Cancer Prevention and Therapy with Polyphenols: Sphingolipid-Mediated Mechanisms
Abstract
1. Polyphenols
1.1. Polyphenols: Chemical Classification
1.2. Distribution of Polyphenols in Food
1.3. Bioavailability, Absorption and Metabolism of Polyphenols
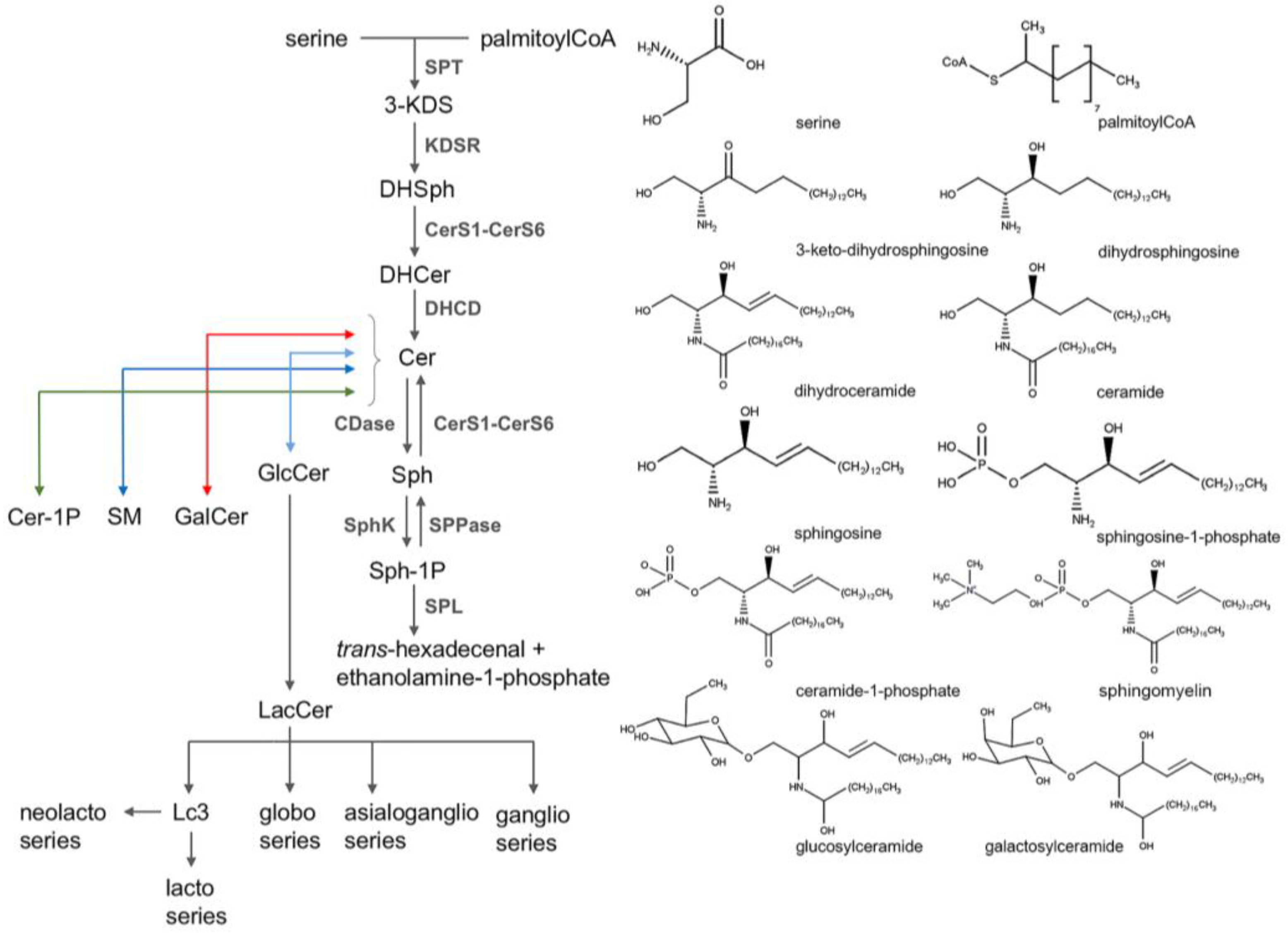
2. Sphingolipids
2.1. Sphingolipid Classification
2.2. Sphingolipid Metabolism
2.3. Sphingolipids Modulation of Cellular Functions
2.4. Sphingolipids and Cancer
3. Focus on Cancer: Dietary Polyphenols and Sphingolipids
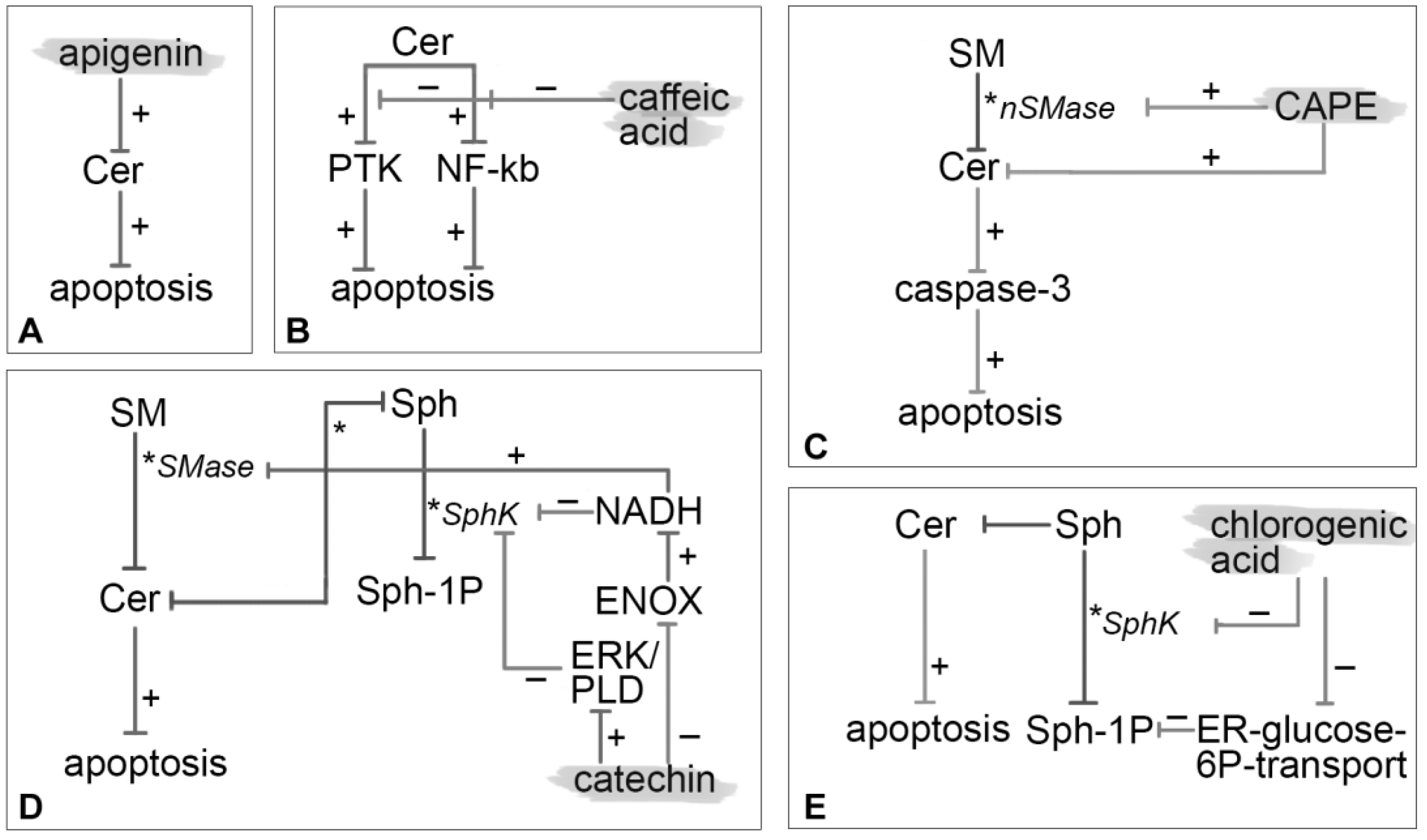
3.1. Apigenin
3.2. Caffeic Acid
3.3. CAPE
3.4. Catechin
3.5. Chlorogenic Acid
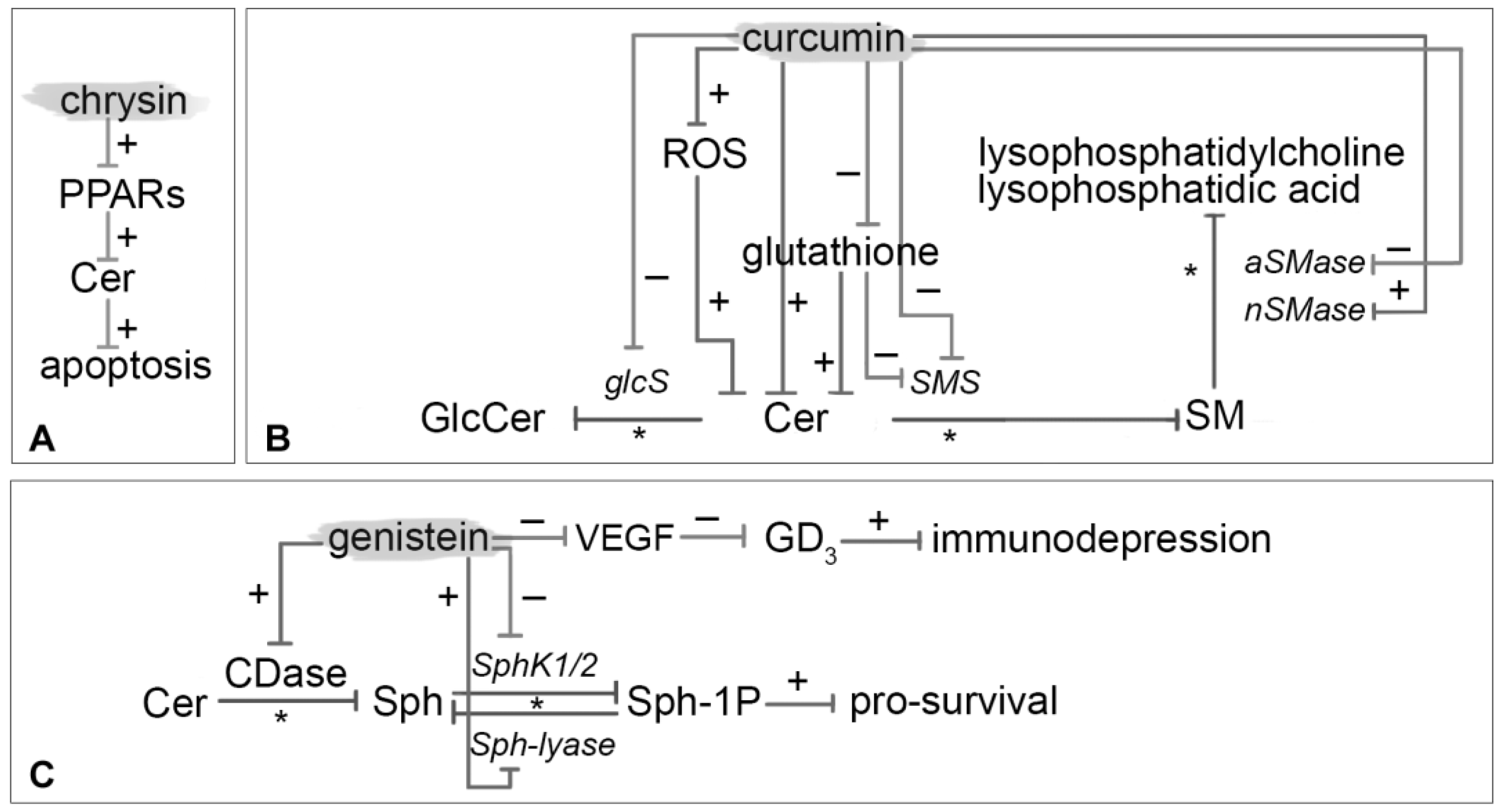
3.6. Chrysin
3.7. Curcumin
3.8. Genistein
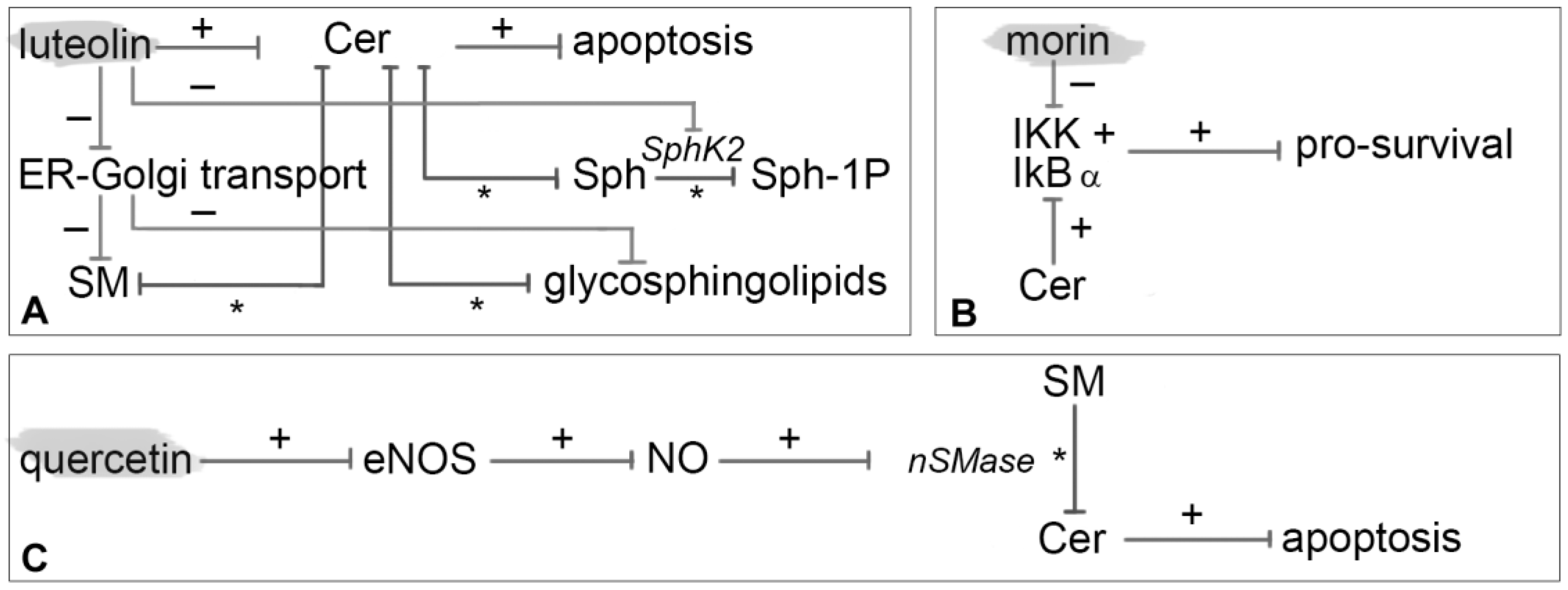
3.9. Luteolin
3.10. Morin
3.11. Quercetin
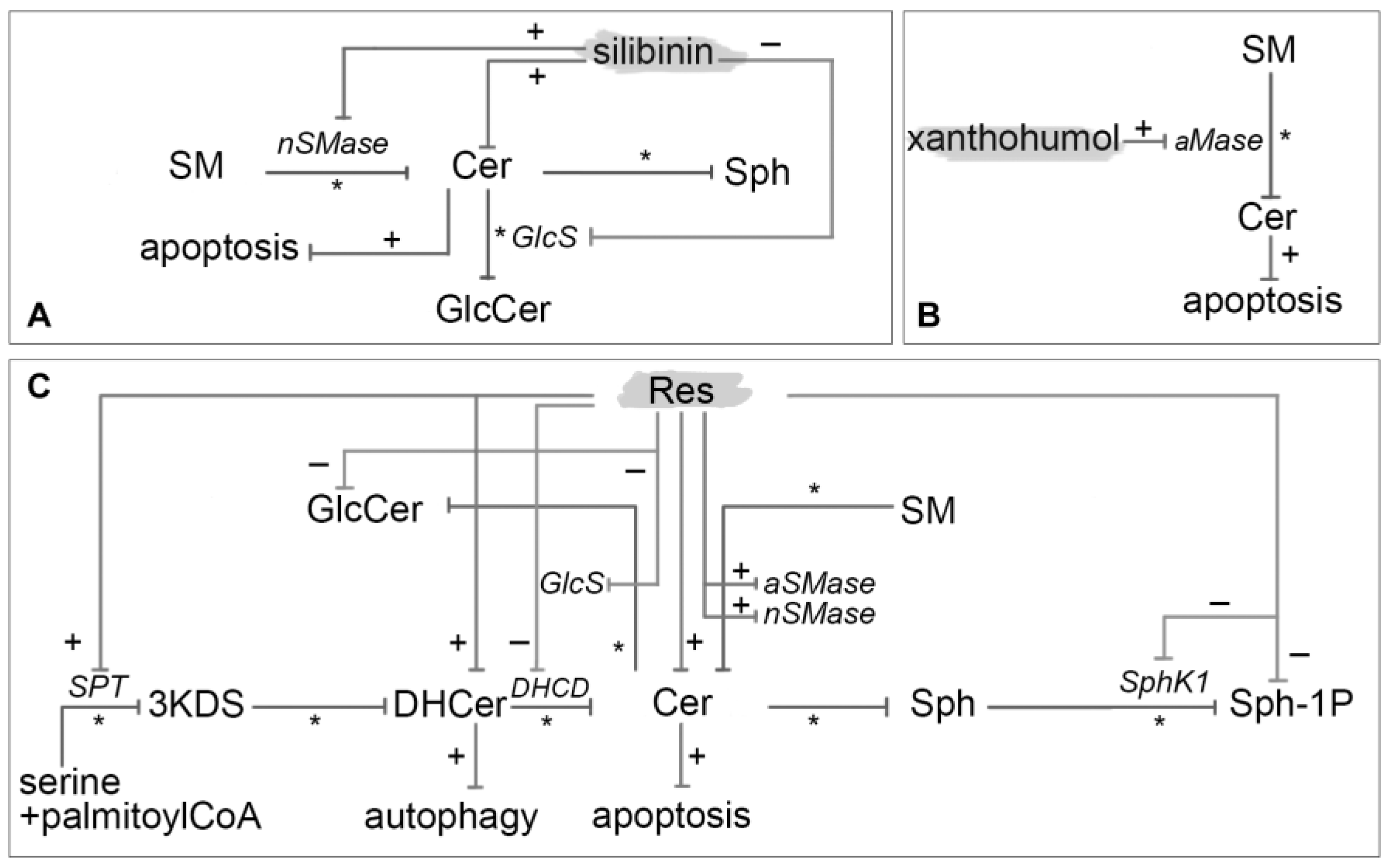
3.12. Resveratrol
3.13. Silibinin
3.14. Xanthohumol
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-KDS | 3-keto dihydrosphingosine |
| 4-HPR | retinamide |
| aCDase | acid ceramidase |
| AIF | apoptosis inducing factor |
| AKT | protein kinase B |
| AP-1 | activator protein 1 |
| aSMase | lysosomal acidic sphingomyelinase |
| ATF6 | activating transcription factor-6 |
| ATM | ataxia-telangiectasia mutated kinase |
| ATR | serine/threonine-protein kinase ATR or ataxia telangiectasia and Rad3-related protein |
| BAX | apoptosis regulator BAX |
| BAX | apoptosis regulator BAX |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-xL | B-cell lymphoma-extra large |
| Bcl-xS | B-cell lymphoma-xS |
| BCR/ABL | Philadelphia chromosome |
| c-FOS | cellular DNA-binding proteins encoded by the c-fos genes |
| CAPE | caffeic acid phenethyl ester |
| CDase | ceramidase |
| Cdc25C | gene for M-phase inducer phosphatase 3 |
| Cdk1 | cyclin-dependent kinase 1 |
| Cer | ceramide |
| CERT | ceramide transfer protein |
| Cer-1P | ceramide-1-phosphate |
| CerK | ceramide kinase |
| CerS | ceramide synthases |
| CERT | ceramide transfer protein |
| cGMP | cyclic guanosine monophosphate |
| Chk1/2 | checkpoint kinase ½ |
| CHOP | C/EBP homology protein |
| CHOP | transcription factor CCAAT-enhancer-binding protein homologous protein |
| COX | cyclooxygenase |
| COX-2 | cyclooxygenase 2 |
| cPLA2 | cytosolic phospholipases A2 |
| CREB | cAMP response element-binding protein |
| DHCD | dihydroceramide desaturase |
| DHCer | dihydroceramides |
| DHSph | dihydrosphingosine or sphinganine |
| EGCG | epigallocatechin-3-gallate |
| EGF | epithelial growth factor |
| ELISA | enzyme-linked immunosorbent assay |
| ENOX | Ecto-NOX disulfide-thiol exchanger |
| ENSA | electrophoretic mobility shift assay |
| ER | estrogen receptor |
| ER | endoplasmic reticulum |
| ERK | extracellular signal regulated kinase |
| FACS | fluorescence activated cell sorted |
| Fas | first apoptosis signal receptor |
| FITC | fluorescein isothiocyanate |
| GalCer | galactosylceramide |
| GC/MS | gas chromatography tandem mass spectrometry |
| GD3 | ganglioside GD3 or disialosyllactosylceramide |
| GlcCer | glucosylceramide |
| GlcS | glucosylceramide synthase |
| GPR30 | G protein-coupled receptor for estrogen |
| GSK-3 | glycogen synthase kinase 3 |
| GT-11 | N-[(1R,2S)-2-hydroxy-1-hydroxymethyl-2-(2-tridecyl-1-cyclopropenyl)ethyl]octanamide |
| GW4869 | N,N′-Bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]-3,3′-p-phenylene-bis-acrylamide |
| HIF-1α | hypoxia-inducible factor-1α |
| HNSCC | head and neck squamous cell carcimona |
| HPLC | high performance liquid chromatography |
| HPTLC | high performance thin layer chromatography |
| I2PP2A | protein phosphatase 2A inhibitor 2 |
| IKK | IκBα kinase |
| IκBα | inhibitory subunit of NF-κB |
| JAK | janus kinase |
| JNK | c-Jun N-terminal kinase |
| KDSR | 3-ketodihydrosphingosine reductase |
| LacCer | lactosylcercamide |
| LASS | longevity assurance genes |
| Lc3 | GlcNAcβ1-3Galβ1-4Glcβ-Cer |
| LPS | lipopolysaccharides |
| MAPK | mitogen activated protein kinase |
| MPM-2 | mitotic protein monoclonal 2 |
| mTOR | mammalian target of rapamycin |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NADH | reduced form of Nicotinamide adenine dinucleotide |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGF | nerve growth factor |
| NGFR | nerve growth factor receptor |
| nSMase | neutral sphingomyelinase Mg-dependent |
| P-gp | permeability glycoprotein |
| p38 MAPK | p38 mitogen activated protein kinase |
| p53 | tumor protein p53 |
| p75NTR | low-affinity nerve growth factor receptor or p75 neurotrophin receptor |
| PA | phosphatidic acid |
| Par-4 | prostate apoptosis response 4 |
| PARP | poly (ADP-ribose) polymerase |
| PDMP | dl-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol |
| PI3K | phosphatidylinositide 3-kinases |
| PKC/PKA | protein kinase C/protein kinase A |
| PLC | phospholipase C |
| PLD | phospholipase D |
| PPAR | peroxisome proliferator-activated receptors |
| PPE | polyphenon E |
| Res | resveratrol |
| ROS | reactive oxygen species |
| S1PR1-5 | sphingosine-1-phosphate receptors 1–5 |
| SEAP | secreted embryonic alkaline phosphatase |
| SKI-II | 2-(p-Hydroxyanilino)-4-(p-chlorophenyl)thiazole |
| SM | sphingomyelin |
| SMase | sphingomyelinase |
| SMS1/2 | sphingomyelin synthase 1/2 |
| Sph | sphingosine |
| Sph-1P | sphingosine-1-phosphate |
| SphK1/2 | sphingosine kinase 1/2 |
| SPL | sphingosine-1-phosphate lyase |
| SPPase1/2 | sphingosine-1-phosphate phosphatases 1/2 |
| SPT | serinepalmitoyl transferase |
| STAT | signal transducer and activator of transcription protein |
| THDF | 5,7,3′-trihydroxy-3,4′-dimethoxyflavone |
| THDF | 5,7,3′-trihydroxy-3,4′-dimethoxyflavone |
| TLC | thin layer chromatography |
| TLC | thin layer chromatography |
| TNF | tumor necrosis factor alpha |
| VDAC | voltage-dependent anion-selective channel |
| VEGF | vascular endothelial growth factor |
| XM462 | Octanoic acid (1S,2S)-(2-hydroxy-1-hydroxymethyl-3-tridecylsulfanyl-propyl)-amide |
References
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3244S–3246S. [Google Scholar] [CrossRef] [PubMed]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.-M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Husain, N.; Gupta, S. A critical study on chemistry and distribution of phenolic compounds in plants, and their role in human health. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015, 1, 57–60. [Google Scholar]
- Cook, N. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Eur. Ceram. Soc. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Kim, K.; Vance, T.M.; Chun, O.K. Estimated intake and major food sources of flavonoids among US adults: Changes between 1999–2002 and 2007–2010 in NHANES. Eur. J. Nutr. 2016, 55, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Chemistry of extra virgin olive oil: Adulteration, oxidative stability, and antioxidants. J. Agric. Food Chem. 2010, 58, 5991–6006. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, M.C.; Barroso, C.G.; Pérez-Bustamante, J.A. Analysis of polyphenolic compounds of different vinegar samples. Z. Lebensm. Unters. Forsch. 1994, 199, 29–31. [Google Scholar] [CrossRef]
- Adisakwattana, S. Cinnamic acid and its derivatives: Mechanisms for prevention and management of diabetes and its complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 2009, 56, 317–333. [Google Scholar] [CrossRef]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Lewandowska, U.; Szewczyk, K.; Hrabec, E.; Janecka, A.; Gorlach, S. Overview of metabolism and bioavailability enhancement of polyphenols. J. Agric. Food Chem. 2013, 61, 12183–12199. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols—Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Sullards, M.C.; Merrill, A.H. An introduction to sphingolipid metabolism and analysis by new technologies. NeuroMol. Med. 2010, 12, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Kollmeyer, J.; Symolon, H.; Momin, A.; Munter, E.; Wang, E.; Kelly, S.; Allegood, J.C.; Liu, Y.; Peng, Q.; et al. Ceramides and other bioactive sphingolipid backbones in health and disease: Lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1864–1884. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–862. [Google Scholar] [CrossRef] [PubMed]
- Slotte, J.P. Biological functions of sphingomyelins. Prog. Lipid Res. 2013, 52, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.M.; Ernst, D.; Wei, Y.; Laurà, M.; Liu, Y.T.; Polke, J.; Blake, J.; Winer, J.; Houlden, H.; Hornemann, T.; Reilly, M.M. Hereditary sensory and autonomic neuropathy type 1 (HSANI) caused by a novel mutation in SPTLC2. Neurology 2013, 80, 2106–2111. [Google Scholar] [CrossRef] [PubMed]
- Park, W.J.; Park, J.W. The effect of altered sphingolipid acyl chain length on various disease models. Biol. Chem. 2015, 396, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Romero, A.; Gehin, C.; Riezman, H. Sphingolipid homeostasis in the web of metabolic routes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, T.; Hanada, K. Sphingolipid metabolism and interorganellar transport: Localization of sphingolipid enzymes and lipid transfer proteins. Traffic 2015, 16, 101–122. [Google Scholar] [CrossRef] [PubMed]
- MacEyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, G.A.; Beverly, L.J.; Siskind, L.J. Sphingolipids and mitochondrial apoptosis. J. Bioenerg. Biomembr. 2016, 48, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Young, M.M.; Kester, M.; Wang, H.-G. Sphingolipids: Regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 2013, 54, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Murayama, T. The role of sphingolipids in arachidonic acid metabolism. J. Pharmacol. Sci. 2014, 124, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Ghidoni, R.; Caretti, A.; Signorelli, P. Role of sphingolipids in the pathobiology of lung inflammation. Mediat. Inflamm. 2015, 2015, 487508. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Futerman, A.H. The metabolism and function of sphingolipids and glycosphingolipids. Cell. Mol. Life Sci. 2007, 64, 2270–2284. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. The ceramide-centric universe of lipid-mediated cell regulation: Stress encounters of the lipid kind. J. Biol. Chem. 2002, 277, 25847–25850. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.A.; Hannun, Y.A.; Obeid, L.M. Sphingosine kinase: Biochemical and cellular regulation and role in disease. J. Biochem. Mol. Biol. 2006, 39, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Cowart, L.A. Sphingolipids: Players in the pathology of metabolic disease. Trends Endocrinol. Metab. 2009, 20, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Sridevi, P.; Alexander, H.; Laviad, E.L.; Min, J.; Mesika, A.; Hannink, M.; Futerman, A.H.; Alexander, S. Stress-induced ER to Golgi translocation of ceramide synthase 1 is dependent on proteasomal processing. Exp. Cell Res. 2010, 316, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Siskind, L.J.; Mullen, T.D.; Rosales, K.R.; Clarke, C.J.; Hernandez-Corbacho, M.J.; Edinger, A.L.; Obeid, L.M. The BCL-2 protein BAK is required for long-chain ceramide generation during apoptosis. J. Biol. Chem. 2010, 285, 11818–11826. [Google Scholar] [CrossRef] [PubMed]
- Karahatay, S.; Thomas, K.; Koybasi, S.; Senkal, C.E.; ElOjeimy, S.; Liu, X.; Bielawski, J.; Day, T.A.; Gillespie, M.B.; Sinha, D.; et al. Clinical relevance of ceramide metabolism in the pathogenesis of human head and neck squamous cell carcinoma (HNSCC): Attenuation of C18-ceramide in HNSCC tumors correlates with lymphovascular invasion and nodal metastasis. Cancer Lett. 2007, 256, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Pettus, B.J.; Bielawska, A.; Subramanian, P.; Wijesinghe, D.S.; Maceyka, M.; Leslie, C.C.; Evans, J.H.; Freiberg, J.; Roddy, P.; Hannun, Y.A.; et al. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J. Biol. Chem. 2004, 279, 11320–11326. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.; Meyers-Needham, M.; Senkal, C.E.; Saddoughi, S.A.; Sentelle, D.; Selvam, S.P.; Salas, A.; Ogretmen, B. Sphingolipids and cancer: Ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 2010, 6, 1603–1624. [Google Scholar] [CrossRef] [PubMed]
- Saddoughi, S.A.; Song, P.; Ogretmen, B. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell. Biochem. 2008, 49, 413–440. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, L.; Dayon, A.; Doumerc, N.; Ader, I.; Golzio, M.; Izard, J.C.; Hara, Y.; Malavaud, B.; Cuvillier, O. The sphingosine kinase-1 survival pathway is a molecular target for the tumor-suppressive tea and wine polyphenols in prostate cancer. FASEB J. 2010, 24, 3882–3894. [Google Scholar] [CrossRef] [PubMed]
- Zbidah, M.; Lupescu, A.; Jilani, K.; Fajol, A.; Michael, D.; Qadri, S.M.; Lang, F. Apigenin-induced suicidal erythrocyte death. J. Agric. Food Chem. 2012, 60, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Lefort, É.C.; Blay, J. Apigenin and its impact on gastrointestinal cancers. Mol. Nutr. Food Res. 2013, 57, 126–144. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.Y.; Choi, J.H.; Lee, J.Y.; Yoon, K.S.; Choe, W.; Ha, J.; Yeo, E.J.; Kang, I. Apigenin protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis. Neurochem. Int. 2010, 57, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zbidah, M.; Lupescu, A.; Shaik, N.; Lang, F. Gossypol-induced suicidal erythrocyte death. Toxicology 2012, 302, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, M. Insight into the Mechanisms by Which Apigenin, Curcumin and Sulfasalazine Induce Apoptosis in Colon Cancer Cells. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2003. [Google Scholar]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic acid derivatives, analogs and applications: A patent review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Leonardi, F.; Scaccini, C.; Virgili, F. Modulation of ceramide-induced NF-κB binding activity and apoptotic response by caffeic acid in U937 cells: Comparison with other antioxidants. Free Radic. Biol. Med. 2001, 30, 722–733. [Google Scholar] [CrossRef]
- Hagiwara, A.; Kokubo, Y.; Takesada, Y.; Tanaka, H.; Tamano, S.; Hirose, M.; Shirai, T.; Ito, N. Inhibitory effects of phenolic compounds on development of naturally occurring preneoplastic hepatocytic foci in long-term feeding studies using male F344 rats. Teratog. Carcinog. Mutagen. 1996, 16, 317–325. [Google Scholar] [CrossRef]
- Tanaka, T.; Kojima, T.; Kawamori, T.; Wang, A.; Suzui, M.; Okamoto, K.; Mori, H. Inhibition of 4-nitroquinoline-1-oxide-induced rat tongue carcinogenesis by the naturally occurring plant phenolics caffeic, ellagic, chlorogenic and ferulic acids. Carcinogenesis 1993, 14, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Scaccini, C.; Packer, L.; Virgili, F. In vitro inhibition of the activity of phosphorylase kinase, protein kinase C and protein kinase A by caffeic acid and a procyanidin-rich pine bark (Pinus marittima) extract. Biochim. Biophys. Acta Gen. Subj. 2000, 1474, 219–225. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.S.; Lee, H.J.; Dong, Z. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2009, 30, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Karim, S.; Akram, M.R.; Khan, S.A.; Azhar, S.; Mumtaz, A.; Bin Asad, M.H.H. Caffeic acid phenethyl ester and therapeutic potentials. Biomed. Res. Int. 2014, 2014, 145342. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.H.; Shen, C.H.; Huang, W.S.; Chen, C.N.; Liang, W.H.; Lin, T.H.; Kuo, H.C. Activation of neutral-sphingomyelinase, MAPKs, and p75 NTR-mediating caffeic acid phenethyl ester-induced apoptosis in C6 glioma cells. J. Biomed. Sci. 2014, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, W.; Jiang, X. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J. Agric. Food Chem. 2008, 56, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Hong, J.; Zhou, J.N.; Pan, Z.; Bose, M.; Liao, J.; Yang, G.Y.; Liu, Y.Y.; Hou, Z.; Lin, Y.; et al. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005, 65, 10623–10631. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Hao, X.; Simi, B.; Ju, J.; Jiang, H.; Reddy, B.S.; Yang, C.S. Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats. Carcinogenesis 2008, 29, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; De Luca, T.; Watanabe, T.; Morré, D.M.; Morré, D.J. Metabolite modulation of HeLa cell response to ENOX2 inhibitors EGCG and phenoxodiol. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Human nutrition and metabolism chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Lee, S.O.; Kim, K.R.; Lee, H.J. Sphingosine kinase-1 involves the inhibitory action of HIF-1α by chlorogenic acid in hypoxic DU145 cells. Int. J. Mol. Sci. 2017, 18, 325. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, A.; Currie, J.C.; Desgagnés, J.; Annabi, B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int. 2006, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Das, A.S.; Majumder, M.; Mukhopadhyay, R. Antiatherogenic roles of dietary flavonoids chrysin, quercetin, and luteolin. J. Cardiovasc. Pharmacol. 2016, 68, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.Y.; Cao, Y.Y.; Chen, C.Y.; Dai, H.Q.; Yu, S.X.; Wei, J.L.; Li, H.; Yang, B. Antioxidant flavonoids from the seed of Oroxylum indicum. Fitoterapia 2011, 82, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Samokhvalov, V.; Zlobine, I.; Jamieson, K.L.; Jurasz, P.; Chen, C.; Lee, K.S.S.; Hammock, B.D.; Seubert, J.M. PPARδ signaling mediates the cytotoxicity of DHA in H9c2 cells. Toxicol. Lett. 2015, 232, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.A.; Gardner, S.H.; Hawcroft, G. Activity of the non-steroidal anti-inflammatory drug indomethacin against colorectal cancer. Cancer Treat. Rev. 2003, 29, 309–320. [Google Scholar] [CrossRef]
- Lestari, M.L.A.D.; Indrayanto, G. Curcumin. Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: New York, NY, USA, 2013; Volume 39, pp. 113–204. ISBN 9780128001738. [Google Scholar]
- Cheng, Y.; Kozubek, A.; Ohlsson, L.; Sternby, B.; Duan, R.D. Curcumin decreases acid sphingomyelinase activity in colon cancer caco-2 cells. Planta Med. 2007, 73, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Koumanov, K.S.; Quinn, P.J.; Béréziat, G.; Wolf, C.; Bereziat, G. Cholesterol relieves the inhibitory effect of sphingomyelin on type II secretory phospholipase A2. Biochem. J. 1998, 336, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Shida, D.; Kitayama, J.; Yamaguchi, H.; Okaji, Y.; Tsuno, N.H.; Watanabe, T.; Takuwa, Y.; Nagawa, H. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003, 63, 1706–1711. [Google Scholar] [PubMed]
- Moussavi, M.; Assi, K.; Gómez-Muñoz, A.; Salh, B. Curcumin mediates ceramide generation via the de novo pathway in colon cancer cells. Carcinogenesis 2006, 27, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Wu, X.Y.; Tao, G.Q.; Liu, C.Y.; Chen, J.; Wang, L.Q.; Lu, P.H. Perifosine sensitizes curcumin-induced anti-colorectal cancer effects by targeting multiple signaling pathways both in vivo and in vitro. Int. J. Cancer 2012, 131, 2487–2498. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, J.; Qiu, Y.; Sun, H. 1-Phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) facilitates curcumin-induced melanoma cell apoptosis by enhancing ceramide accumulation, JNK activation, and inhibiting PI3K/AKT activation. Mol. Cell. Biochem. 2012, 361, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.A.; Sordillo, P.P.; Helson, L. Curcumin for the treatment of glioblastoma. Anticancer Res. 2015, 35, 6373–6378. [Google Scholar] [PubMed]
- Thayyullathil, F.; Rahman, A.; Pallichankandy, S.; Patel, M.; Galadari, S. ROS-dependent prostate apoptosis response-4 (Par-4) up-regulation and ceramide generation are the prime signaling events associated with curcumin-induced autophagic cell death in human malignant glioma. FEBS Open Bio. 2014, 4, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Furlong, S.J.; Sutton, K.; Richardson, A.; Robichaud, M.R.J.; Giacomantonio, C.A.; Ridgway, N.D.; Hoskin, D.W. Curcumin-induced apoptosis in PC3 prostate carcinoma cells is caspase-independent and involves cellular ceramide accumulation and damage to mitochondria. Nutr. Cancer 2010, 62, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Kizhakkayil, J.; Thayyullathil, F.; Chathoth, S.; Hago, A.; Patel, M.; Galadari, S. Glutathione regulates caspase-dependent ceramide production and curcumin-induced apoptosis in human leukemic cells. Free Radic. Biol. Med. 2012, 52, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Scharstuhl, A.; Mutsaers, H.A.M.; Pennings, S.W.C.; Russel, F.G.M.; Wagener, F.A.D.T.G. Involvement of VDAC, Bax and ceramides in the efflux of AIF from mitochondria during curcumin-induced apoptosis. PLoS ONE 2009, 4, e6688. [Google Scholar] [CrossRef] [PubMed]
- Abdel Shakor, A.B.; Atia, M.; Ismail, I.A.; Alshehri, A.; El-Refaey, H.; Kwiatkowska, K.; Sobota, A. Curcumin induces apoptosis of multidrug-resistant human leukemia HL60 cells by complex pathways leading to ceramide accumulation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Abdel Shakor, A.B.; Atia, M.; Alshehri, A.S.; Sobota, A.; Kwiatkowska, K. Ceramide generation during curcumin-induced apoptosis is controlled by crosstalk among Bcl-2, Bcl-xL, caspases and glutathione. Cell Signal. 2015, 27, 2220–2230. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Ji, C.; Cheng, L.; He, L.; Lu, C.C.; Wang, R.; Bi, Z.G. Sphingosine kinase-1 inhibition sensitizes curcumin-induced growth inhibition and apoptosis in ovarian cancer cells. Cancer Sci. 2012, 103, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yu, T.; Wang, W.; Pan, K.; Shi, D.; Sun, H. Curcumin-induced melanoma cell death is associated with mitochondrial permeability transition pore (mPTP) opening. Biochem. Biophys. Res. Commun. 2014, 448, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. Int. Rev. J. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Engel, N.; Lisec, J.; Piechulla, B.; Nebe, B. Metabolic Profiling reveals sphingosine-1-phosphate kinase 2 and lyase as key targets of (phyto-) estrogen action in the breast cancer cell line MCF-7 and not in MCF-12A. PLoS ONE 2012, 7, e47833. [Google Scholar] [CrossRef] [PubMed]
- Lucki, N.C.; Sewer, M.B. Genistein stimulates MCF-7 breast cancer cell growth by inducing acid ceramidase (ASAH1) gene expression. J. Biol. Chem. 2011, 286, 19399–19409. [Google Scholar] [CrossRef] [PubMed]
- Engel, N.; Adamus, A.; Schauer, N.; Kühn, J.; Nebe, B.; Seitz, G.; Kraft, K. Synergistic action of genistein and calcitriol in immature osteosarcoma MG-63 cells by SGPL1 up-regulation. PLoS ONE 2017, 12, e0169742. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Yang, Y.L.; He, L.; Gu, B.; Xia, J.P.; Sun, W.L.; Su, Z.L.; Chen, B.; Bi, Z.G. Increasing ceramides sensitizes genistein-induced melanoma cell apoptosis and growth inhibition. Biochem. Biophys. Res. Commun. 2012, 421, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Tiper, I.V.; Temkin, S.M.; Spiegel, S.; Goldblum, S.E.; Giuntoli, R.L.; Oelke, M.; Schneck, J.P.; Webb, T.J. VEGF potentiates GD3-mediated immunosuppression by human ovarian cancer cells. Clin. Cancer Res. 2016, 22, 4249–4258. [Google Scholar] [CrossRef] [PubMed]
- Zebrowski, B.K.; Liu, W.; Ramirez, K.; Akagi, Y.; Mills, G.B.; Ellis, L.M. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann. Surg. Oncol. 1999, 6, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Bamias, A.; Koutsoukou, V.; Terpos, E.; Tsiatas, M.L.; Liakos, C.; Tsitsilonis, O.; Rodolakis, A.; Voulgaris, Z.; Vlahos, G.; Papageorgiou, T.; et al. Correlation of NK T-like CD3+CD56+ cells and CD4+CD25+(hi) regulatory T cells with VEGF and TNFα in ascites from advanced ovarian cancer: Association with platinum resistance and prognosis in patients receiving first-line, platinum-based chemotherapy. Gynecol. Oncol. 2008, 108, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.R.; Xia, P.; Hahn, C.N.; Drew, J.J.; Drogemuller, C.J.; Brown, D.; Vadas, M.A. Phenoxodiol, an experimental anticancer drug, shows potent antiangiogenic properties in addition to its antitumour effects. Int. J. Cancer 2006, 118, 2412–2420. [Google Scholar] [CrossRef] [PubMed]
- Limaye, V.; Li, X.; Hahn, C.; Xia, P.; Berndt, M.C.; Vadas, M.A.; Gamble, J.R. Sphingosine kinase-1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. Blood 2005, 105, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Plano, D.; Amin, S.; Sharma, A.K. Importance of sphingosine kinase (SphK) as a target in developing cancer therapeutics and recent developments in the synthesis of novel SphK inhibitors. J. Med. Chem. 2014, 57, 5509–5524. [Google Scholar] [CrossRef] [PubMed]
- Hadi, L.A.; Di Vito, C.; Marfia, G.; Ferraretto, A.; Tringali, C.; Viani, P.; Riboni, L. Sphingosine kinase 2 and ceramide transport as key targets of the natural flavonoid luteolin to induce apoptosis in colon cancer cells. PLoS ONE 2015, 10, e0143384. [Google Scholar] [CrossRef]
- Madankumar, P.; Naveenkumar, P.; Devaraj, H.; Niranjalidevaraj, S. Morin, a dietary flavonoid, exhibits anti-fibrotic effect and induces apoptosis of activated hepatic stellate cells by suppressing canonical NF-κB signaling. Biochimie 2015, 110, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.K.; Aggarwal, R.S.; Sethi, G.; Aggarwal, B.B.; Ramesh, G.T. Morin (3,5,7,2′,4′-pentahydroxyflavone) abolishes nuclear factor-κB activation induced by various carcinogens and inflammatory stimuli, leading to suppression of nuclear factor-κB—Regulated gene expression and up-regulation of apoptosis. Clin. Cancer Res. 2007, 13, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, P.; Asensi, M.; Priego, S.; Benlloch, M.; Mena, S.; Ortega, A.; Obrador, E.; Esteve, J.M.; Estrela, J.M. Nitric oxide mediates natural polyphenol-induced Bcl-2 down-regulation and activation of cell death in metastatic B16 melanoma. J. Biol. Chem. 2007, 282, 2880–2890. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.; Quintana, J.; Estévez, F. 5,7,3′-Trihydroxy-3,4′-Dimethoxyflavone Inhibits the Tubulin Polymerization and Activates the Sphingomyelin Pathway. Mol. Carcinog. 2011, 50, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Chiou, Y.S.; Ho, C.T.; Pan, M.H. Chemoprevention by resveratrol and pterostilbene: Targeting on epigenetic regulation. BioFactors 2018, 44, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, A.; Caretti, A.; Signorelli, P.; Ghidoni, R. Resveratrol: A potential challenger against gastric cancer. World J. Gastroenterol. 2015, 21, 10636–10643. [Google Scholar] [CrossRef] [PubMed]
- Xianfeng, H.; Zhu, H.-L. Resveratrol and Its Analogues: Promising Antitumor Agents. Anticancer. Agents Med. Chem. 2011, 11, 479–490. [Google Scholar] [CrossRef]
- Signorelli, P.; Ghidoni, R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 2005, 16, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, P.; Munoz-Olaya, J.M.; Gagliostro, V.; Casas, J.; Ghidoni, R.; Fabriàs, G. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 2009, 282, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.-O.; Park, N.-Y.; Seo, C.-H.; Hong, S.-P.; Oh, K.-W.; Hong, J.-T.; Han, S.-K.; Lee, Y.-M. Inhibition of sphingolipid metabolism enhances resveratrol chemotherapy in human gastric cancer cells. Biomol. Ther. 2012, 20, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Delmas, D.; Vang, O.; Hsieh, T.C.; Lin, S.; Cheng, G.Y.; Chiang, H.L.; Chen, C.E.; Tang, H.Y.; Crawford, D.R.; et al. Mechanisms of ceramide-induced COX-2-dependent apoptosis in human ovarian cancer OVCAR-3 cells partially overlapped with resveratrol. J. Cell. Biochem. 2013, 114, 1940–1954. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D. A Dysregulated post-transcriptional control of COX-2 gene expression in cancer. Curr. Pharm. Des. 2004, 10, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Zahner, G.; Wolf, G.; Ayoub, M.; Reinking, R.; Panzer, U.; Shankland, S.J.; Stahl, R.A. Cyclooxygenase-2 overexpression inhibits platelet-derived growth factor- induced mesangial cell proliferation through induction of the tumor suppressor gene p53 and the cyclin-dependent kinase inhibitors p21waf-1/cip-1 and p27kip-1. J. Biol. Chem. 2002, 277, 9763–9771. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.G.; Gray, A.I.; Pyne, S.; Pyne, N.J. Resveratrol dimers are novel sphingosine kinase 1 inhibitors and affect sphingosine kinase 1 expression and cancer cell growth and survival. Br. J. Pharmacol. 2012, 166, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yu, Z. Resveratrol induces apoptosis of leukemia cell line K562 by modulation of sphingosine kinase-1 pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 2755–2762. [Google Scholar] [PubMed]
- Cakir, Z.; Saydam, G.; Sahin, F.; Baran, Y. The roles of bioactive sphingolipids in resveratrol-induced apoptosis in HL60 acute myeloid leukemia cells. J. Cancer Res. Clin. Oncol. 2011, 137, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Kartal, M.; Saydam, G.; Sahin, F.; Baran, Y. Resveratrol triggers apoptosis through regulating ceramide metabolizing genes in human K562 chronic myeloid leukemia cells. Nutr. Cancer 2011, 63, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.E.; Kao, C.H.; Liu, Y.T.A.; Cheng, M.L.; Yang, Y.W.; Huang, Y.K.; Hsu, C.C.; Wang, J.S. Resveratrol induced ER expansion and ER caspase-mediated apoptosis in human nasopharyngeal carcinoma cells. Apoptosis 2014, 19, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, T.; Zhu, C.; Sun, H.; Qiu, Y.; Zhu, X.; Li, J. Resveratrol triggers protective autophagy through the ceramide/Akt/mTOR pathway in melanoma B16 cells. Nutr. Cancer 2014, 66, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, N.; Omori, Y.; Kawamoto, Y.; Sobue, S.; Ichihara, M.; Suzuki, M.; Kyogashima, M.; Nakamura, M.; Tamiya-Koizumi, K.; Nozawa, Y.; et al. Resveratrol-induced transcriptional up-regulation of ASMase (SMPD1) of human leukemia and cancer cells. Biochem. Biophys. Res. Commun. 2016, 470, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Scarlatti, F.; Sala, G.; Somenzi, G.; Signorelli, P.; Sacchi, N.; Ghidoni, R. Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signaling. FASEB J. 2003, 17, 2339–2341. [Google Scholar] [CrossRef] [PubMed]
- Scarlatti, F.; Sala, G.; Ricci, C.; Maioli, C.; Milani, F.; Minella, M.; Botturi, M.; Ghidoni, R. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett. 2007, 253, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Dolfini, E.; Roncoroni, L.; Dogliotti, E.; Sala, G.; Erba, E.; Sacchi, N.; Ghidoni, R. Resveratrol impairs the formation of MDA-MB-231 multicellular tumor spheroids concomitant with ceramide accumulation. Cancer Lett. 2007, 249, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, F.; Sala, G.; Bagnacani, A.; Bertini, S.; Carboni, I.; Placanica, G.; Prota, G.; Rapposelli, S.; Sacchi, N.; Macchia, M.; et al. Synthesis of a resveratrol analogue with high ceramide-mediated proapoptotic activity on human breast cancer cells. J. Med. Chem. 2005, 48, 6783–6786. [Google Scholar] [CrossRef] [PubMed]
- Davis-Searles, P.R.; Nakanishi, Y.; Kim, N.C.; Graf, T.N.; Oberlies, N.H.; Wani, M.C.; Wall, M.E.; Agarwal, R.; Kroll, D.J. Milk thistle and prostate cancer: Differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005, 65, 4448–4457. [Google Scholar] [CrossRef] [PubMed]
- Boojar, M.M.A.; Hassanipour, M.; Mehr, S.E.; Boojar, M.M.A.; Dehpour, A.R. New aspects of silibinin stereoisomers and their 3-o-galloyl derivatives on cytotoxicity and ceramide metabolism in hep G2hepatocarcinoma cell line. Iran. J. Pharm. Res. 2016, 15, 421–433. [Google Scholar]
- Xuan, N.T.; Shumilina, E.; Gulbins, E.; Gu, S.; Götz, F.; Lang, F. Triggering of dendritic cell apoptosis by xanthohumol. Mol. Nutr. Food Res. 2010, 54. [Google Scholar] [CrossRef] [PubMed]
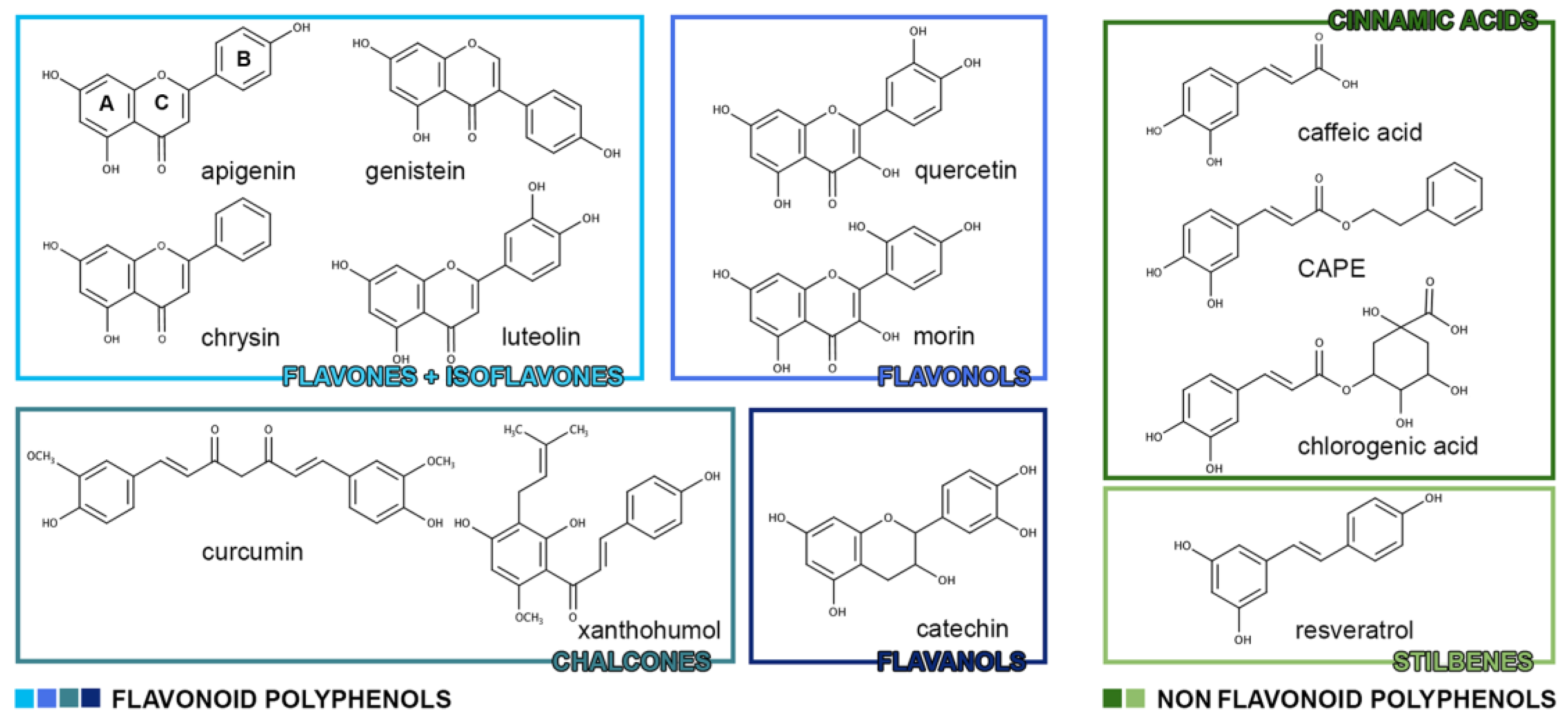
| Flavonoid Polyphenols | |
| Flavones | apigenin, chrysin, diosmin, luteolin, baicalein |
| Isoflavones | daidzein, daidzin, genistein |
| Flavanones | hesperetin, narigenin |
| Flavonols | kaempferol, quercetin, rutine, myricetin, morin |
| Anthocyanidins | cyanidin, dephinidin, malvidin, pelargonidin, peonidin |
| Chalcones | butein, curcumin, xanthohumol |
| Flavanols | catechins, tannins |
| Non-Flavonoid Polyphenols | |
| Benzoic acids | vanillic acid, gallic acid, syringic acid |
| Cinnamic acids | caffeic acid, chlorogenic acid, CAPE, tannic acid |
| Stilbenes | resveratrol, piceatannol, isorhapontigenin, oxyresveratrol |
| Sphingolipids | Biological Target | Effect in Cancer | References |
|---|---|---|---|
| Cer | PKC, I2PP2A, cathepsin D, caspases, telomerase | Apoptosis, growth arrest, senescence | [23,26,27,28,29,30,31,34,35,36,37] |
| Cer-1P | cPLA2 | Release of arachidonic acid and activation of inflammatory cascade | [31,33,37] |
| DAG (from SM) | PKC | Cellular proliferation | [38,39] |
| Sph-1P | NFKB, COX-2, ERK | Malignant transformation, anti-apoptosis, angiogenesis, survival, metastatization | [31,32,33,40] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dei Cas, M.; Ghidoni, R. Cancer Prevention and Therapy with Polyphenols: Sphingolipid-Mediated Mechanisms. Nutrients 2018, 10, 940. https://doi.org/10.3390/nu10070940
Dei Cas M, Ghidoni R. Cancer Prevention and Therapy with Polyphenols: Sphingolipid-Mediated Mechanisms. Nutrients. 2018; 10(7):940. https://doi.org/10.3390/nu10070940
Chicago/Turabian StyleDei Cas, Michele, and Riccardo Ghidoni. 2018. "Cancer Prevention and Therapy with Polyphenols: Sphingolipid-Mediated Mechanisms" Nutrients 10, no. 7: 940. https://doi.org/10.3390/nu10070940
APA StyleDei Cas, M., & Ghidoni, R. (2018). Cancer Prevention and Therapy with Polyphenols: Sphingolipid-Mediated Mechanisms. Nutrients, 10(7), 940. https://doi.org/10.3390/nu10070940





