Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity
Abstract
1. Introduction
2. Appetite, Energy Intake and Appetite-Related Hormone Responses to Exercise Interventions
2.1. Acute Exercise
2.2. Chronic Exercise
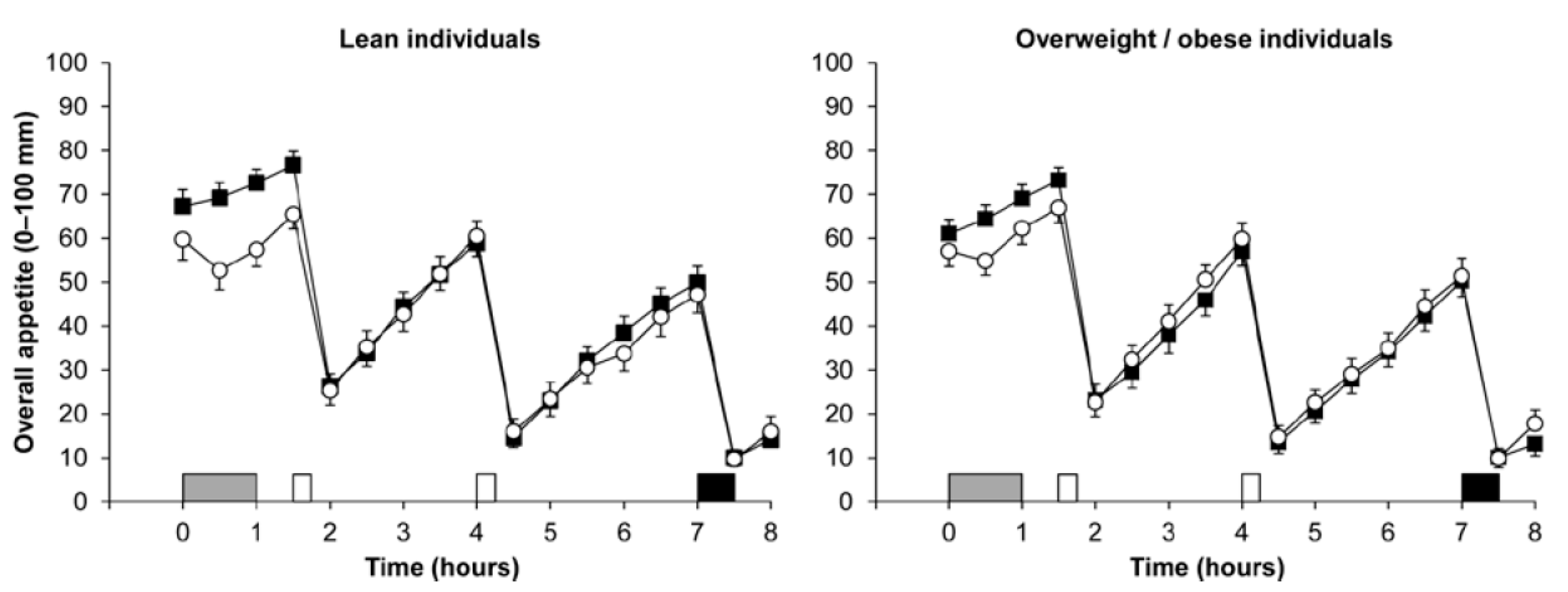
3. Body Adiposity
3.1. Acute Exercise
3.2. Chronic Exercise
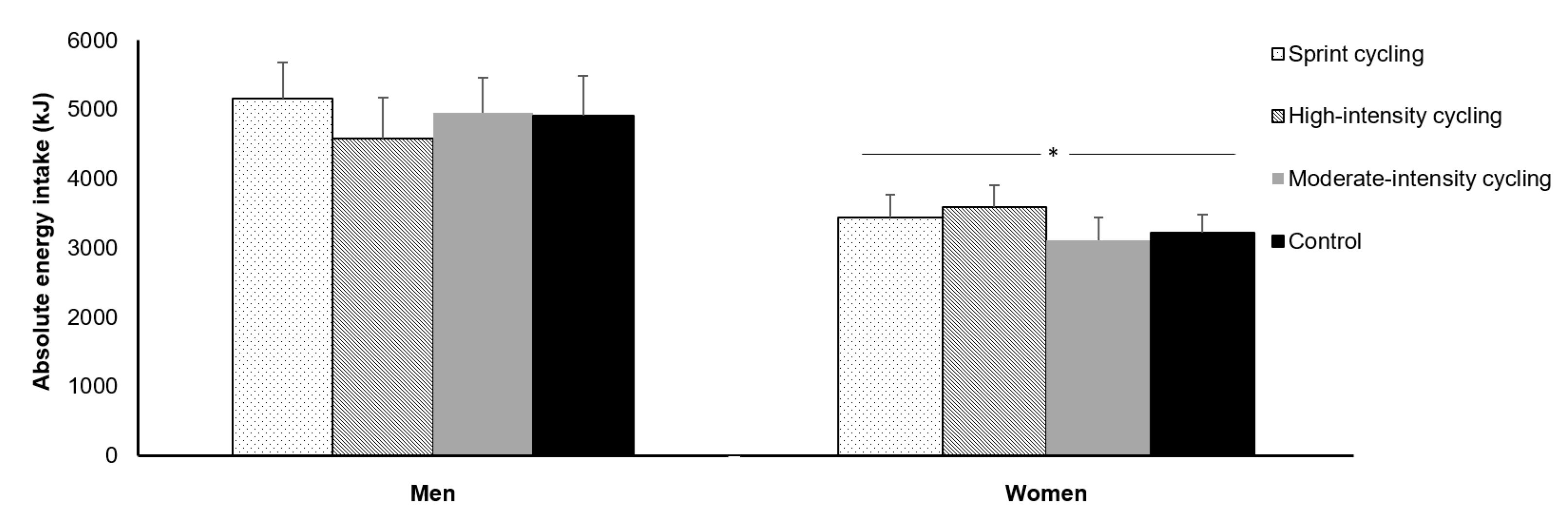
4. Sex
4.1. Acute Exercise
4.2. Chronic Exercise
5. Habitual Physical Activity and Exercise
5.1. Acute Exercise
5.2. Chronic Exercise
6. Implications and Areas of Future Study
7. Methodological Issues
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Cesare, M.; Bentham, J.; Stevens, G.A.; Zhou, B.; Danaei, G.; Lu, Y.; Bixby, H.; Cowan, M.J.; Riley, L.M.; Hajifathalian, K.; et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; McPherson, K.; Marsh, T.; Gortmaker, S.L.; Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011, 378, 815–825. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, W.M.; Bloomfield, S.A.; Little, K.D.; Nelson, M.E.; Yingling, V.R. American College of Sports Medicine Position Stand: Physical activity and bone health. Med. Sci. Sports Exerc. 2004, 36, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K. American college of sports medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sport. Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Tremblay, A.; Després, J.P.P.; Thériault, G.; Nadeauf, A.; Lupien, P.J.; Moorjani, S.; Prudhomme, D.; Fournier, G. The response to exercise with constant energy intake in identical twins. Obes. Res. 1994, 2, 400–410. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Hopkins, M.; Caudwell, P.; Stubbs, R.J.; Blundell, J.E. Individual variability following 12 weeks of supervised exercise: Identification and characterization of compensation for exercise-induced weight loss. Int. J. Obes. 2008, 32, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Roy, P.; Mitra, K.P. Relation between caloric intake, body weight, and physical work: Studies in an industrial male population in West Bengal. Am. J. Clin. Nutr. 1956, 4, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Caudwell, P.; Gibbons, C.; Hopkins, M.; Naslund, E.; King, N.; Finlayson, G. Role of resting metabolic rate and energy expenditure in hunger and appetite control: A new formulation. Dis. Model. Mech. 2012, 5, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Caudwell, P.; Finlayson, G.; Gibbons, C.; Hopkins, M.; King, N.; Näslund, E.; Blundell, J.E. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am. J. Clin. Nutr. 2013, 97, 7–14. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Deighton, K.; Broom, D.R.; Wasse, L.K.; Douglas, J.A.; Burns, S.F.; Cordery, P.A.; Petherick, E.S.; Batterham, R.L.; Goltz, F.R.; et al. Individual variation in hunger, energy intake, and ghrelin responses to acute exercise. Med. Sci. Sports Exerc. 2017, 49, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; De Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; Van Der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, K.; Hopkins, M.; Blundell, J.E.; Finlayson, G. Homeostatic and non-homeostatic appetite control along the spectrum of physical activity levels: An updated perspective. Physiol. Behav. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Wasse, L.K.; Stensel, D.J.; Nimmo, M.A. Exercise and ghrelin. A narrative overview of research. Appetite 2013, 68, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.; Batterham, R.L. The role of gut hormone peptide YY in energy and glucose homeostasis: Twelve years on. Annu. Rev. Physiol. 2014, 76, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, K.; Hopkins, M.; Blundell, J.; Finlayson, G. Does habitual physical activity increase the sensitivity of the appetite control system? A systematic review. Sports Med. 2016, 46, 1897–1919. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Stensel, D.J. Creating an acute energy deficit without stimulating compensatory increases in appetite: Is there an optimal exercise protocol? Proc. Nutr. Soc. 2014, 73, 352–358. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Burley, V.J.; Blundell, J.E. Exercise-induced suppression of appetite: Effects on food intake and implications for energy balance. Eur. J. Clin. Nutr. 1994, 48, 715–724. [Google Scholar] [PubMed]
- King, J.A.; Wasse, L.K.; Ewens, J.; Crystallis, K.; Emmanuel, J.; Batterham, R.L.; Stensel, D.J. Differential acylated ghrelin, peptide YY3-36, appetite, and food intake responses to equivalent energy deficits created by exercise and food restriction. J. Clin. Endocrinol. MeTable 2011, 96, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Miyashita, M.; Wasse, L.K.; Stensel, D.J. Influence of prolonged treadmill running on appetite, energy intake and circulating concentrations of acylated ghrelin. Appetite 2010, 54, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.R.; Stensel, D.J.; Bishop, N.C.; Burns, S.F.; Miyashita, M. Exercise-induced suppression of acylated ghrelin in humans. J. Appl. Physiol. 2007, 102, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Barry, R.; Connon, C.E.; Stensel, D.J. Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise. Eur. J. Appl. Physiol. 2013, 113, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.R.; Batterham, R.L.; King, J.A.; Stensel, D.J. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R29–R35. [Google Scholar] [CrossRef] [PubMed]
- Laan, D.J.; Leidy, H.J.; Lim, E.; Campbell, W.W. Effects and reproducibility of aerobic and resistance exercise on appetite and energy intake in young, physically active adults. Appl. Physiol. Nutr. Metab. 2010, 35, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.S.; Donges, C.E.; Guelfi, K.J.; Smith, G.C.; Adams, D.R.; Duffield, R. Effects of aerobic, strength or combined exercise on perceived appetite and appetite-related hormones in inactive middle-aged men. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Ballard, T.P.; Melby, C.L.; Camus, H.; Cianciulli, M.; Pitts, J.; Schmidt, S.; Hickey, M.S. Effect of resistance exercise, with or without carbohydrate supplementation, on plasma ghrelin concentrations and postexercise hunger and food intake. Metabolism 2009, 58, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Morgan, L.M.; Bloom, S.R.; Robertson, M.D. Effects of exercise on gut peptides, energy intake and appetite. J. Endocrinol. 2007, 193, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.Y.; Yoshikawa, T.; Katsura, Y.; Usui, T.; Nakao, H.; Fujimoto, S. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. J. Endocrinol. 2009, 201, 151–159. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Lluch, A.; Stubbs, R.J.; Blundell, J.E. High dose exercise does not increase hunger or energy intake in free living males. Eur. J. Clin. Nutr. 1997, 51, 478–483. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Garnham, J.O.; Jackson, A.P.; Kelly, B.M.; Xenophontos, S.; Nimmo, M.A. Appetite-regulatory hormone responses on the day following a prolonged bout of moderate-intensity exercise. Physiol. Behav. 2015, 141, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.A.; King, J.A.; Clayton, D.J.; Jackson, A.P.; Sargeant, J.A.; Thackray, A.E.; Davies, M.J.; Stensel, D.J. Acute effects of exercise on appetite, ad libitum energy intake and appetite-regulatory hormones in lean and overweight/obese men and women. Int. J. Obes. 2017, 41, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Alajmi, N.; Deighton, K.; King, J.A.; Reischak-Oliveira, A.; Wasse, L.K.; Jones, J.; Batterham, R.L.; Stensel, D.J. Appetite and energy intake responses to acute energy deficits in females versus males. Med. Sci. Sports Exerc. 2016, 48, 412–420. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Wasse, L.K.; Broom, D.R.; Stensel, D.J. Influence of brisk walking on appetite, energy intake, and plasma acylated ghrelin. Med. Sci. Sports Exerc. 2010, 42, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Balaguera-Cortes, L.; Wallman, K.E.; Fairchild, T.J.; Guelfi, K.J. Energy intake and appetite-related hormones following acute aerobic and resistance exercise. Appl. Physiol. Nutr. Metab. 2011, 36, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Jokisch, E.; Coletta, A.; Raynor, H.A. Acute energy compensation and macronutrient intake following exercise in active and inactive males who are normal weight. Appetite 2012, 58, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Cadieux, S.; McNeil, J.; Lapierre, M.P.; Riou, M.È.; Doucet, É. Resistance and aerobic exercises do not affect post-exercise energy compensation in normal weight men and women. Physiol. Behav. 2014, 130, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.G.; Bloom, S.R. Gut hormones and the regulation of energy homeostasis. Nature 2006, 444, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Sliwowski, Z.; Lorens, K.; Konturek, S.J.; Bielanski, W.; Zoladz, J.A. Leptin, gastrointestinal and stress hormones in response to exercise in fasted or fed subjects and before or after blood donation. J. Physiol. Pharmacol. 2001, 52, 53–70. [Google Scholar] [PubMed]
- Ueda, S.Y.; Yoshikawa, T.; Katsura, Y.; Usui, T.; Fujimoto, S. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. J. Endocrinol. 2009, 203, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Mineta, M.; Asaka, M.; Miyashita, M.; Numao, S.; Gando, Y.; Ando, T.; Sakamoto, S.; Higuchi, M. Effects of different modes of exercise on appetite and appetite-regulating hormones. Appetite 2013, 66, 26–33. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Caudwell, P.P.; Hopkins, M.; Stubbs, J.R.; Naslund, E.; Blundell, J.E. Dual-process action of exercise on appetite control: Increase in orexigenic drive but improvement in meal-induced satiety. Am. J. Clin. Nutr. 2009, 90, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Caudwell, P.; Gibbons, C.; Hopkins, M.; King, N.; Finlayson, G.; Blundell, J. No sex difference in body fat in response to supervised and measured exercise. Med. Sci. Sports Exerc. 2013, 45, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Kulseng, B.; King, N.A.; Holst, J.J.; Blundell, J.E. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J. Clin. Endocrinol. Metab. 2010, 95, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Kulseng, B.; Rehfeld, J.F.; King, N.A.; Blundell, J.E. Effect of chronic exercise on appetite control in overweight and obese individuals. Med. Sci. Sports Exerc. 2013, 45, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Morishima, T.; Kurihara, T.; Hamaoka, T.; Goto, K. Whole body, regional fat accumulation, and appetite-related hormonal response after hypoxic training. Clin. Physiol. Funct. Imaging 2014, 34, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Truby, H.; Morgan, L.M. Short-term appetite control in response to a 6-week exercise programme in sedentary volunteers. Br. J. Nutr. 2007, 98, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Guelfi, K.J.; Donges, C.E.; Duffield, R. Beneficial effects of 12 weeks of aerobic compared with resistance exercise training on perceived appetite in previously sedentary overweight and obese men. Metabolism 2013, 62, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Rosenkilde, M.; Reichkendler, M.H.; Auerbach, P.; Torang, S.; Gram, A.S.; Ploug, T.; Holst, J.J.; Sjodin, A.; Stallknecht, B. Appetite regulation in overweight, sedentary men after different amounts of endurance exercise: A randomized controlled trial. J. Appl. Physiol. 2013, 115, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Bryant, E.J.; Caudwell, P.; Hopkins, M.E.; King, N.A.; Blundell, J.E. Psycho-markers of weight loss. The roles of TFEQ disinhibition and restraint in exercise-induced weight management. Appetite 2012, 58, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Melanson, E.L.; Salzberg, A.K.; Bechtell, J.L.; Tregellas, J.R. The effects of exercise on the neuronal response to food cues. Physiol. Behav. 2012, 105, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Pil-Byung, C.; Shin-Hwan, Y.; Il-Gyu, K.; Gwang-Suk, H.; Jae-Hyun, Y.; Han-Joon, L.; Sung-Eun, K.; Yong-Seok Korea, J. Effects of exercise program on appetite-regulating hormones, inflammatory mediators, lipid profiles, and body composition in healthy men. J. Sports Med. Phys. Fitness 2011, 51, 654–663. [Google Scholar] [PubMed]
- Kanaley, J.A.; Heden, T.D.; Liu, Y.; Whaley-Connell, A.T.; Chockalingam, A.; Dellsperger, K.C.; Fairchild, T.J. Short-term aerobic exercise training increases postprandial pancreatic polypeptide but not peptide YY concentrations in obese individuals. Int. J. Obes. 2014, 38, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, W.; Sun, D.; Fernandez, C.; Li, J.; Kelly, T.; He, J.; Krousel-Wood, M.; Whelton, P.K. Variability and rapid increase in body mass index during childhood are associated with adult obesity. Int. J. Epidemiol. 2015, 44, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Karra, E.; Batterham, R.L. The role of gut hormones in the regulation of body weight and energy homeostasis. Mol. Cell. Endocrinol. 2010, 316, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, S.R.; Rehfeld, J.F.; Holst, J.J.; Kulseng, B.; Martins, C. Impact of weight loss achieved through a multidisciplinary intervention on appetite in patients with severe obesity. Am. J. Physiol. Endocrinol. Metab. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Bagdade, J.D.; Bierman, E.L.; Porte, D.J. The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J. Clin. Investig. 1967, 46, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Holmstrup, M.E.; Fairchild, T.J.; Keslacy, S.; Weinstock, R.S.; Kanaley, J.A. Satiety, but not total PYY, is increased with continuous and intermittent exercise. Obesity 2013, 21, 2014–2020. [Google Scholar] [CrossRef] [PubMed]
- Holliday, A.; Blannin, A.K. Very low volume sprint interval exercise suppresses subjective appetite, lowers acylated ghrelin, and elevates GLP-1 in overweight individuals: A pilot study. Nutrients 2017, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Sim, A.Y.; Wallman, K.E.; Fairchild, T.J.; Guelfi, K.J. High-intensity intermittent exercise attenuates ad-libitum energy intake. Int. J. Obes. 2014, 38, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Stensvold, D.; Finlayson, G.; Holst, J.; Wisloff, U.; Kulseng, B.; Morgan, L.; King, N.A. Effect of moderate- and high-intensity acute exercise on appetite in obese individuals. Med. Sci. Sports Exerc. 2015, 47, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Unick, J.L.; Otto, A.D.; Goodpaster, B.H.; Helsel, D.L.; Pellegrini, C.A.; Jakicic, J.M. Acute effect of walking on energy intake in overweight/obese women. Appetite 2010, 55, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Tsofliou, F.; Pitsiladis, Y.P.; Malkova, D.; Wallace, A.M.; Lean, M.E.J. Moderate physical activity permits acute coupling between serum leptin and appetite-satiety measures in obese women. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Karra, E.; Batterham, R.L.; Stensel, D.J. Appetite, energy intake, and PYY3-36 responses to energy-matched continuous exercise and submaximal high-intensity exercise. Appl. Physiol. Nutr. Metab. 2013, 38, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Kissileff, H.R.; Pi-Sunyer, F.X.; Segal, K.; Meltzer, S.; Foelsch, P.A. Acute effects of exercise on food intake in obese and nonobese women. Am. J. Clin. Nutr. 1990, 52, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Marzullo, P.; Salvadori, A.; Brunani, A.; Verti, B.; Walker, G.E.; Fanari, P.; Tovaglieri, I.; Medici, C.D.; Savia, G.; Liuzzi, A. Acylated ghrelin decreases during acute exercise in the lean and obese state. Clin. Endocrinol. (Oxf.) 2008, 69, 970–971. [Google Scholar] [CrossRef] [PubMed]
- Mihalache, L.; Gherasim, A.; Niță, O.; Ungureanu, M.C.; Pădureanu, S.S.; Gavril, R.S.; Arhire, L.I. Effects of ghrelin in energy balance and body weight homeostasis. Hormones 2016, 15, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.; Leichner, P.; Wong, A.C.K.; Ghatei, M.A.; Kieffer, T.J.; Bloom, S.R.; Chanoine, J.P. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J. Clin. Endocrinol. Metab. 2005, 90, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Aschehoug, I.; Ludviksen, M.; Holst, J.; Finlayson, G.; Wisloff, U.; Morgan, L.; King, N.; Kulseng, B. High-intensity interval training, appetite, and reward value of food in the obese. Med. Sci. Sport Exerc. 2017, 49, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, H.; Kojima, M.; Mizushima, T.; Shimizu, S.; Kangawa, K. Structural divergence of human ghrelin: Identification of multiple ghrelin-derived molecules produced by post-translational processing. J. Biol. Chem. 2003, 278, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Blundell, J.E.; Caudwell, P.; Webb, D.L.; Hellström, P.M.; Näslund, E.; Finlayson, G. The Role of Episodic Postprandial Peptides in Exercise-Induced Compensatory Eating. J. Clin. Endocrinol. Metab. 2017, 102, 4051–4059. [Google Scholar] [CrossRef] [PubMed]
- Ramel, A.; Halldorsson, T.I.; Tryggvadottir, E.A.; Martinez, J.A.; Kiely, M.; Bandarra, N.M.; Thorsdottir, I. Relationship between BMI and body fatness in three European countries. Eur. J. Clin. Nutr. 2013, 67, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, E.; Gormsen, L.C.; Nellemann, B.; Vestergaard, E.T.; Christiansen, J.S.; Nielsen, S. Visceral fat mass is a strong predictor of circulating ghrelin levels in premenopausal women. Eur. J. Endocrinol. 2009, 160, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Brennan, I.M.; Feltrin, K.L.; Nair, N.S.; Hausken, T.; Little, T.J.; Gentilcore, D.; Wishart, J.M.; Jones, K.L.; Horowitz, M.; Feinle-Bisset, C. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G602–G610. [Google Scholar] [CrossRef] [PubMed]
- Lissner, L.; Stevens, J.; Levitsky, D.A.; Rasmussen, K.M.; Strupp, B.J. Variation in energy intake during the menstrual cycle: Implications for food-intake research. Am. J. Clin. Nutr. 1988, 48, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Wade, G.N.; Jones, J.E. Neuroendocrinology of nutritional infertility. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1277–R1296. [Google Scholar] [CrossRef] [PubMed]
- Thackray, A.E.; Deighton, K.; King, J.A.; Stensel, D.J. Exercise, appetite and weight control: Are there differences between men and women? Nutrients 2016, 8, 583. [Google Scholar] [CrossRef] [PubMed]
- Reger, W.E.; Allison, T.A.; Kurucz, R.L. Exercise, postexercise metabolic rate and appetite. Sport Heal. Nutr. 1986, 2, 117–123. [Google Scholar]
- Howe, S.M.; Hand, T.M.; Larson-Meyer, D.E.; Austin, K.J.; Alexander, B.M.; Manore, M.M. No effect of exercise intensity on appetite in highly-trained endurance women. Nutrients 2016, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Tiryaki-Sonmez, G.; Ozen, S.; Bugdayci, G.; Karli, U.; Ozen, G.; Cogalgil, S.; Schoenfeld, B.; Sozbir, K.; Aydin, K. Effect of exercise on appetite-regulating hormones in overweight women. Biol. Sport 2013, 30, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Hazell, T.J.; Townsend, L.K.; Hallworth, J.R.; Doan, J.; Copeland, J.L. Sex differences in the response of total PYY and GLP-1 to moderate-intensity continuous and sprint interval cycling exercise. Eur. J. Appl. Physiol. 2017, 117, 431–440. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Snell, L.; Smith, R.D.; Blundell, J.E. Effects of short-term exercise on appetite responses in unrestrained females. Eur. J. Cin. Nutr. 1996, 50, 663–667. [Google Scholar]
- Hallworth, J.R.; Copeland, J.L.; Doan, J.; Hazell, T.J. The effect of exercise intensity on total PYY and GLP-1 in healthy females: A pilot study. J. Nutr. Metab. 2017, 2017, 4823102. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Palm, S.; Bansal, A.; Austin, K.J.; Hart, A.M.; Alexander, B.M. Influence of running and walking on hormonal regulators of appetite in women. J. Obes. 2012, 2012, 730409. [Google Scholar] [CrossRef] [PubMed]
- Panissa, V.L.G.; Julio, U.F.; Hardt, F.; Kurashima, C.; Lira, F.S.; Takito, M.Y.; Franchini, E. Effect of exercise intensity and mode on acute appetite control in men and women. Appl. Physiol. Nutr. Metab. 2016, 41, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Hagobian, T.; Yamashiro, M.; Hinkel-Lipsker, J.; Streder, K.; Evero, N.; Hackney, T. Effects of acute exercise on appetite hormones and ad libitum energy intake in men and women. Appl. Physiol. Nutr. Metab. 2013, 38, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Shamlan, G.; Bech, P.; Robertson, M.D.; Collins, A.L. Acute effects of exercise intensity on subsequent substrate utilisation, appetite and energy balance in men and women. Appl. Physiol. Nutr. Metab. 2017, 42, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Chandarana, K.; Drew, M.E.; Emmanuel, J.; Karra, E.; Gelegen, C.; Chan, P.; Cron, N.J.; Batterham, R.L. Subject standardization, acclimatization, and sample processing affect gut hormone levels and appetite in humans. Gastroenterology 2009, 136, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Hagobian, T.A.; Sharoff, C.G.; Stephens, B.R.; Wade, G.N.; Silva, J.E.; Chipkin, S.R.; Braun, B. Effects of exercise on energy-regulating hormones and appetite in men and women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R233–R242. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.J.; Sepp, A.; Hughes, D.A.; Johnstone, A.M.; Horgan, G.; King, N.; Blundell, J.E. The effect of graded levels of exercise on energy intake and balance in free-living women. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.; Sepp, A.; Hughes, D.; Johnstone, A.; King, N.; Horgan, G.; Blundell, J. The effect of graded levels of exercise on energy intake and balance in free-living men, consuming their normal diet. Eur. J. Clin. Nutr. 2002, 56, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Whybrow, S.; Hughes, D.A.; Ritz, P.; Johnstone, A.M.; Horgan, G.W.; King, N.; Blundell, J.E.; Stubbs, R.J. The effect of an incremental increase in exercise on appetite, eating behaviour and energy balance in lean men and women feeding ad libitum. Br. J. Nutr. 2008, 100, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Church, T.S.; Martin, C.K.; Thompson, A.M.; Earnest, C.P.; Mikus, C.R.; Blair, S.N. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, coverweight postmenopausal women. PLoS ONE 2009, 4, e4515. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.E.; Markovitch, D.; Betts, J.A.; Thompson, D. Nonprescribed physical activity energy expenditure is maintained with structured exercise and implicates a compensatory increase in energy intake. Am. J. Clin. Nutr. 2010, 92, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.S.; Houmard, J.A.; Considine, R.V.; Tyndall, G.L.; Midgette, J.B.; Gavigan, K.E.; Weidner, M.L.; McCammon, M.R.; Israel, R.G.; Caro, J.F. Gender-dependent effects of exercise training on serum leptin levels in humans. Am. J. Physiol. 1997, 272, E562–E566. [Google Scholar] [CrossRef] [PubMed]
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; MacEra, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Gibbons, C.; Caudwell, P.; Finlayson, G.; Hopkins, M. Appetite control and energy balance: Impact of exercise. Obes. Rev. 2015, 16, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Ishibashi, A.; Ebi, K.; Goto, K. The effect of a 20 km run on appetite regulation in long distance runners. Nutrients 2016, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, G.; Bryant, E.; Blundell, J.E.; King, N.A. Acute compensatory eating following exercise is associated with implicit hedonic wanting for food. Physiol. Behav. 2009, 97, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.; Blundell, J.E.; King, N.A. Individual variability in compensatory eating following acute exercise in overweight and obese women. Br. J. Sports Med. 2013, 48, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Mercier, J. Balance of carbohydrate and lipid utilization during exercise: The “crossover” concept. J. Appl. Physiol. 1994, 76, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, E.; Kristal, A.R.; Li, S.; Patterson, R.E. Demographic and Psychosocial Predictors of Fruit and Vegetable Intakes Differ. J. Am. Diet. Assoc. 1998, 98, 1412–1417. [Google Scholar] [CrossRef]
- Ayala, G.X.; Ornelas, I.; Rhodes, S.D.; Amell, J.W.; Dodds, J.M.; Mebane, E.; Horton, E.; Montano, J.; Armstrong-Brown, J.; Eng, E. Correlates of dietary intake among men involved in the MAN for health study. Am. J. Men Health 2009, 3, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.; Owen, N.; Crawford, D.; Bauman, A.; Sallis, J.F. Physical activity and sedentary behavior: A population-based study of barriers, enjoyment, and preference. Health Psychol. 2003, 22, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; King, N.A. Effects of exercise on appetite control: Loose coupling between energy expenditure and energy intake. Int. J. Obes. 1998, 22, 22–29. [Google Scholar]
- Charlot, K.; Chapelot, D. Energy compensation after an aerobic exercise session in high-fat/low-fit and low-fat/high-fit young male subjects. Br. J. Nutr. 2013, 110, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Paxman, J.; Dalton, C.; Winter, E.; Broom, D. Effects of an acute bout of aerobic exercise on immediate and subsequent three-day food intake and energy expenditure in active and inactive pre-menopausal women taking oral contraceptives. Appetite 2015, 89, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Paxman, J.R.; Dalton, C.F.; Hopkins, M.; Broom, D.R. An acute bout of cycling does not induce compensatory responses in pre- menopausal women not using hormonal contraceptives. Appetite 2018, 128, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Paxman, J.; Dalton, C.; Winter, E.; Broom, D. Effects of an acute bout of aerobic exercise on immediate and subsequent three-day food intake and energy expenditure in active and inactive men. Appetite 2013, 71, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, N.V.; Schoeller, D.; Brown, A.W.; Heymsfield, S.B.; Thomas, D.; Sørensen, T.I.A.; Speakman, J.R.; Jeansonne, M.; Allison, D.B. Energy Balance Measurement Working Group Energy balance measurement: When something is not better than nothing. Int. J. Obes. 2015, 39, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, N.T.; Moller, B.K.; Raben, A.; Kristensen, S.T.; Holm, L.; Flint, A.; Astrup, A. Determinants of appetite ratings: The role of age, gender, BMI, physical activity, smoking habits, and diet/weight concern. Food Nutr. Res. 2011, 55. [Google Scholar] [CrossRef] [PubMed]
- Van Walleghen, E.L.; Orr, J.S.; Gentile, C.L.; Davy, K.P.; Davy, B.M. Habitual physical activity differentially affects acute and short-term energy intake regulation in young and older adults. Int. J. Obes. (Lond.) 2007, 31, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Long, S.J.; Hart, K.; Morgan, L.M. The ability of habitual exercise to influence appetite and food intake in response to high- and low-energy preloads in man. Br. J. Nutr. 2002, 87, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, K.; Hopkins, M.; Long, C.; Blundell, J.; Finlayson, G. High habitual physical activity improves acute energy compensation in nonobese adults. Med. Sci. Sports Exerc. 2017, 49, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.T.; Taudorf, L.; Hartmann, B.; Helge, J.W.; Holst, J.J.; Dela, F. Meal induced gut hormone secretion is altered in aerobically trained compared to sedentary young healthy males. Eur. J. Appl. Physiol. 2013, 113, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.; Proietto, J.; Hargreves, M. Effect of detraining on GLUT-4 protein in human skeletal muscle. J. Appl. Physiol. 1994, 77, 1532–1536. [Google Scholar] [CrossRef] [PubMed]
- Kraniou, G.N.; Cameron-Smith, D.; Hargreaves, M. Effect of short-term training on GLUT-4 mRNA and protein expression in human skeletal muscle. Exp. Physiol. 2004, 89, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Caudwell, P.; Gibbons, C.; Hopkins, M.; Näslund, E.; King, N.A.; Finlayson, G. Body composition and appetite: Fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. Br. J. Nutr. 2012, 107, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Van Der Klaauw, A.A.; Farooqi, I.S. The hunger genes: Pathways to obesity. Cell 2015, 161, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.B.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Karra, E.; Daly, O.G.O.; Choudhury, A.I.; Yousseif, A.; Millership, S.; Neary, M.T.; Scott, W.R.; Chandarana, K.; Manning, S.; Hess, M.E.; et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J. Clin. Investig. 2013, 123, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cecil, J.E.; Tavendale, R.; Watt, P.; Hetherington, M.M.; Palmer, C.N.A. An obesity-associated FTO gene variant and increased energy intake in children. N. Engl. J. Med. 2008, 359, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Shimokata, H.; Tobin, J.D.; Muller, D.C.; Elahi, D.; Coon, P.J.; Andres, R. Studies in the distribution of body fat: Effects of age, sex, and obesity. J. Gerontol. 1989, 44, M66–M73. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh, C.G.; Andrews, J.M.; Jones, K.L.; Wishart, J.M.; Morris, H.A.; Jansen, J.B.M.J.; Morley, J.E.; Horowitz, M.; Chapman, I.M. Effects of age on concentrations of plasma cholecystokinin, glucagon-like peptide 1, and peptide YY and their relation appetite and pyloric motility. Am. J. Clin. Nutr. 1999, 69, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Atalayer, D.; Astbury, N.M. Anorexia of aging and gut hormones. Aging Dis. 2013, 4, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, D.A. Limitations in the assessment of dietary energy intake by self-report. Metabolism 1995, 44, 18–22. [Google Scholar] [CrossRef]
- Hosoda, H.; Doi, K.; Nagaya, N.; Okumura, H.; Nakagawa, E.; Enomoto, M.; Ono, F.; Kangawa, K. Optimum collection and storage conditions for ghrelin measurements: Octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clin. Chem. 2004, 50, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorling, J.; Broom, D.R.; Burns, S.F.; Clayton, D.J.; Deighton, K.; James, L.J.; King, J.A.; Miyashita, M.; Thackray, A.E.; Batterham, R.L.; et al. Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity. Nutrients 2018, 10, 1140. https://doi.org/10.3390/nu10091140
Dorling J, Broom DR, Burns SF, Clayton DJ, Deighton K, James LJ, King JA, Miyashita M, Thackray AE, Batterham RL, et al. Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity. Nutrients. 2018; 10(9):1140. https://doi.org/10.3390/nu10091140
Chicago/Turabian StyleDorling, James, David R. Broom, Stephen F. Burns, David J. Clayton, Kevin Deighton, Lewis J. James, James A. King, Masashi Miyashita, Alice E. Thackray, Rachel L. Batterham, and et al. 2018. "Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity" Nutrients 10, no. 9: 1140. https://doi.org/10.3390/nu10091140
APA StyleDorling, J., Broom, D. R., Burns, S. F., Clayton, D. J., Deighton, K., James, L. J., King, J. A., Miyashita, M., Thackray, A. E., Batterham, R. L., & Stensel, D. J. (2018). Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity. Nutrients, 10(9), 1140. https://doi.org/10.3390/nu10091140






