The Effect of Digestion and Digestibility on Allergenicity of Food
Abstract
1. Introduction
2. Digestion of Carbohydrates: Amylase Action Critical for Starch Digestion and Microbiome
- (1)
- It may result in epitope modification of plant food allergens or reveal neo-epitopes;
- (2)
- Starch and other food matrix compounds may form stable complexes during food processing, supporting the transit of intact allergens;
- (3)
- Amylase action affects the composition of fermentation products with significant effect on microbiota composition.
3. Digestion and Digestibility of Proteins Associated with Lipids or Carbohydrates
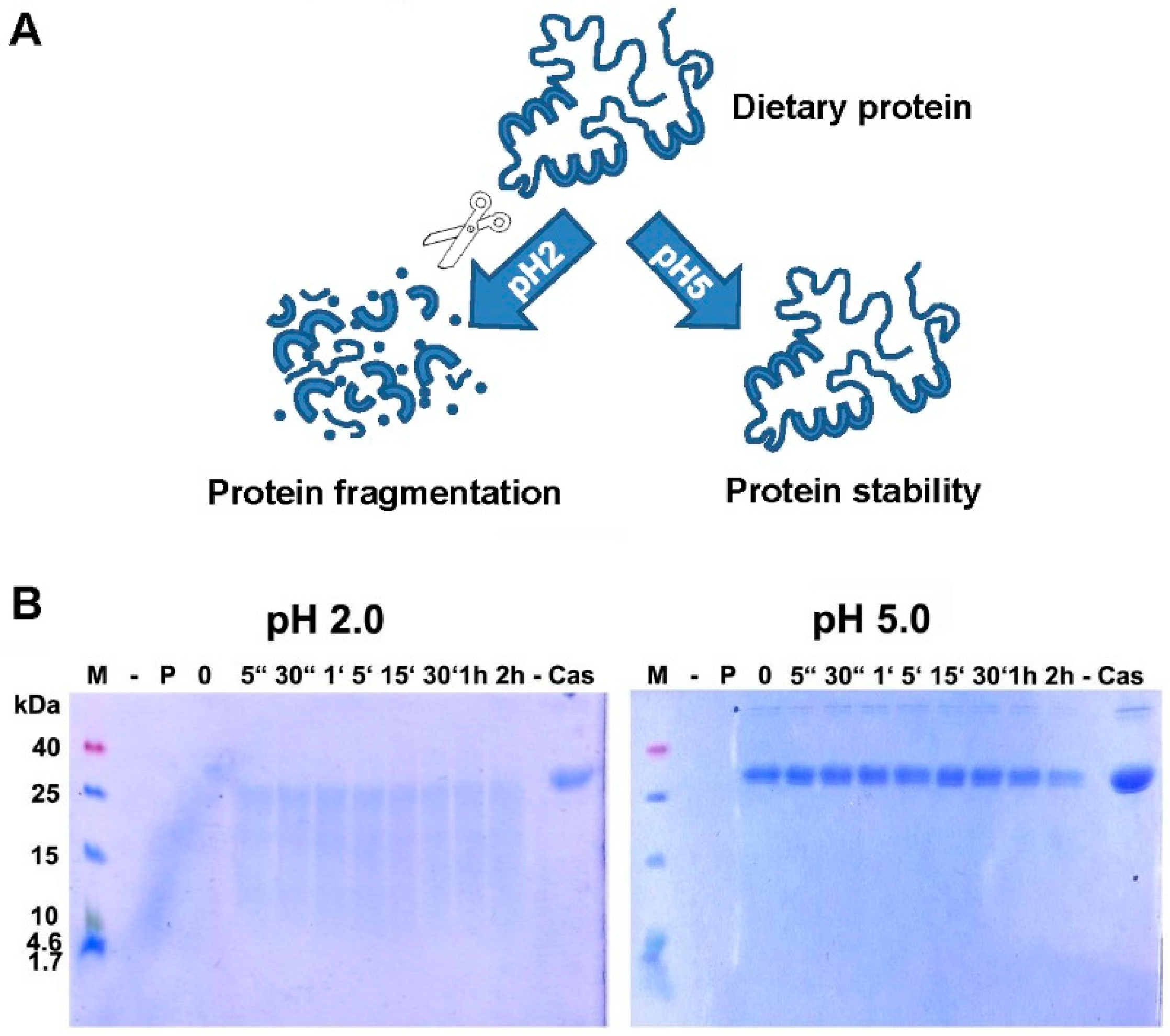
4. Digestion of Proteins: Gastric Acid is Critical for Adequate Protein Digestion and Prevention of Food Allergy
5. Summary
| What Is Well Established? | What Should be Further Investigated? |
| Amylase action influences the resulting remnants of ingested starch and thereby the microbiome | Whether different amylase action and concentration, e.g., in stress situations, also leads to different outcome regarding allergenicity of the food |
| Food processing changes protein structure and digestibility | Whether food processing might impact on gastrointestinal pH levels |
| Heating can lead to glycation and Maillard products, and thereby influences digestibility of involved proteins | Whether proteins become more able to sensitize, or to elicit reactions in allergic patients |
| Anti-ulcer medication and antacids elevate gastric pH levels and consequently influence food protein digestion | Whether gastric acid suppression influences also intestinal pH levels and small intestinal protein digestion |
| Loading of lipid transfer proteins (LTP) with ligands changes their digestibility | Whether loading of LTP changes their immunogenicity and allergenicity in vivo |
| Blocking of gastric digestion increases the risk for allergic sensitization | Whether the subsequent intestinal digestion is also influenced by the changed gastric pH. Whether a functional intestinal digestion could equalize the detrimental sensitizing effect of a blocked gastric digestion |
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGE | advanced glycated end products |
| ATI | amylase trypsin inhibitor |
| DBPCFC | double-blind placebo-controlled food challenge |
| GIT | gastrointestinal tract |
| MR | Maillard reaction |
| MRP | Maillard reaction products |
| NCGS | non-celiac gluten-sensitivity |
| OVA | ovalbumin |
| PC | phosphatidylcholine |
| PPI | proton pump inhibitor |
| RDS | rapidly-digestible starch |
| RS | resistant starch |
| SDS | slowly-digestible starch |
References
- U.S. Department of Health and Human Services. National Health Interview Survey. Available online: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_C-2.pdf (accessed on 27 June 2018).
- Saulyte, J.; Regueira, C.; Montes-Martinez, A.; Khudyakov, P.; Takkouche, B. Active or passive exposure to tobacco smoking and allergic rhinitis, allergic dermatitis, and food allergy in adults and children: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001611. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Diesner, S.C.; Oostingh, G.J.; Selzle, K.; Pfaller, T.; Schultz, C.; Zhang, Y.; Krishnamurthy, D.; Starkl, P.; Knittelfelder, R.; et al. Nitration of the egg-allergen ovalbumin enhances protein allergenicity but reduces the risk for oral sensitization in a murine model of food allergy. PLoS ONE 2010, 5, e14210. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Koplin, J.J.; Ponsonby, A.L.; Gurrin, L.C.; Wake, M.; Vuillermin, P.; Martin, P.; Matheson, M.; Lowe, A.; Robinson, M.; et al. Vitamin d insufficiency is associated with challenge-proven food allergy in infants. J. Allergy Clin. Immunol. 2013, 131, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Sampson, H.A.; Plaut, M.; Burks, A.W.; Akdis, C.A.; Lack, G. Food allergy: Update on prevention and tolerance. J. Allergy Clin. Immunol. 2018, 141, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Michel, S.; Busato, F.; Genuneit, J.; Pekkanen, J.; Dalphin, J.C.; Riedler, J.; Mazaleyrat, N.; Weber, J.; Karvonen, A.M.; Hirvonen, M.R.; et al. Farm exposure and time trends in early childhood may influence DNA methylation in genes related to asthma and allergy. Allergy 2013, 68, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Mitre, E.; Susi, A.; Kropp, L.E.; Schwartz, D.J.; Gorman, G.H.; Nylund, C.M. Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatr. 2018, 172, e180315. [Google Scholar] [CrossRef] [PubMed]
- Jeurink, P.V.; Knipping, K.; Wiens, F.; Baranska, K.; Stahl, B.; Garssen, J.; Krolak-Olejnik, B. Importance of maternal diet in the training of the infant’s immune system during gestation and lactation. Crit. Rev. Food Sci. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- West, C. Introduction of complementary foods to infants. Ann. Nutr. Metab. 2017, 70 (Suppl. S2), 47–54. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellof, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary feeding: A position paper by the european society for paediatric gastroenterology, hepatology, and nutrition (espghan) committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Katz, Y.; Sasieni, P.; Mesher, D.; Maleki, S.J.; Fisher, H.R.; Fox, A.T.; Turcanu, V.; Amir, T.; Zadik-Mnuhin, G.; et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J. Allergy Clin. Immunol. 2008, 122, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Fisher, H.; Toit, G.D.; Bahnson, H.T.; Lack, G. The challenges of preventing food allergy: Lessons learned from leap and eat. Ann. Allergy Asthma Immunol. 2018, in press. [Google Scholar]
- du Toit, G.; Sayre, P.H.; Roberts, G.; Lawson, K.; Sever, M.L.; Bahnson, H.T.; Fisher, H.R.; Feeney, M.; Radulovic, S.; Basting, M.; et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the learning early about peanut allergy study cohort. J. Allergy Clin. Immunol. 2018, 141, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.H. Structure and digestion of common complementary food starches. J. Pediatr. Gastroenterol. Nutr. 2018, 66, S35–S38. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Honnda, Y. Amylases. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 159–173. [Google Scholar]
- Shulman, R.J. Starch malabsorption in infants. J. Pediatr. Gastroenterol. Nutr. 2018, 66 (Suppl. S3), S65–S67. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.C.; Barroso, I.G.; Ferreira, J.M.J.; Dias, R.O.; Ferreira, C.; Terra, W.R. Molecular machinery of starch digestion and glucose absorption along the midgut of musca domestica. J. Insect Physiol. 2018, 109, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Pierzynowska, K.; Valverde-Piedra, J.; Szymanczyk, S.; Prykhod’ko, O.; Pieszka, M.; Kardas, M.; Grochowska-Niedworok, E.; Grabowski, T.; Winiarczyk, M.; Pierzynowski, S. Pancreatic-like enzymes of microbial origin restore growth and normalize lipid absorption in a pig model with exocrine pancreatic insufficiency. Arch. Med. Sci. 2018, 14, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Meijster, T.; Tielemans, E.; Heederik, D. Effect of an intervention aimed at reducing the risk of allergic respiratory disease in bakers: Change in flour dust and fungal alpha-amylase levels. Occup. Environ. Med. 2009, 66, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Peyrot des Gachons, C.; Breslin, P.A. Salivary amylase: Digestion and metabolic syndrome. Curr. Diabetes Rep. 2016, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.; Le Feunteun, S.; Panouille, M.; Souchon, I. The important role of salivary alpha-amylase in the gastric digestion of wheat bread starch. Food Funct. 2018, 9, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Skoluda, N.; Strahler, J. Biomarkers of stress in behavioural medicine. Curr. Opin. Psychiatry 2013, 26, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Bobel, T.S.; Hackl, S.B.; Langgartner, D.; Jarczok, M.N.; Rohleder, N.; Rook, G.A.; Lowry, C.A.; Gundel, H.; Waller, C.; Reber, S.O. Less immune activation following social stress in rural vs. Urban participants raised with regular or no animal contact, respectively. Proc. Natl. Acad. Sci. USA 2018, 115, 5259–5264. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Warren, F.J.; Butterworth, P.J.; Ellis, P.R.; Gidley, M.J. Mechanisms of starch digestion by alpha-amylase-structural basis for kinetic properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Lin, A.H.; Hamaker, B.R.; Gidley, M.J.; Muniandy, A. Mammalian mucosal alpha-glucosidases coordinate with alpha-amylase in the initial starch hydrolysis stage to have a role in starch digestion beyond glucogenesis. PLoS ONE 2013, 8, e62546. [Google Scholar] [CrossRef] [PubMed]
- Lapis, T.J.; Penner, M.H.; Balto, A.S.; Lim, J. Oral digestion and perception of starch: Effects of cooking, tasting time, and salivary alpha-amylase activity. Chem. Senses 2017, 42, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Hirota, S. Interactions of flavonoids with alpha-amylase and starch slowing down its digestion. Food Funct. 2018, 9, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.M.; Dhital, S.; Li, C.; Zhang, B.; Huang, Q. Effects of palm oil on structural and in vitro digestion properties of cooked rice starches. Int. J. Biol. Macromol. 2018, 107, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.; Cordiner, S.B.; McGhie, T.K. Kiwifruit actinidin digests salivary amylase but not gastric lipase. Food Funct. 2017, 8, 3339–3345. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.; Pan, X.; Bellido, V.; Toole, G.A.; Gates, F.K.; Wickham, M.S.; Shewry, P.R.; Bakalis, S.; Padfield, P.; Mills, E.N. Digestibility of gluten proteins is reduced by baking and enhanced by starch digestion. Mol. Nutr. Food Res. 2015, 59, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Pickert, G.; Ashfaq-Khan, M.; Zevallos, V. Non-celiac wheat sensitivity: Differential diagnosis, triggers and implications. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Bellinghausen, I.; Weigmann, B.; Zevallos, V.; Maxeiner, J.; Reissig, S.; Waisman, A.; Schuppan, D.; Saloga, J. Wheat amylase-trypsin inhibitors exacerbate intestinal and airway allergic immune responses in humanized mice. J. Allergy Clin. Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zevallos, V.F.; Raker, V.K.; Maxeiner, J.; Scholtes, P.; Steinbrink, K.; Schuppan, D. Dietary wheat amylase trypsin inhibitors exacerbate murine allergic airway inflammation. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. Wheat-based foods and non celiac gluten/wheat sensitivity: Is drastic processing the main key issue? Med. Hypotheses 2015, 85, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, E.; Young, W.; Reichert-Grimm, V.; Weis, S.; Riedel, C.U.; Rosendale, D.; Stoklosinski, H.; Hunt, M.; Egert, M. In vivo assessment of resistant starch degradation by the caecal microbiota of mice using rna-based stable isotope probing—A proof-of-principle study. Nutrients 2018, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Bello-Perez, L.A.; Flores-Silva, P.C.; Agama-Acevedo, E.; Tovar, J. Starch digestibility: Past, present, and future. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef] [PubMed]
- Warren, F.J.; Fukuma, N.M.; Mikkelsen, D.; Flanagan, B.M.; Williams, B.A.; Lisle, A.T.; Ó’Cuív, P.; Morrison, M.; Gidley, M.J. Food starch structure impacts gut microbiome composition. mSphere 2018, 3, e00086-18. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.A.; Petri, R.M.; Grull, D.; Zebeli, Q.; Metzler-Zebeli, B.U. Transglycosylated starch modulates the gut microbiome and expression of genes related to lipid synthesis in liver and adipose tissue of pigs. Front. Microbiol. 2018, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.V.; Lucio, M.; Lee, L.H.; VerBerkmoes, N.C.; Brislawn, C.J.; Bernhardt, J.; Lamendella, R.; McDermott, J.E.; Bergeron, N.; Heinzmann, S.S.; et al. Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. mBio 2017, 8, e01343-17. [Google Scholar] [CrossRef] [PubMed]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.Y.; Cao, S.; Theriault, B.R.; et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.E.; Bunyavanich, S. Role of the microbiome in food allergy. Curr. Allergy Asthma Rep. 2018, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities-2017 practall document of the American academy of allergy, asthma & immunology and the European academy of allergy and clinical immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar] [PubMed]
- Bogh, K.L.; Barkholt, V.; Madsen, C.B. The sensitising capacity of intact beta-lactoglobulin is reduced by co-administration with digested beta-lactoglobulin. Int. Arch. Allergy Immunol. 2013, 161, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Berecz, B.; Clare Mills, E.N.; Paradi, I.; Lang, F.; Tamas, L.; Shewry, P.R.; Mackie, A.R. Stability of sunflower 2s albumins and ltp to physiologically relevant in vitro gastrointestinal digestion. Food Chem. 2013, 138, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Bartra, J.; Garcia-Moral, A.; Enrique, E. Geographical differences in food allergy. Bundesgesundheitsblatt Gesundheitsforsch. Gesundheitssch. 2016, 59, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.J.; Mackie, A.R.; Mills, E.N. Phospholipid interactions protect the milk allergen alpha-lactalbumin from proteolysis during in vitro digestion. J. Agric. Food Chem. 2005, 53, 9810–9816. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Adel-Patient, K.; Barkholt, V.; Baro, C.; Bennett, L.; Bublin, M.; Gaier, S.; Graser, G.; Ladics, G.S.; Mierzejewska, D.; et al. In vitro digestibility of beta-casein and beta-lactoglobulin under simulated human gastric and duodenal conditions: A multi-laboratory evaluation. Regul. Toxicol. Pharmacol. 2009, 55, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.U.; Alexeev, Y.; Johnson, P.E.; Rigby, N.M.; Mackie, A.R.; Dhaliwal, B.; Mills, E.N. Ligand binding to an allergenic lipid transfer protein enhances conformational flexibility resulting in an increase in susceptibility to gastroduodenal proteolysis. Sci. Rep. 2016, 6, 30279. [Google Scholar] [CrossRef] [PubMed]
- Pastorello, E.A.; Farioli, L.; Conti, A.; Pravettoni, V.; Bonomi, S.; Iametti, S.; Fortunato, D.; Scibilia, J.; Bindslev-Jensen, C.; Ballmer-Weber, B.; et al. Wheat ige-mediated food allergy in european patients: Alpha-amylase inhibitors, lipid transfer proteins and low-molecular-weight glutenins. Allergenic molecules recognized by double-blind, placebo-controlled food challenge. Int. Arch. Allergy Immunol. 2007, 144, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Palacin, A.; Quirce, S.; Armentia, A.; Fernandez-Nieto, M.; Pacios, L.F.; Asensio, T.; Sastre, J.; Diaz-Perales, A.; Salcedo, G. Wheat lipid transfer protein is a major allergen associated with baker’s asthma. J. Allergy Clin. Immunol. 2007, 120, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, L.; Picariello, G.; Mongiello, M.; Nocerino, R.; Berni Canani, R.; Bavaro, S.; Monaci, L.; Ferranti, P.; Mamone, G. Peanut digestome: Identification of digestion resistant ige binding peptides. Food Chem. Toxicol. 2017, 107, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.C.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Roth-Walter, F.; Berin, M.C.; Arnaboldi, P.; Escalante, C.R.; Dahan, S.; Rauch, J.; Jensen-Jarolim, E.; Mayer, L. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through peyer’s patches. Allergy 2008, 63, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Luz Sanz, M.; Corzo-Martinez, M.; Rastall, R.A.; Olano, A.; Moreno, F.J. Characterization and in vitro digestibility of bovine beta-lactoglobulin glycated with galactooligosaccharides. J. Agric. Food Chem. 2007, 55, 7916–7925. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; L’Hocine, L.; Karboune, S. Allergenicity of potato proteins and of their conjugates with galactose, galactooligosaccharides, and galactan in native, heated, and digested forms. J. Agric. Food Chem. 2014, 62, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Watanabe, K.; Ojima, T.; Ahn, D.H.; Saeki, H. Effect of maillard reaction on allergenicity of scallop tropomyosin. J. Agric. Food Chem. 2005, 53, 7559–7564. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Schmitt, D.A.; Galeano, M.; Hurlburt, B.K. Comparison of the digestibility of the major peanut allergens in thermally processed peanuts and in pure form. Foods 2014, 3, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Simonato, B.; Pasini, G.; Giannattasio, M.; Peruffo, A.D.; De Lazzari, F.; Curioni, A. Food allergy to wheat products: The effect of bread baking and in vitro digestion on wheat allergenic proteins. A study with bread dough, crumb, and crust. J. Agric. Food Chem. 2001, 49, 5668–5673. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Saiz, R.; Belloque, J.; Molina, E.; Lopez-Fandino, R. Human immunoglobulin e (ige) binding to heated and glycated ovalbumin and ovomucoid before and after in vitro digestion. J. Agric. Food Chem. 2011, 59, 10044–10051. [Google Scholar] [CrossRef] [PubMed]
- Downs, M.L.; Baumert, J.L.; Taylor, S.L.; Mills, E.N. Mass spectrometric analysis of allergens in roasted walnuts. J. Proteomics 2016, 142, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, F.K.; Alli, I.; Yaylayan, V.A.; Yasuo, K.; Chowdhury, S.F.; Purisima, E.O. Effect of limited solid-state glycation on the conformation of lysozyme by esi-msms peptide mapping and molecular modeling. Bioconjug. Chem. 2004, 15, 27–34. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, H.H.; Taylor, S.L.; Koppelman, S.J. Controlling the aggregation propensity and thereby digestibility of allergens by maillardation as illustrated for cod fish parvalbumin. J. Biosci. Bioeng. 2011, 111, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martinez, M.; Soria, A.C.; Belloque, J.; Villamiel, M.; Moreno, F.J. Effect of glycation on the gastrointestinal digestibility and immunoreactivity of bovine b-lactoglobulin. Int. Dairy J. 2012, 20, 742–752. [Google Scholar] [CrossRef]
- Scheijen, J.; Hanssen, N.M.J.; van Greevenbroek, M.M.; Van der Kallen, C.J.; Feskens, E.J.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: The codam study. Clin. Nutr. 2018, 37, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Bunzel, D.; Huch, M.; Franz, C.M.; Kulling, S.E.; Henle, T. Stability of individual maillard reaction products in the presence of the human colonic microbiota. J. Agric. Food Chem. 2015, 63, 6723–6730. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gupta, K.; Sharma, A.; Das, M.; Ansari, I.A.; Dwivedi, P.D. Maillard reaction in food allergy: Pros and cons. Crit. Rev. Food Sci. Nutr. 2018, 58, 208–226. [Google Scholar] [CrossRef] [PubMed]
- Buttari, B.; Profumo, E.; Capozzi, A.; Facchiano, F.; Saso, L.; Sorice, M.; Rigano, R. Advanced glycation end products of human beta(2) glycoprotein i modulate the maturation and function of dcs. Blood 2011, 117, 6152–6161. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.E.; Hillson, W.R.; Noti, M.; Gartlan, K.H.; Johnson, S.; Thomas, B.; Artis, D.; Sattentau, Q.J. Dry roasting enhances peanut-induced allergic sensitization across mucosal and cutaneous routes in mice. J. Allergy Clin. Immunol. 2014, 134, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.A.; Maleki, S.J.; Johnson, K.; Hurlburt, B.K.; Cheng, H.; Ruan, S.; Nesbit, J.B.; Pomes, A.; Edwards, L.L.; Schorzman, A.; et al. Identification of maillard reaction products on peanut allergens that influence binding to the receptor for advanced glycation end products. Allergy 2013, 68, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Maleki, S.J.; Cheng, H.; Novak, N. Differences in the uptake of ara h 3 from raw and roasted peanut by monocyte-derived dendritic cells. Int. Arch. Allergy Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hilmenyuk, T.; Bellinghausen, I.; Heydenreich, B.; Ilchmann, A.; Toda, M.; Grabbe, S.; Saloga, J. Effects of glycation of the model food allergen ovalbumin on antigen uptake and presentation by human dendritic cells. Immunology 2010, 129, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Ilchmann, A.; Burgdorf, S.; Scheurer, S.; Waibler, Z.; Nagai, R.; Wellner, A.; Yamamoto, Y.; Yamamoto, H.; Henle, T.; Kurts, C.; et al. Glycation of a food allergen by the maillard reaction enhances its t-cell immunogenicity: Role of macrophage scavenger receptor class a type I and II. J. Allergy Clin. Immunol. 2010, 125, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Heilmann, M.; Ilchmann, A.; Vieths, S. The maillard reaction and food allergies: Is there a link? Clin. Chem. Lab. Med. 2014, 52, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Seiquer, I.; Diaz-Alguacil, J.; Delgado-Andrade, C.; Lopez-Frias, M.; Munoz Hoyos, A.; Galdo, G.; Navarro, M.P. Diets rich in maillard reaction products affect protein digestibility in adolescent males aged 11–14 y. Am. J. Clin. Nutr. 2006, 83, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, M.; Wellner, A.; Gadermaier, G.; Ilchmann, A.; Briza, P.; Krause, M.; Nagai, R.; Burgdorf, S.; Scheurer, S.; Vieths, S.; et al. Ovalbumin modified with pyrraline, a maillard reaction product, shows enhanced t-cell immunogenicity. J. Biol. Chem. 2014, 289, 7919–7928. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Y.; Yang, H.; Rao, S.T.; Liu, G.Y.; Hu, M.J.; Zeng, B.C.; Cao, M.J.; Liu, G.M. The maillard reaction reduced the sensitization of tropomyosin and arginine kinase from scylla paramamosain, simultaneously. J. Agric. Food Chem. 2018, 66, 2934–2943. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kassai, M.; Hirose, T.; Katayama, S.; Nakamura, K.; Akiyama, H.; Teshima, R.; Nakamura, S. Modulation of immunoresponse in balb/c mice by oral administration of fag e 1-glucomannan conjugate. J. Agric. Food Chem. 2009, 57, 9787–9792. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Raghav, A.; Sharma, A.; Gupta, K.; Neelabh; Mandal, P.; Tripathi, A.; Ansari, I.A.; Das, M.; Dwivedi, P.D. Glycation of clinically relevant chickpea allergen attenuates its allergic immune response in balb/c mice. Food Chem. 2017, 235, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Conformational changes of beta-lactoglobulin induced by shear, heat, and ph-effects on antigenicity. J. Dairy Sci. 2015, 98, 4255–4265. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, S.; Lauer, I.; Foetisch, K.; San Miguel Moncin, M.; Retzek, M.; Hartz, C.; Enrique, E.; Lidholm, J.; Cistero-Bahima, A.; Vieths, S. Strong allergenicity of pru av 3, the lipid transfer protein from cherry, is related to high stability against thermal processing and digestion. J. Allergy Clin. Immunol. 2004, 114, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Malanin, K.; Lundberg, M.; Johansson, S.G. Anaphylactic reaction caused by neoallergens in heated pecan nut. Allergy 1995, 50, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Codina, R.; Oehling, A.G., Jr.; Lockey, R.F. Neoallergens in heated soybean hull. Int. Arch. Allergy Immunol. 1998, 117, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Gruber, P.; Vieths, S.; Wangorsch, A.; Nerkamp, J.; Hofmann, T. Maillard reaction and enzymatic browning affect the allergenicity of pru av 1, the major allergen from cherry (prunus avium). J. Agric. Food Chem. 2004, 52, 4002–4007. [Google Scholar] [CrossRef] [PubMed]
- Cucu, T.; De Meulenaer, B.; Bridts, C.; Devreese, B.; Ebo, D. Impact of thermal processing and the maillard reaction on the basophil activation of hazelnut allergic patients. Food Chem. Toxicol. 2012, 50, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gong, Y.; Gern, J.E.; Ikeda, S.; Lucey, J.A. Glycation of whey protein with dextrans of different molar mass: Effect on immunoglobulin e-binding capacity with blood sera obtained from patients with cow milk protein allergy. J. Dairy Sci. 2018, 101, 6823–6834. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Chung, S.Y.; Champagne, E.T.; Raufman, J.P. The effects of roasting on the allergenic properties of peanut proteins. J. Allergy Clin. Immunol. 2000, 106, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.S.; Ballmer-Weber, B.K.; Luttkopf, D.; Skov, P.S.; Wuthrich, B.; Bindslev-Jensen, C.; Vieths, S.; Poulsen, L.K. Roasted hazelnuts—Allergenic activity evaluated by double-blind, placebo-controlled food challenge. Allergy 2003, 58, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Teodorowicz, M.; van Neerven, J.; Savelkoul, H. Food processing: The influence of the maillard reaction on immunogenicity and allergenicity of food proteins. Nutrients 2017, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Hantusch, B.; Knittelfelder, R.; Wallmann, J.; Krieger, S.; Szalai, K.; Untersmayr, E.; Vogel, M.; Stadler, B.M.; Scheiner, O.; Boltz-Nitulescu, G.; et al. Internal images: Human anti-idiotypic fab antibodies mimic the ige epitopes of grass pollen allergen phl p 5a. Mol. Immunol. 2006, 43, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, C.; Ballmer-Weber, B.K.; Hansen, K.S.; Ispano, M.; Wuthrich, B.; Bindslev-Jensen, C.; Ansaloni, R.; Vannucci, L.; Pravettoni, V.; Scibilia, J.; et al. Hazelnut allergy: A double-blind, placebo-controlled food challenge multicenter study. J. Allergy Clin. Immunol. 2000, 105, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.; Gao, Y.; Storer, N. Acid-induced unfolding kinetics in simulated gastric digestion of proteins. Regul. Toxicol. Pharmacol. 2006, 46, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Schöll, I.; Untersmayr, E.; Bakos, N.; Roth-Walter, F.; Gleiss, A.; Boltz-Nitulescu, G.; Scheiner, O.; Jensen-Jarolim, E. Antiulcer drugs promote oral sensitization and hypersensitivity to hazelnut allergens in balb/c mice and humans. Am. J. Clin. Nutr. 2005, 81, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Vestergaard, H.; Malling, H.J.; Jensen, L.B.; Platzer, M.H.; Boltz-Nitulescu, G.; Scheiner, O.; Skov, P.S.; Jensen-Jarolim, E.; Poulsen, L.K. Incomplete digestion of codfish represents a risk factor for anaphylaxis in patients with allergy. J. Allergy Clin. Immunol. 2007, 119, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Bakos, N.; Scholl, I.; Kundi, M.; Roth-Walter, F.; Szalai, K.; Riemer, A.B.; Ankersmit, H.J.; Scheiner, O.; Boltz-Nitulescu, G.; et al. Anti-ulcer drugs promote ige formation toward dietary antigens in adult patients. FASEB J. 2005, 19, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.D.; Ciociola, A.A.; Robinson, M. Measurement of meal-stimulated gastric acid secretion by in vivo gastric autotitration. J. Appl. Physiol. 2002, 92, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Prichard, P.J.; Yeomans, N.D.; Mihaly, G.W.; Jones, D.B.; Buckle, P.J.; Smallwood, R.A.; Louis, W.J. Omeprazole: A study of its inhibition of gastric ph and oral pharmacokinetics after morning or evening dosage. Gastroenterology 1985, 88, 64–69. [Google Scholar] [CrossRef]
- Diesner, S.C.; Bergmayr, C.; Pfitzner, B.; Assmann, V.; Krishnamurthy, D.; Starkl, P.; Endesfelder, D.; Rothballer, M.; Welzl, G.; Rattei, T.; et al. A distinct microbiota composition is associated with protection from food allergy in an oral mouse immunization model. Clin. Immunol. 2016, 173, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Riemer, A.B.; Gruber, S.; Pali-Scholl, I.; Kinaciyan, T.; Untersmayr, E.; Jensen-Jarolim, E. Suppression of gastric acid increases the risk of developing immunoglobulin e-mediated drug hypersensitivity: Human diclofenac sensitization and a murine sensitization model. Clin. Exp. Allergy 2010, 40, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Brunner, R.; Wallmann, J.; Szalai, K.; Karagiannis, P.; Altmeppen, H.; Riemer, A.B.; Jensen-Jarolim, E.; Pali-Scholl, I. Aluminium per se and in the anti-acid drug sucralfate promotes sensitization via the oral route. Allergy 2009, 64, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Diesner, S.C.; Knittelfelder, R.; Krishnamurthy, D.; Pali-Scholl, I.; Gajdzik, L.; Jensen-Jarolim, E.; Untersmayr, E. Dose-dependent food allergy induction against ovalbumin under acid-suppression: A murine food allergy model. Immunol. Lett. 2008, 121, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Pali-Scholl, I.; Yildirim, A.O.; Ackermann, U.; Knauer, T.; Becker, C.; Garn, H.; Renz, H.; Jensen-Jarolim, E.; Fehrenbach, H. Anti-acids lead to immunological and morphological changes in the intestine of balb/c mice similar to human food allergy. Exp. Toxicol. Pathol. 2008, 60, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Brunner, R.; Wallmann, J.; Szalai, K.; Karagiannis, P.; Kopp, T.; Scheiner, O.; Jensen-Jarolim, E.; Pali-Scholl, I. The impact of aluminium in acid-suppressing drugs on the immune response of balb/c mice. Clin. Exp. Allergy 2007, 37, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Scholl, I.; Ackermann, U.; Ozdemir, C.; Blumer, N.; Dicke, T.; Sel, S.; Sel, S.; Wegmann, M.; Szalai, K.; Knittelfelder, R.; et al. Anti-ulcer treatment during pregnancy induces food allergy in mouse mothers and a th2-bias in their offspring. FASEB J. 2007, 21, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, E.; Cabanas, R.; Laserna, L.S.; Fiandor, A.; Tong, H.; Prior, N.; Calderon, O.; Medrano, N.; Bobolea, I.; Frias, J.; et al. Proton pump inhibitors are associated with hypersensitivity reactions to drugs in hospitalized patients: A nested case-control in a retrospective cohort study. Clin. Exp. Allergy 2013, 43, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Pali-Scholl, I.; Herzog, R.; Wallmann, J.; Szalai, K.; Brunner, R.; Lukschal, A.; Karagiannis, P.; Diesner, S.C.; Jensen-Jarolim, E. Antacids and dietary supplements with an influence on the gastric ph increase the risk for food sensitization. Clin. Exp. Allergy 2010, 40, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Scholl, I.; Swoboda, I.; Beil, W.J.; Forster-Waldl, E.; Walter, F.; Riemer, A.; Kraml, G.; Kinaciyan, T.; Spitzauer, S.; et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: A fish allergy model in balb/c mice. J. Allergy Clin. Immunol. 2003, 112, 616–623. [Google Scholar] [CrossRef]
- Untersmayr, E. Acid suppression therapy and allergic reactions. Allergo J. Int. 2015, 24, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Pali-Scholl, I.; Jensen-Jarolim, E. Anti-acid medication as a risk factor for food allergy. Allergy 2011, 66, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Burks, A.W.; Williams, L.W.; Thresher, W.; Connaughton, C.; Cockrell, G.; Helm, R.M. Allergenicity of peanut and soybean extracts altered by chemical or thermal denaturation in patients with atopic dermatitis and positive food challenges. J. Allergy Clin. Immunol. 1992, 90, 889–897. [Google Scholar] [CrossRef]
- Untersmayr, E.; Poulsen, L.K.; Platzer, M.H.; Pedersen, M.H.; Boltz-Nitulescu, G.; Skov, P.S.; Jensen-Jarolim, E. The effects of gastric digestion on codfish allergenicity. J. Allergy Clin. Immunol. 2005, 115, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Diesner, S.C.; Bramswig, K.H.; Knittelfelder, R.; Bakos, N.; Gundacker, C.; Lukschal, A.; Wallmann, J.; Szalai, K.; Pali-Scholl, I.; et al. Characterization of intrinsic and extrinsic risk factors for celery allergy in immunosenescence. Mech. Ageing Dev. 2008, 129, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Aalberse, R.C. Structural biology of allergens. J. Allergy Clin. Immunol. 2000, 106, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Bakos, N.; Scholl, I.; Szalai, K.; Kundi, M.; Untersmayr, E.; Jensen-Jarolim, E. Risk assessment in elderly for sensitization to food and respiratory allergens. Immunol. Lett. 2006, 107, 15–21. [Google Scholar] [CrossRef] [PubMed]
- DeMuth, K.; Stecenko, A.; Sullivan, K.; Fitzpatrick, A. Relationship between treatment with antacid medication and the prevalence of food allergy in children. Allergy Asthma Proc. 2013, 34, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Trikha, A.; Baillargeon, J.G.; Kuo, Y.F.; Tan, A.; Pierson, K.; Sharma, G.; Wilkinson, G.; Bonds, R.S. Development of food allergies in patients with gastroesophageal reflux disease treated with gastric acid suppressive medications. Pediatr. Allergy Immunol. 2013, 24, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Dehlink, E.; Yen, E.; Leichtner, A.M.; Hait, E.J.; Fiebiger, E. First evidence of a possible association between gastric acid suppression during pregnancy and childhood asthma: A population-based register study. Clin. Exp. Allergy 2009, 39, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Mulder, B.; Schuiling-Veninga, C.C.; Bos, H.J.; De Vries, T.W.; Jick, S.S.; Hak, E. Prenatal exposure to acid-suppressive drugs and the risk of allergic diseases in the offspring: A cohort study. Clin. Exp. Allergy 2014, 44, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Wu, M.; Liu, J.; Luo, M.; He, L.; Wang, X.; Wu, B.; Ying, S.; Chen, Z.; Li, W.; et al. Acid-suppressive drug use during pregnancy and the risk of childhood asthma: A meta-analysis. Pediatrics 2018, 141, e20170889. [Google Scholar] [CrossRef] [PubMed]
- Cea Soriano, L.; Hernandez-Diaz, S.; Johansson, S.; Nagy, P.; Garcia-Rodriguez, L.A. Exposure to acid-suppressing drugs during pregnancy and the risk of asthma in childhood: An observational cohort study. Aliment. Pharmacol. Ther. 2016, 43, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M.; Kroh, M.; Chand, B.; Mikami, D.; Reavis, K.; Khaitan, L. Sages review of endoscopic and minimally invasive bariatric interventions: A review of endoscopic and non-surgical bariatric interventions. Surg. Endosc. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Shakeri-Leidenmuhler, S.; Lukschal, A.; Schultz, C.; Bohdjalian, A.; Langer, F.; Birsan, T.; Diesner, S.C.; Greisenegger, E.K.; Scheiner, O.; Kopp, T.; et al. Surgical elimination of the gastric digestion by roux-en-y gastric bypass impacts on food sensitization—A pilot study. Obes. Surg. 2015, 25, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Pekar, J.; Ret, D.; Untersmayr, E. Stability of allergens. Mol. Immunol. 2018, 100, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Jensen-Jarolim, E. The role of protein digestibility and antacids on food allergy outcomes. J. Allergy Clin. Immunol. 2008, 121, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pali-Schöll, I.; Untersmayr, E.; Klems, M.; Jensen-Jarolim, E. The Effect of Digestion and Digestibility on Allergenicity of Food. Nutrients 2018, 10, 1129. https://doi.org/10.3390/nu10091129
Pali-Schöll I, Untersmayr E, Klems M, Jensen-Jarolim E. The Effect of Digestion and Digestibility on Allergenicity of Food. Nutrients. 2018; 10(9):1129. https://doi.org/10.3390/nu10091129
Chicago/Turabian StylePali-Schöll, Isabella, Eva Untersmayr, Martina Klems, and Erika Jensen-Jarolim. 2018. "The Effect of Digestion and Digestibility on Allergenicity of Food" Nutrients 10, no. 9: 1129. https://doi.org/10.3390/nu10091129
APA StylePali-Schöll, I., Untersmayr, E., Klems, M., & Jensen-Jarolim, E. (2018). The Effect of Digestion and Digestibility on Allergenicity of Food. Nutrients, 10(9), 1129. https://doi.org/10.3390/nu10091129




