AIC 2 | 15,233.2 |
| Male | −0.4563 | 0.0247 | −0.5047, −0.4078 | <0.0001 | Residue | 0.8323 | 0.9123 | ||
| Number of children | −0.0223 | 0.0105 | −0.0429, −0.0017 | 0.0339 | |||||
| Birth weight | 0.0006 | <0.0001 | 5.4 × 10−4, 6.5 × 10−4 | <0.0001 | |||||
| Income 1 | 0.0001 | <0.0001 | 6.5 × 10−5, 1.8 × 10−4 | <0.0001 | |||||
| Gestational age (weeks) | 0.0173 | 0.0080 | 0.0016, 0.0331 | 0.0312 | |||||
| Hemoglobin 3 | 0.2288 | 0.0121 | 0.2306, 0.2775 | 0.0000 | |||||
| Maternal age (years) | 0.0081 | 0.0030 | 0.0022, 0.0140 | 0.0072 | |||||
| Residential location | 0.0075 | 0.0261 | −0.0436, 0.0586 | 0.7736 | |||||
| Maternal schooling (years) | −0.0016 | 0.0046 | −0.0105, 0.0074 | 0.7312 | |||||
| Breastfeeding | 0.0041 | 0.0022 | −0.0001, 0.0084 | 0.0579 | |||||
| HHgM 4 | 0.0006 | 0.0015 | −2.3662, 0.0037 | 0.6656 | |||||
| Weekly fish intake | −0.0107 | 0.0064 | −0.0232, 0.0017 | 0.0921 | |||||
| W/A | |||||||||
| Intercept | −2.939 | 0.0982 | −3.131, −2.746 | <0.0001 | Intercept | 0.2744 | 0.5238 | −2 Log-Similarity | −6983.1 |
| child age | 0.1103 | 0.3321 | AIC 2 | 13,986.2 | |||||
| Male | −0.4076 | 0.0236 | −0.4538, −0.3613 | <0.0001 | Residue | 0.5776 | 0.7600 | ||
| Birth weight | 0.0008 | <0.0001 | 7.8 × 10−4, 8.9 × 10−4 | <0.0001 | |||||
| Income 1 | <0.0001 | <0.0001 | 2.8 × 10−5, 1.3 × 10−4 | 0.0027 | |||||
| Maternal age | 0.0048 | 0.0020 | 0.0009, 0.0087 | 0.0147 | |||||
| Hemoglobin 3 | 0.2442 | 0.0183 | 0.2880, 0.3584 | 0.0000 | |||||
| Maternal age (years) | 0.0078 | 0.0077 | −0.0073, 0.0229 | 0.3122 | |||||
| Residence location | −0.0298 | 0.0246 | −0.0780, 0.0185 | 0.2272 | |||||
| Maternal schooling (years) | 0.0054 | 0.0044 | −0.0031, 0.0141 | 0.2137 | |||||
| Breastfeeding | 0.0032 | 0.0020 | −0.0008, 0.0072 | 0.1227 | |||||
| Number of children | −0.0182 | 0.0100 | −0.0379, 0.0146 | 0.0697 | |||||
| Gestational age (weeks) | 0.0078 | 0.0077 | −0.0073, 0.0229 | 0.3122 | |||||
| HHgM 4 | 0.0006 | 0.0015 | −0.0023, 0.0036 | 0.6824 | |||||
| Weekly fish intake | −0.0043 | 0.0063 | −0.0167, 0.0081 | 0.4958 | |||||
| W/H | |||||||||
| Intercept | −1.050 | 0.0689 | −1.185, −0.915 | <0.0001 | Intercept | <0.000 | <0.000 | −2 Log-Similarity | −8474.0 |
| Age | 0.0162 | 0.0006 | 0.0150, 0.0174 | <0.0001 | Child age | <0.000 | <0.000 | AIC | 16,970.0 |
| Male | −0.0743 | 0.0304 | −0.1339, −0.0147 | 0.0145 | Residue | 1.264 | 1.124 | ||
| Urban area | −0.0743 | 0.0319 | −0.1368, −0.0118 | 0.0198 | |||||
| Breastfeeding | 0.0053 | 0.0027 | 7.1 × 10−5, 0.0105 | 0.0470 | |||||
| Maternal age (years) | 0.0062 | 0.0025 | 0.0012, 0.0112 | 0.0151 | |||||
| Maternal Schooling (years) | 0.0181 | 0.0051 | 0.0081, 0.0281 | 0.0004 | |||||
| Hemoglobin 3 | 0.2955 | 0.0220 | 0.3159, 0.3984 | 0.0000 | |||||
| Income 1 | <0.0001 | <0.0001 | −5.31 × 10−6, 0.0001 | 0.0681 | |||||
| Number of children | −0.0188 | 0.0130 | −0.0445, 0.0063 | 0.1403 | |||||
| Gestational age (weeks) | −0.0058 | 0.0101 | −0.0255, 0.0140 | 0.5669 | |||||
| Birth weight | <0.0001 | <0.0001 | −2.81 × 10−5, 0.0001 | 0.2661 | |||||
| HHgM 4 | −0.0014 | 0.0015 | −0.0044, 0.0017 | 0.3765 | |||||
| Weekly fish intake | 0.0151 | 0.0099 | −0.0043, 0.0345 | 0.1280 | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, M.P.L.; Marques, R.C.; Dórea, J.G. Influence of Maternal Fish Intake on the Anthropometric Indices of Children in the Western Amazon. Nutrients 2018, 10, 1146. https://doi.org/10.3390/nu10091146
Cunha MPL, Marques RC, Dórea JG. Influence of Maternal Fish Intake on the Anthropometric Indices of Children in the Western Amazon. Nutrients. 2018; 10(9):1146. https://doi.org/10.3390/nu10091146
Chicago/Turabian StyleCunha, Mônica P. L., Rejane C. Marques, and José G. Dórea. 2018. "Influence of Maternal Fish Intake on the Anthropometric Indices of Children in the Western Amazon" Nutrients 10, no. 9: 1146. https://doi.org/10.3390/nu10091146
APA StyleCunha, M. P. L., Marques, R. C., & Dórea, J. G. (2018). Influence of Maternal Fish Intake on the Anthropometric Indices of Children in the Western Amazon. Nutrients, 10(9), 1146. https://doi.org/10.3390/nu10091146





