Influence of Physical Activity on Bone Mineral Content and Density in Overweight and Obese Children with Low Adherence to the Mediterranean Dietary Pattern
Abstract
1. Introduction
2. Methods
2.1. Study Design
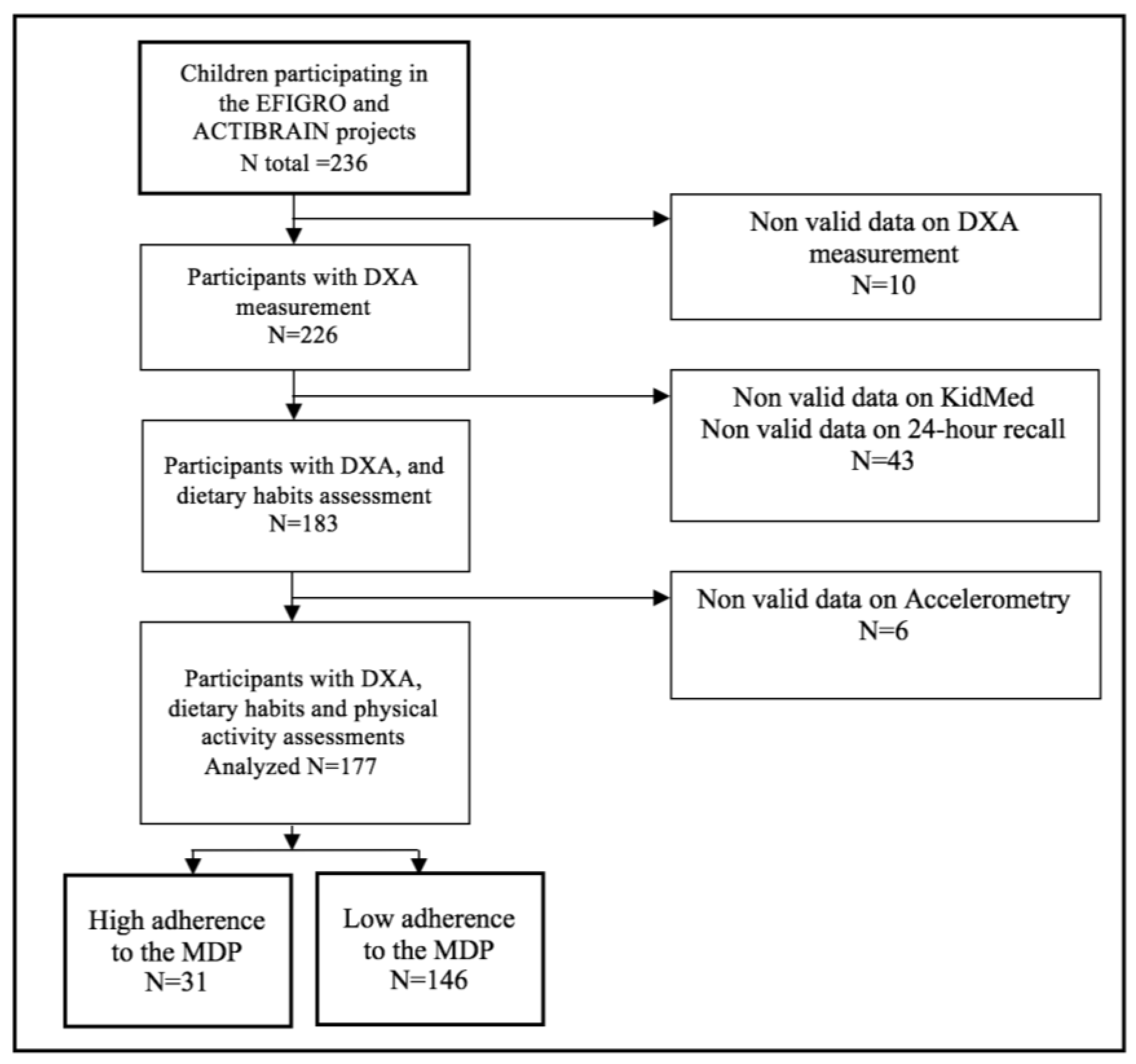
2.2. Participants
2.3. Anthropometry and Body Composition
2.4. Dietary Assessment
2.5. Physical Activity and Sedentary Behavior
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMD | Body mass density |
| BMC | Body mass content |
| LM | Lean mass |
| MDP | Mediterranean dietary pattern |
| DXA | Dual-Energy X-ray Absorptiometry |
| PA | Total physical activity |
| MPA | Moderate physical activity |
| MVPA | Moderate to vigorous physical activity |
| VP | Vigorous physical activity |
| ST | Sedentary time |
References
- Nutrition and Prevention of Chronic Diseases. Available online: http://apps.who.int/iris/bitstream/handle/10665/42665/WHO_TRS_916.pdf;jsessionid=D3684C83FB5242A01CDC01EB7A0E9745?sequence=1 (accessed on 30 June 2018).
- Lupsa, B.C.; Insogna, K. Bone health and osteoporosis. Endocrinol. Metab. Clin. 2015, 44, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Fernández García, M.; Martínez, J.; Olmos, J.; González Macías, J.; Hernández, J. Review of the incidences of hip fracture in Spain. Osteoporos. Metab. Miner. 2015, 7, 115–120. [Google Scholar]
- Kindler, J.M.; Lewis, R.D.; Hamrick, M.W. Skeletal muscle and pediatric bone development. Endocrinol. Diabetes Obes. 2015, 22, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Theintz, G.; Buchs, B.; Rizzoli, R.; Slosman, D.; Clavien, H.; Sizonenko, P.C.; Bonjour, J.P. Longitudinal monitoring of bone mass accumulation in healthy adolescents: Evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J. Clin. Endocrinol. Metab. 1992, 75, 1060–1065. [Google Scholar]
- Mitchell, J.A.; Chesi, A.; Elci, O.; Mccormack, S.E.; Kalkwarf, H.J.; Lappe, J.M.; Gilsanz, V.; Oberfield, S.E.; Shepherd, J.A.; Kelly, A.; et al. Genetics of Bone Mass in Childhood and Adolescence: Effects of Sex and Maturation Interactions. J. Bone Miner. Res. 2015, 30, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, D.; Vandenput, L.; Boonen, S. Reversing sex steroid deficiency and optimizing skeletal development in the adolescent with gonadal failure. Endocr. Dev. 2005, 8, 150–165. [Google Scholar] [PubMed]
- Mouratidou, T.; Vicente-Rodriguez, G.; Garcia-Marco, L.; Huybrechts, I.; Sioen, I.; Widhalm, K.; Valtuena, J.; Gonzalez-Gross, M.; Moreno, L.A.; Groups, H.S. Associations of dietary calcium, vitamin D., milk intakes, and 25-hydroxyvitamin D with bone mass in Spanish adolescents: The HELENA study. J. Clin. Densitom. 2013, 16, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Valtuena, J.; Garcia-Marco, L.; Vicente-Rodriguez, G.; Gonzalez-Gross, M.; Huybrechts, I.; Rey-Lopez, J.P.; Mouratidou, T.; Sioen, I.; Mesana, M.I.; Martinez, A.E.; et al. Vitamin D status and physical activity interact to improve bone mass in adolescents. The HELENA Study. Osteoporos. Int. 2012, 23, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Vlachpoulos, D.; Ubago-Guisado, E.; Barker, A.R.; Metcalf, B.S.; Fatouros, I.G.; Avloniti, A.; Knapp, K.M.; Moreno, L.A.; Williams, C.A.; Garcia-Marco, L. Determinants of Bone Outcomes in Adolescent Athletes at Baseline: The PRO-BONE Study. Med. Sci. Sports Exerc. 2017, 49, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, D.; Barker, A.R.; Ubago-Guisado, E.; Fatouros, I.G.; Knapp, K.M.; Williams, C.A.; Gracia-Marco, L. Longitudinal Adaptations of Bone Mass, Geometry, and Metabolism in Adolescent Male Athletes: The PRO-BONE Study. J. Bone Miner. Res. 2017, 32, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, D.; Barker, A.R.; Ubago-Guisado, E.; Ortega, F.B.; Krustrup, P.; Metcalf, B.; Castro Pinero, J.; Ruiz, J.R.; Knapp, K.M.; Williams, C.A.; et al. The effect of 12-month participation in osteogenic and non-osteogenic sports on bone development in adolescent male athletes. The PRO-BONE study. J. Sci. Med. Sport 2018, 21, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Ubago-Guisado, E.; Gómez-Cabello, A.; Sánchez-Sánchez, J.; García-Unanue, J.; Gallardo, L. Influence of different sports on bone mass in growing girls. J. Sports Sci. 2015, 33, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Bianchi, M.L.; Garabédian, M.; McKay, H.A.; Moreno, L.A. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone 2010, 46, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.I.; Petit, M.A.; Vigna, Y.M.; Prior, J.C. Eating attitudes and habitual calcium intake in peripubertal girls are associated with initial bone mineral content and its change over 2 years. J. Bone Miner. 2001, 16, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Specker, B.L.; Mulligan, L.; Ho, M. Longitudinal study of calcium intake, physical activity, and bone mineral content in infants 6–18 months of age. J. Bone Miner. 1999, 14, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Rauch, F.; Bailey, D.A.; Baxter-Jones, A.; Mirwald, R.; Faulkner, R. The ‘muscle-bone unit’ during the pubertal growth spurt. Bone 2004, 34, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Koedijk, J.B.; van Rijswijk, J.; Oranje, W.A.; van den Bergh, J.P.; Bours, S.P.; Savelberg, H.H.; Schaper, N.C. Sedentary behaviour and bone health in children, adolescents and young adults: A systematic review. Osteoporos. Int. 2017, 28, 2507–2519. [Google Scholar] [CrossRef] [PubMed]
- Teegarden, D.; Proulx, W.R.; Martin, B.R.; Zhao, J.; McCabe, G.P.; Lyle, R.M.; Peacock, M.; Slemenda, C.; Johnston, C.C.; Weaver, C.M. Peak bone mass in young women. J. Bone Miner. Res. 1995, 10, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Vogel, K.A.; Martin, B.R.; McCabe, L.D.; Peacock, M.; Warden, S.J.; McCabe, G.P.; Weaver, C.M. The effect of dairy intake on bone mass and body composition in early pubertal girls and boys: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 1214–1229. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.M.; Watson, P.; Gilsanz, V.; Hangartner, T.; Kalkwarf, H.J.; Oberfield, S.; Shepherd, J.; Winer, K.K.; Zemel, B. The longitudinal effects of physical activity and dietary calcium on bone mass accrual across stages of pubertal development. J. Bone Miner. Res. 2015, 30, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.H.; Evans, B.A.J.; Gregory, J.W. Bone mass acquisition in healthy children. Arch. Dis. Child. 2005, 90, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.C.; Cole, Z.A.; Crozier, S.R.; Kim, M.; Ntani, G.; Goodfellow, L.; Robinson, S.M.; Inskip, H.M.; Godfrey, K.M.; Dennison, E.M.; et al. Physical activity, calcium intake and childhood bone mineral: A population-based cross-sectional study. Osteoporos. Int. 2012, 23, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health: Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Prynne, C.J.; Mishra, G.D.; O’Connell, M.A.; Muniz, G.; Laskey, M.A.; Yan, L.; Prentice, A.; Ginty, F. Fruit and vegetable intakes and bone mineral status: A cross sectional study in 5 age and sex cohorts. Am. J. Clin. Nutr. 2006, 83, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Mistretta, A.; Marventano, S.; Antoci, M.; Cagnetti, A.; Giogianni, G.; Nolfo, F.; Rametta, S.; Pecora, G.; Marranzano, M. Mediterranean diet adherence and body composition among Southern Italian adolescents. Obes. Res. Clin. Pract. 2017, 11, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.V.; Bunn, D.K.; Hayhoe, R.P.; Appleyard, W.O.; Lenaghan, E.A.; Welch, A.A. Relationship between the Mediterranean dietary pattern and musculoskeletal health in children, adolescents, and adults: Systematic review and evidence map. Nutr. Rev. 2017, 75, 830–857. [Google Scholar] [CrossRef] [PubMed]
- Julian, C.; Huybrechts, I.; Gracia-Marco, L.; Gonzalez-Gil, E.M.; Gutierrez, A.; Gonzalez-Gross, M.; Marcos, A.; Widhalm, K.; Kafatos, A.; Vicente-Rodriguez, G.; et al. Mediterranean diet, diet quality, and bone mineral content in adolescents: The HELENA study. Osteoporos. Int. 2018, 29, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-D.; Dong, X.-W.; Zhu, Y.-Y.; Tian, H.-Y.; He, J.; Chen, Y.-M. Adherence to the Mediterranean diet is associated with a higher BMD in middle-aged and elderly Chinese. Sci. Rep. 2016, 6, 25662. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, M.D.; Melistas, L.; Yannakoulia, M.; Malagaris, I.; Panagiotakos, D.B.; Yiannakouris, N. Association between dietary patterns and indices of bone mass in a sample of Mediterranean women. Nutrition 2009, 25, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Puel, C.; Coxam, V.; Davicco, M.-J. Mediterranean diet and osteoporosis prevention. Med. Sci. 2007, 23, 756–760. [Google Scholar]
- Cadenas-Sanchez, C.; Mora-Gonzalez, J.; Migueles, J.H.; Martin-Matillas, M.; Gomez-Vida, J.; Escolano-Margarit, M.V.; Maldonado, J.; Enriquez, G.M.; Pastor-Villaescusa, B.; de Teresa, C.; et al. An exercise-based randomized controlled trial on brain, cognition, physical health and mental health in overweight/obese children (ActiveBrains project): Rationale, design and methods. Contemp. Clin. Trials 2016, 47, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Medrano, M.; Maiz, E.; Maldonado-Martín, S.; Arenaza, L.; Rodríguez-Vigil, B.; Ortega, F.B.; Ruiz, J.R.; Larrarte, E.; Diez-López, I.; Sarasúa-Miranda, A.; et al. The effect of a multidisciplinary intervention program on hepatic adiposity in overweight-obese children: Protocol of the EFIGRO study. Contemp. Clin. Trials 2015, 45, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; Garcia, A.; Perez-Rodrigo, C.; Aranceta, J. Food, youth and the Mediterranean diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in children and adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef] [PubMed]
- García Cabrera, S.; Herrera Fernández, N.; Rodríguez Hernández, C.; Nissensohn, M.; Román-Viñas, B.; Serra-Majem, L. Kidmed Test; Prevalence of Low Adherence To the Mediterranean Diet in Children and Young; a Systematic Review. Nutr. Hosp. 2015, 32, 2390–2399. [Google Scholar] [PubMed]
- Labayen, I.; Arenaza, L.; Medrano, M.; García, N.; Cadenas-Sanchez, C.; Ortega, F.B. Associations between the adherence to the Mediterranean diet and cardiorespiratory fitness with total and central obesity in preschool children: The PREFIT project. Eur. J. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M.; VAN Hees, V.T.; Hansen, B.H.; Ekelund, U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med. Sci. Sports Exerc. 2014, 46, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Specker, B.; Thiex, N.W.; Sudhagoni, R.G. Does Exercise Influence Pediatric Bone? A Systematic Review. Clin. Orthop. Relat. Res. 2015, 473, 3658–3672. [Google Scholar] [CrossRef] [PubMed]
- Gabel, L.; Macdonald, H.M.; Nettlefold, L.; McKay, H.A. Bouts of Vigorous Physical Activity and Bone Strength Accrual during Adolescence. Pediatr. Exerc. Sci. 2017, 29, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Gabel, L.; Macdonald, H.M.; Nettlefold, L.; McKay, H.A. Physical Activity, Sedentary Time, and Bone Strength from Childhood to Early Adulthood: A Mixed Longitudinal HR-pQCT study. J. Bone Miner. Res. 2017, 32, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Chaplais, E.; Naughton, G.; Greene, D.; Dutheil, F.; Pereira, B.; Thivel, D.; Courteix, D. Effects of interventions with a physical activity component on bone health in obese children and adolescents: A systematic review and meta-analysis. J. Bone Miner. Metab. 2018, 36, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Owen, N.; Bauman, A.; Brown, W. Too much sitting: A novel and important predictor of chronic disease risk? Br. J. Sports Med. 2009, 43, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Ivuskans, A.; Maestu, J.; Jurimae, T.; Latt, E.; Purge, P.; Saar, M.; Maasalu, K.; Jurimae, J. Sedentary time has a negative influence on bone mineral parameters in peripubertal boys: A 1-year prospective study. J. Bone Miner. Metab. 2015, 33, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Vaitkeviciute, D.; Latt, E.; Maestu, J.; Jurimae, T.; Saar, M.; Purge, P.; Maasalu, K.; Jurimae, J. Physical activity and bone mineral accrual in boys with different body mass parameters during puberty: A longitudinal study. PLoS ONE 2014, 9, e107759. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, M.; Molgaard, C.; Husby, S.; Schou, A.J.; Klakk, H.; Moller, N.C.; Holst, R.; Wedderkopp, N. The intensity of physical activity influences bone mineral accrual in childhood: The childhood health, activity and motor performance school (the CHAMPS) study, Denmark. BMC Pediatr. 2013, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Monjardino, T.; Lucas, R.; Ramos, E.; Barros, H. Associations between a priori-defined dietary patterns and longitudinal changes in bone mineral density in adolescents. Public Health Nutr. 2014, 17, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Manz, F. Potential renal acid load of foods and its influence on urine pH. J. Am. Diet Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef]
- Bushinsky, D.A. Acid-base imbalance and the skeleton. Eur. J. Nutr. 2001, 40, 238–244. [Google Scholar] [CrossRef] [PubMed]
- McGartland, C.P.; Robson, P.J.; Murray, L.J.; Cran, G.W.; Savage, M.J.; Watkins, D.C.; Rooney, M.M.; Boreham, C.A. Fruit and vegetable consumption and bone mineral density: The Northern Ireland Young Hearts Project. Am. J. Clin. Nutr. 2004, 80, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, K.; Heaney, R.P. Nutrient effects on the calcium economy: Emphasizing the potassium controversy. J. Nutr. 2008, 138, 166S–171S. [Google Scholar] [CrossRef] [PubMed]
- Wosje, K.S.; Khoury, P.R.; Claytor, R.P.; Copeland, K.A.; Hornung, R.W.; Daniels, S.R.; Kalkwarf, H.J. Dietary patterns associated with fat and bone mass in young children. Am. J. Clin. Nutr. 2010, 92, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Seiquer, I.; Mesias, M.; Hoyos, A.M.; Galdo, G.; Navarro, M.P. A Mediterranean dietary style improves calcium utilization in healthy male adolescents. J. Am. Coll. Nutr. 2008, 27, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, J. Prevention of overweight and obesity in early life. Proc Nutr. Soc. 2018, 77, 247–256. [Google Scholar] [CrossRef] [PubMed]
| High Adherence to the MDP (N = 31) | Low Adherence to the MDP (N = 146) | p1 | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Girls (n, %) | 19, 61.3 | 61, 41.8 | 0.047 | ||
| Age (years) | 10.2 | 1.2 | 10.4 | 1.2 | 0.495 |
| Puberty stage (n) 2 I and II III, IV and V | 21, 10 | 105, 37 | 0.697 | ||
| Height (cm) | 146.4 | 8.8 | 145.2 | 8.0 | 0.444 |
| Weight(Kg) | 56.6 | 8.7 | 55.0 | 10.8 | 0.385 |
| Body mass index (Kg/m2) | 26.4 | 3.2 | 25.9 | 3.4 | 0.458 |
| Body mass index z-score | 2.8 | 0.9 | 2.7 | 0.8 | 0.591 |
| Body fat percent | 41.6 | 5.8 | 41.1 | 4.7 | 0.657 |
| Lean mass (Kg) | 31.0 | 4.7 | 30.5 | 5.1 | 0.598 |
| Areal Bone Mineral Density (g/cm2) | |||||
| TBLH | 0.80 | 0.05 | 0.80 | 0.06 | 0.801 |
| Upper limbs | 0.62 | 0.05 | 0.62 | 0.05 | 0.859 |
| Lower limbs | 0.95 | 0.07 | 0.95 | 0.09 | 0.993 |
| Bone Mineral Content (g) | |||||
| TBLH | 1025.4 | 179.8 | 999.2 | 203.3 | 0.476 |
| Upper limbs | 87.0 | 17.5 | 85.2 | 18.3 | 0.607 |
| Lower limbs | 259.0 | 50.2 | 255.7 | 56.2 | 0.745 |
| Dietary characteristics | |||||
| Energy intake (kcal/day) | 1806 | 384 | 1769 | 378 | 0.630 |
| MDP index | 8.6 | 0.7 | 5.1 | 1.3 | <0.001 |
| Calcium intake (mg/day) | 660 | 217 | 661 | 226 | 0.968 |
| D vitamin intake (µg/day) | 1.92 | 2.23 | 1.87 | 1.97 | 0.902 |
| Total Body Less Head | Upper Limbs | Lower Limbs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | |
| Areal Bone Mineral Density (g/cm2) | |||||||||
| High adherence to the Mediterranean dietary pattern (N = 31) | |||||||||
| MPA (min/day) | 0.115 | −0.001; 0.002 | 0.52 | 0.087 | −0.001; 0.001 | 0.651 | −0.001 | −0.001; 0.001 | 0.997 |
| MVPA (min/day) | 0.084 | −0.001; 0.001 | 0.630 | 0.051 | −0.001; 0.001 | 0.784 | −0.012 | −0.001; 0.001 | 0.93 |
| VPA (min/day) | −0.018 | −0.004; 0.003 | 0.911 | −0.057 | −0.004; 0.003 | 0.739 | −0.040 | −0.005; 0.003 | 0.768 |
| ST (min/day) | −0.015 | 0.000; 0.000 | 0.942 | 0.072 | 0.000; 0.000 | 0.735 | −0.001 | 0.000; 0.000 | 0.996 |
| Total PA (mg/5 sec) | 0.030 | −0.001; 0.001 | 0.871 | −0.049 | −0.001; 0.001 | 0.802 | −0.016 | −0.002; 0.001 | 0.918 |
| Low adherence to the Mediterranean dietary pattern (N = 146) | |||||||||
| MPA (min/day) | 0.169 | 0.004; 0.001 | 0.004 | 0.203 | 0.000; 0.001 | 0.007 | 0.123 | 0.000; 0.001 | 0.027 |
| MVPA (min/day) | 0.185 | 0.000; 0.001 | 0.002 | 0.204 | 0.000; 0.001 | 0.007 | 0.142 | 0.000; 0.001 | 0.011 |
| VPA (min/day) | 0.183 | 0.001; 0.004 | 0.002 | 0.142 | 0.00; 0.003 | 0.062 | 0.167 | 0.001; 0.005 | 0.002 |
| ST (min/day) | −0.150 | 0.000; 0.000 | 0.012 | −0.140 | 0.000; 0.000 | 0.072 | −0.109 | 0.000; 0.000 | 0.056 |
| Total PA (mg/5 sec) | 0.210 | 0.000; 0.001 | <0.001 | 0.206 | 0.000; 0.001 | 0.006 | 0.172 | 0.000; 0.002 | 0.002 |
| Bone Mineral Content (g) | |||||||||
| High adherence to the Mediterranean dietary pattern (N = 31) | |||||||||
| MPA(min/day) | 0.175 | −0.512; 4.112 | 0.121 | 0.063 | −0.215; 0.341 | 0.642 | 0.205 | −0.127; 1.306 | 0.102 |
| MVPA (min/day) | 0.146 | −0.642; 3.088 | 0.188 | 0.026 | −0.200; 0.243 | 0.844 | 0.176 | −0.158; 0.995 | 0.146 |
| VPA (min/day) | 0.037 | −6.265; 8.928 | 0.720 | −0.82 | −1.150; 0.576 | 0.498 | 0.071 | −1.637; 3.070 | 0.535 |
| ST (min/day) | −0.062 | −0.805; 0.498 | 0.630 | 0.006 | −0.074; 0.076 | 0.969 | −0.137 | −0.295: 0.105 | 0.335 |
| Total PA (mg/5 sec) | 0.118 | −1.379; 4.118 | 0.313 | 0.018 | −0.301; 0.342 | 0.896 | 0.162 | −0.317;1.375 | 0.209 |
| Low adherence to the Mediterranean dietary pattern (N = 146) | |||||||||
| MPA(min/day) | 0.100 | 0.322; 1.998 | 0.007 | 0.159 | 0.063; 0.269 | 0.002 | 0.102 | 0.083; 0.573 | 0.009 |
| MVPA (min/day) | 0.109 | 0.355; 1.754 | 0.003 | 0.159 | 0.050; 0.223 | 0.002 | 0.112 | 0.096; 0.504 | 0.004 |
| VPA (min/day) | 0.108 | 1.509; 7.637 | 0.004 | 0.098 | −0.013; 0.760 | 0.058 | 0.113 | 0.431; 2.220 | 0.004 |
| ST (min/day) | −0.092 | −0.509; −0.056 | 0.015 | −0.205 | −0.083; −0.029 | <0.001 | −0.064 | −0.121; 0.013 | 0.114 |
| Total PA (mg/5 sec) | 0.126 | 0.723; 2.596 | 0.001 | 0.182 | 0.101; 0.333 | <0.001 | 0.116 | 0.148; 0.700 | 0.003 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Hernandez, V.; Arenaza, L.; Gracia-Marco, L.; Medrano, M.; Merchan Ramirez, E.; D. Martinez Avila, W.; Oses, M.; R. Ruiz, J.; B. Ortega, F.; Labayen, I. Influence of Physical Activity on Bone Mineral Content and Density in Overweight and Obese Children with Low Adherence to the Mediterranean Dietary Pattern. Nutrients 2018, 10, 1075. https://doi.org/10.3390/nu10081075
Muñoz-Hernandez V, Arenaza L, Gracia-Marco L, Medrano M, Merchan Ramirez E, D. Martinez Avila W, Oses M, R. Ruiz J, B. Ortega F, Labayen I. Influence of Physical Activity on Bone Mineral Content and Density in Overweight and Obese Children with Low Adherence to the Mediterranean Dietary Pattern. Nutrients. 2018; 10(8):1075. https://doi.org/10.3390/nu10081075
Chicago/Turabian StyleMuñoz-Hernandez, Victoria, Lide Arenaza, Luis Gracia-Marco, Maria Medrano, Elisa Merchan Ramirez, Wendy D. Martinez Avila, Maddi Oses, Jonatan R. Ruiz, Francisco B. Ortega, and Idoia Labayen. 2018. "Influence of Physical Activity on Bone Mineral Content and Density in Overweight and Obese Children with Low Adherence to the Mediterranean Dietary Pattern" Nutrients 10, no. 8: 1075. https://doi.org/10.3390/nu10081075
APA StyleMuñoz-Hernandez, V., Arenaza, L., Gracia-Marco, L., Medrano, M., Merchan Ramirez, E., D. Martinez Avila, W., Oses, M., R. Ruiz, J., B. Ortega, F., & Labayen, I. (2018). Influence of Physical Activity on Bone Mineral Content and Density in Overweight and Obese Children with Low Adherence to the Mediterranean Dietary Pattern. Nutrients, 10(8), 1075. https://doi.org/10.3390/nu10081075








