Cutaneous and Mucosal Manifestations Associated with Celiac Disease
Abstract
:1. Introduction
2. Immunopathogenesis of Skin and Oral Lesions Associated with CD
3. Dermatitis Herpetiformis
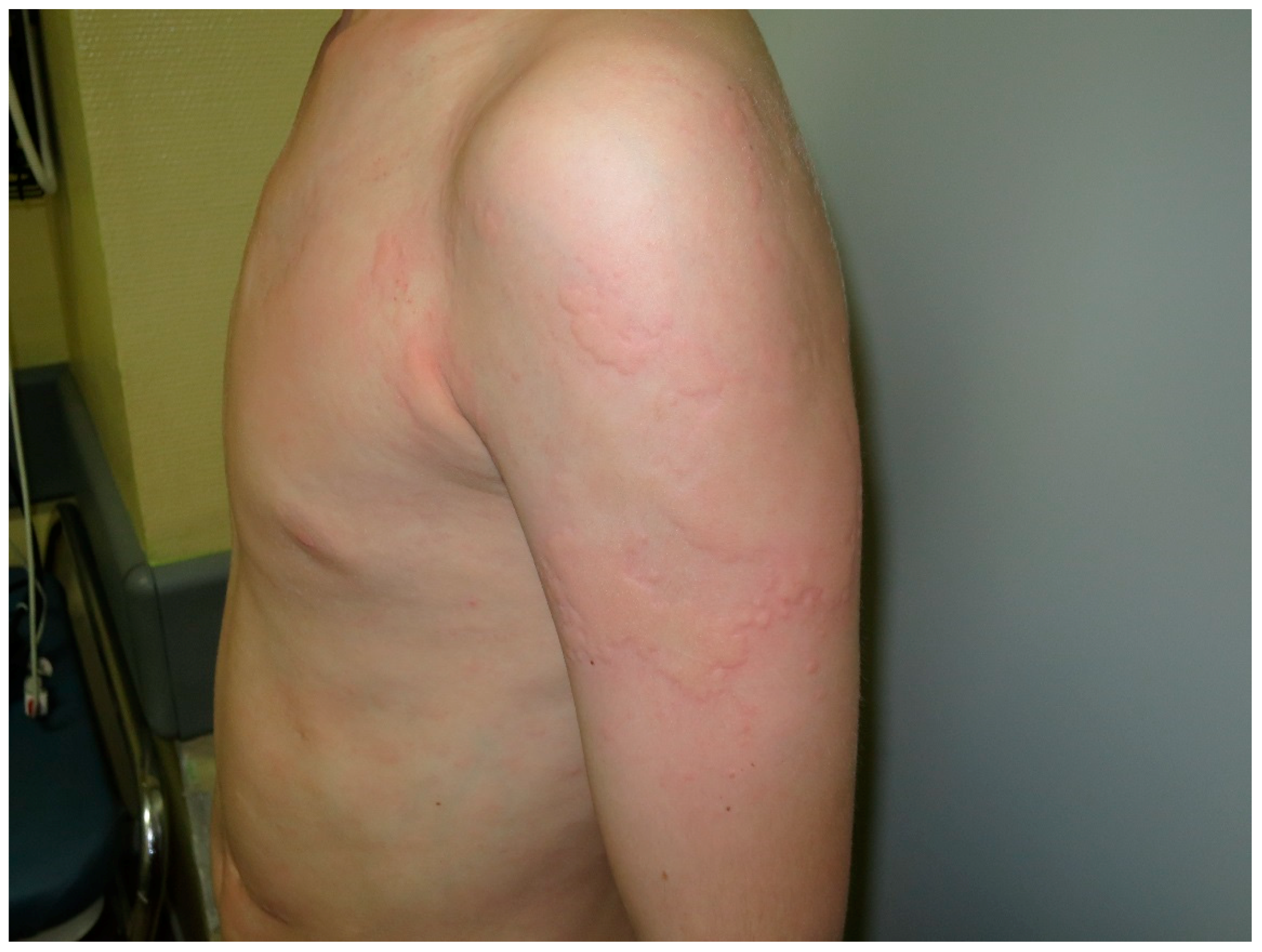
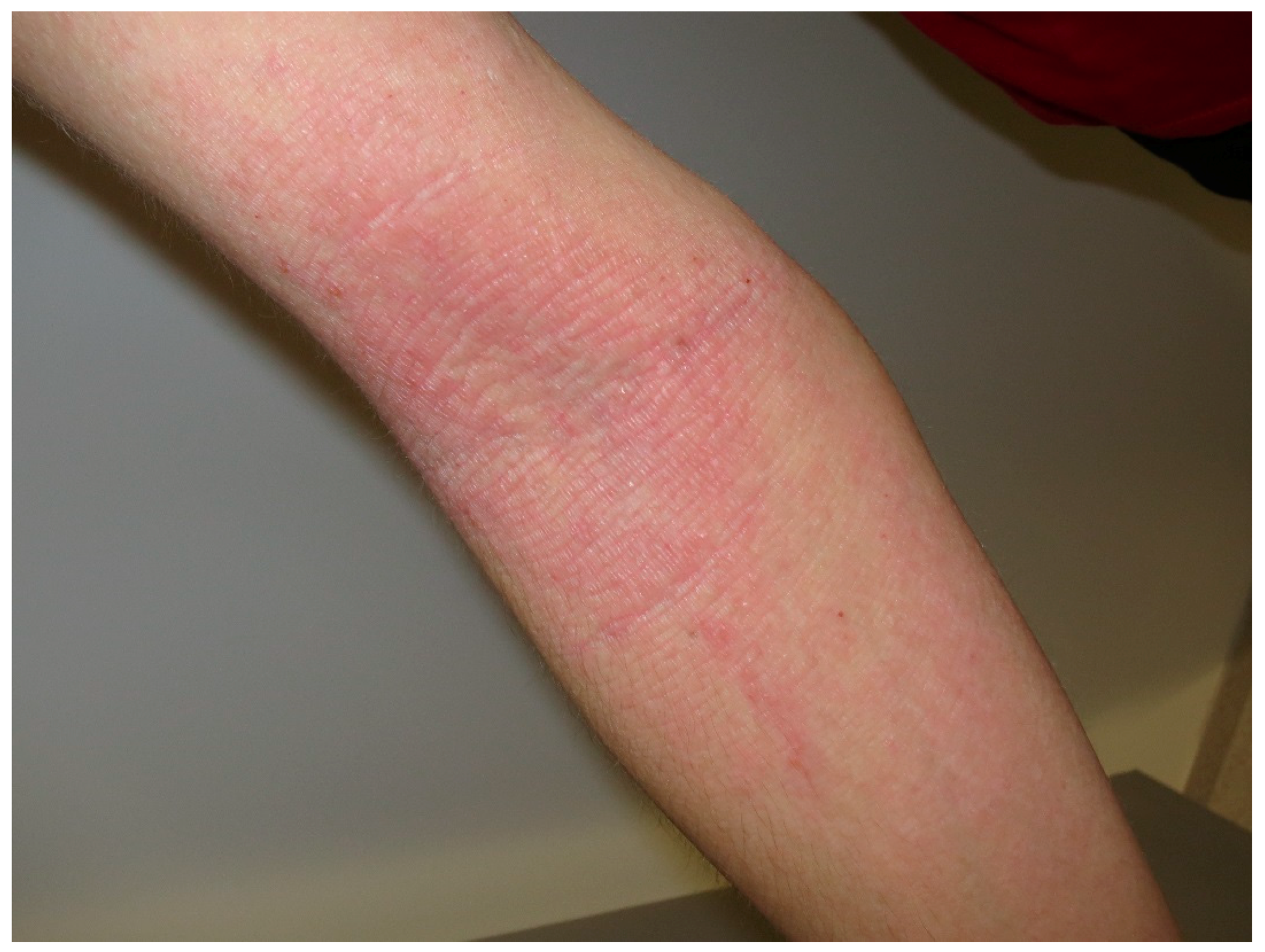
4. Urticaria
5. Atopic Dermatitis
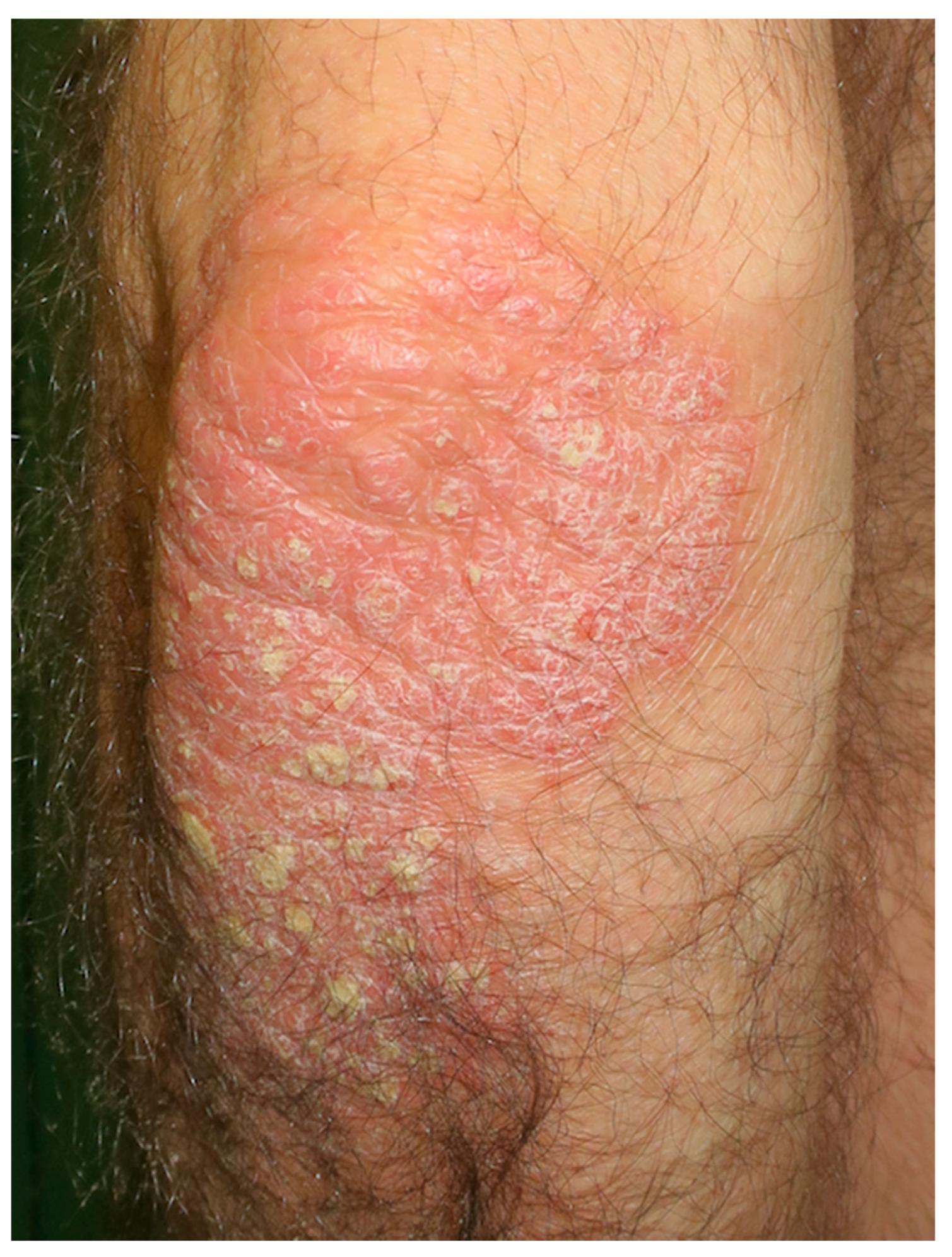
6. Psoriasis
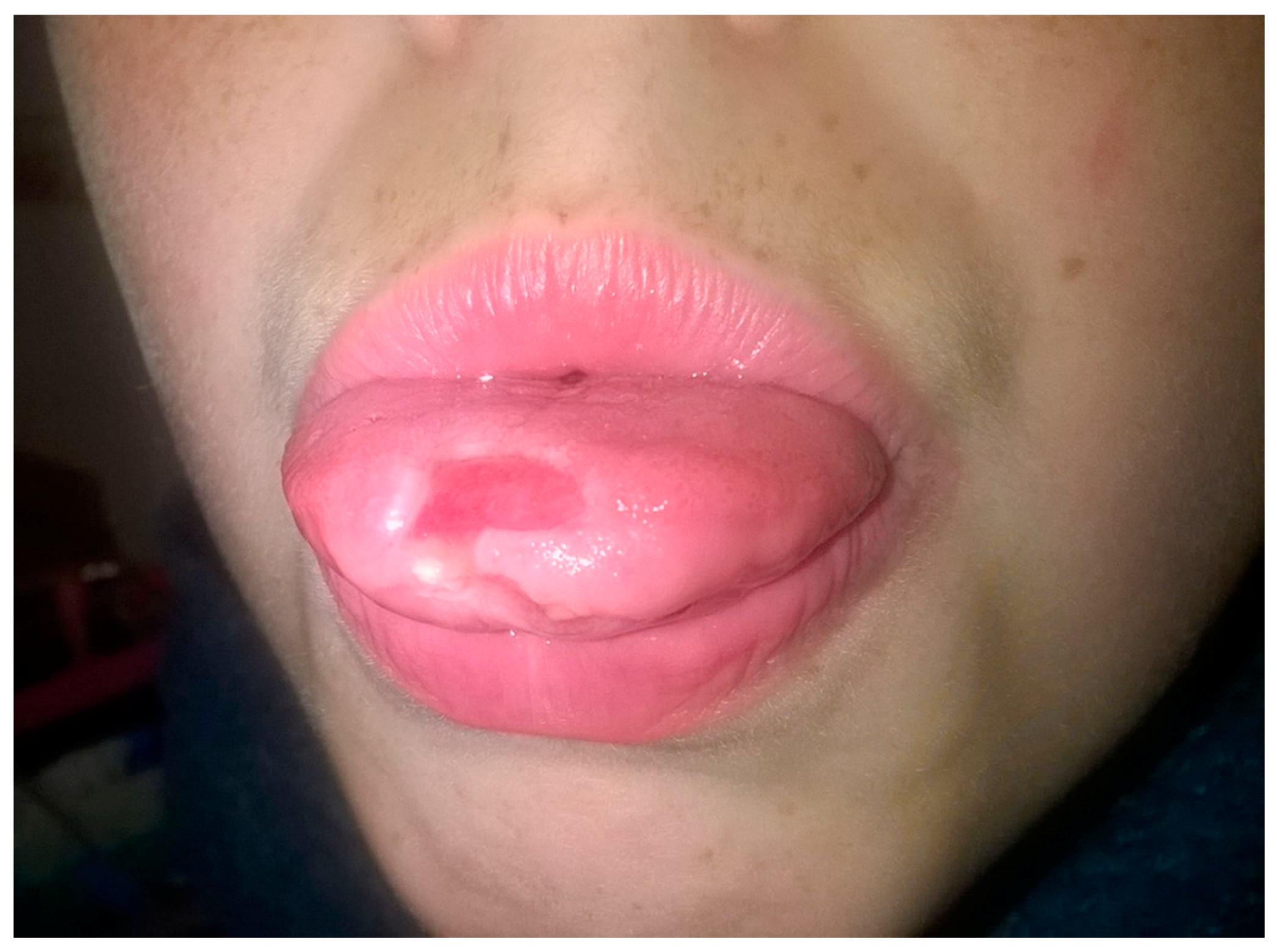
7. Aphthous Stomatitis
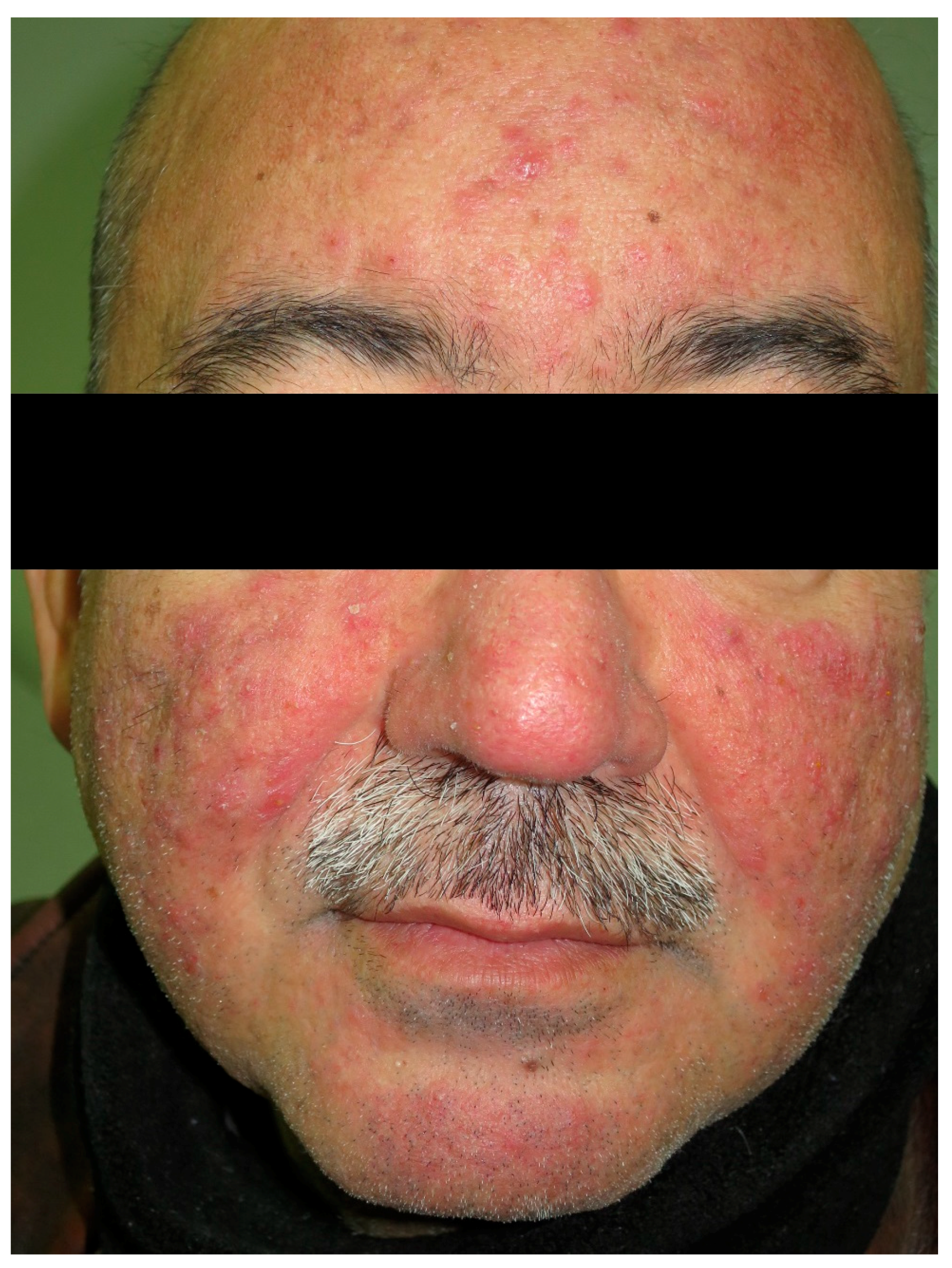
8. Rosacea
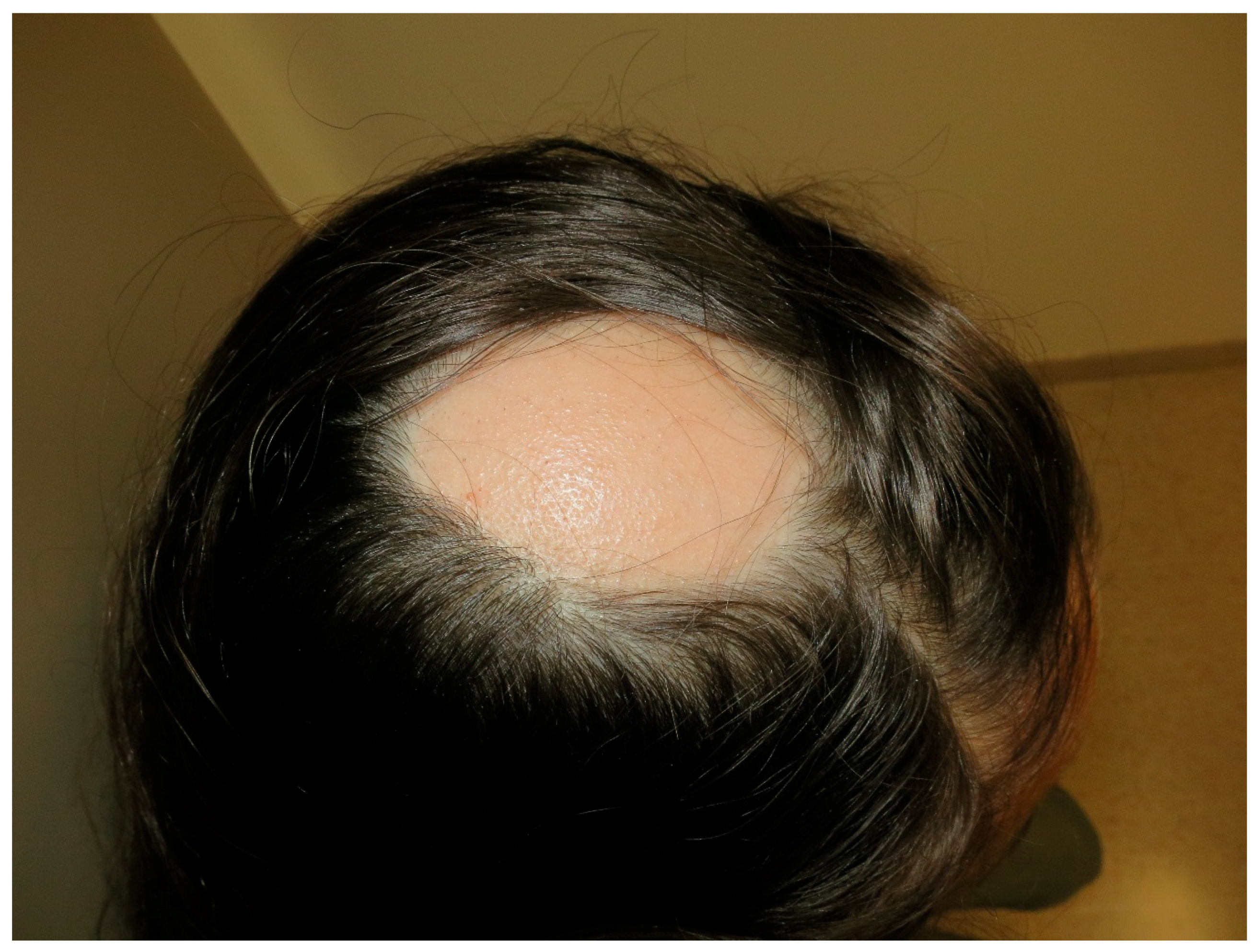
9. Alopecia Areata
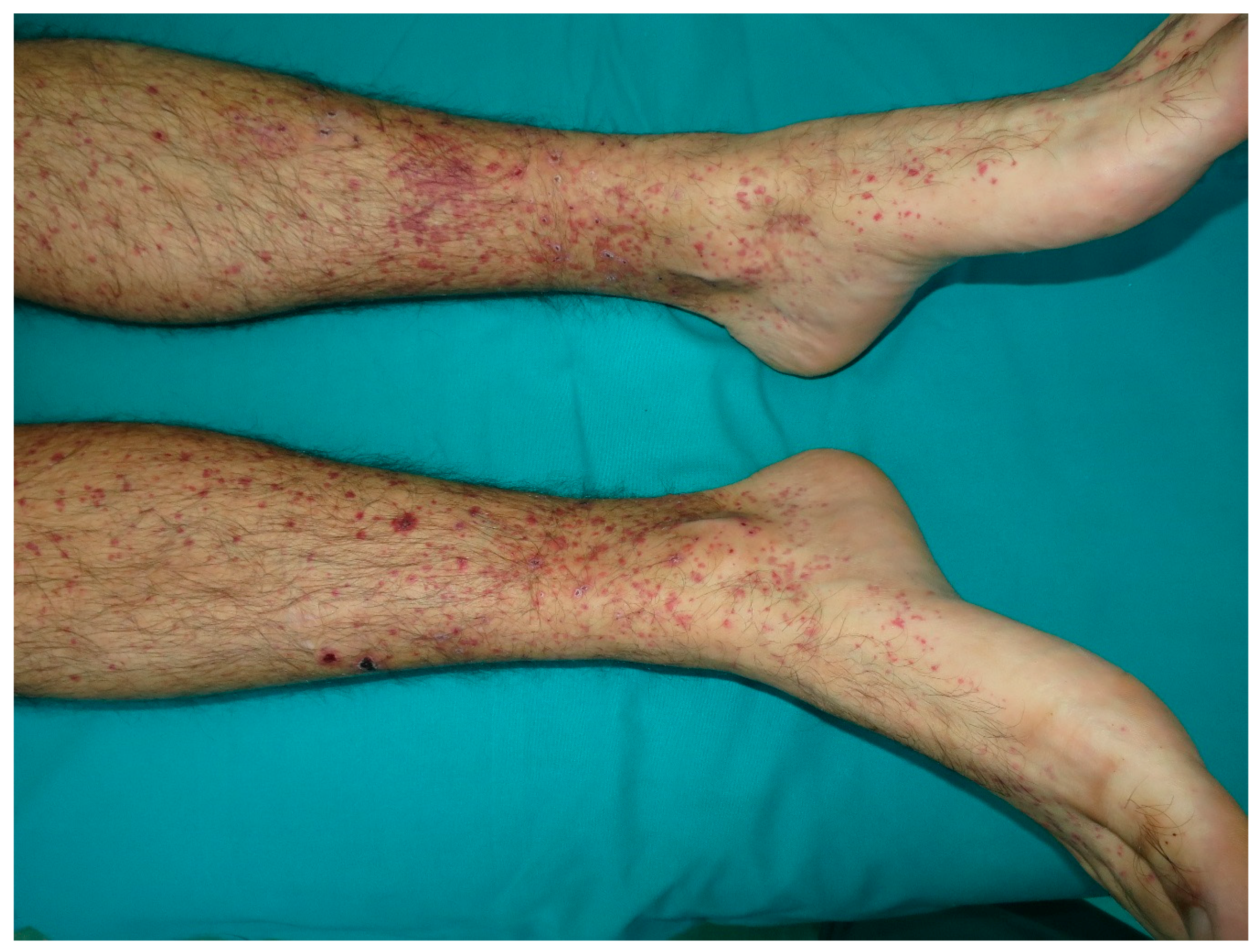
10. Cutaneous Vasculitis
11. Other Skin Conditions Found in Patients with CD
12. Other Oral Cavity Disorders
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bai, J.; Fried, M.; Corazza, G.; Schuppan, D.; Farthing, M.; Catassi, C.; Greco, L.; Cohen, H.; Ciacci, C.; Eliakim, R.; et al. World Gastroenterology Organisation Global Guidelines on celiac disease. J. Clin. Gastroenterol. 2013, 47, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- Collin, P.; Vilppula, A.; Luostarinen, L.; Holmes, G.K.T.; Kaukinen, K. Review article: Coeliac disease in later life must not be missed. Aliment. Pharmacol. Ther. 2018, 47, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, L. Celiac disease. World J. Gastroenterol. 2006, 12, 6585–6593. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Endocrine manifestations in celiac disease. World J. Gastroenterol. 2016, 22, 8472–8479. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Bonura, A.; Garavaglia, D.; Rulli, E.; Floriani, I.; Tagliabue, G.; Contiero, P.; Bardella, M. Immunological comorbity in coeliac disease: Associations, risk factors and clinical implications. J. Clin. Immunol. 2012, 32, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; Kruzliak, P.; Cangemi, G.; Pohanka, M.; Betti, E.; Lauret, E.; Rodrigo, L. The spectrum of differences between childhood and adulthood celiac disease. Nutrients 2015, 7, 8733–8751. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.; Cellier, C. Celiac disease. N. Engl. J. Med. 2007, 357, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Proietti, I.; Leggio, L.; Ferrulli, A.; Vonghia, L.; Capizzi, R.; Rotoli, M.; Amerio, P.L.; Gasbarrini, G.; Addolorato, G. Cutaneous manifestations in celiac disease. World J. Gastroenterol. 2006, 12, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Humbert, P.; Pelletier, F.; Dreno, B.; Puzenat, E.; Aubin, F. Gluten intolerance and skin diseases. Eur. J. Dermatol. 2006, 16, 4–11. [Google Scholar] [PubMed]
- Caproni, M.; Bonciolini, V.; D’Errico, A.; Antiga, E.; Fabbri, P. Celiac disease and dermatologic manifestations: Many skin clue to unfold gluten-sensitive enteropathy. Gastroenterol. Res. Pract. 2012, 2012, 952753. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Lindelöf, B.; Rashtak, S.; Rubio-Tapia, A.; Murray, J.A. Does urticaria risk increase in patients with celiac disease? A large population-based cohort study. Eur. J. Dermatol. 2013, 23, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, C.; Cavallaro, R.; Iovino, P.; Sabbatini, F.; Palumbo, A.; Amoruso, D.; Tortora, R.; Mazzacca, G. Allergy prevalence in adult celiac disease. J. Allergy Clin. Immunol. 2004, 113, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Lindelöf, B.; Zingone, F.; Ciacci, C. Psoriasis in a nationwide cohort study of patients with celiac disease. J. Investig. Dermatol. 2011, 131, 2010–2016. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Griffiths, C.E.M.; Mallbris, L.; Gislason, G.H.; Skov, L. The association between psoriasis and coeliac disease. Br. J. Dermatol. 2017, 177, e329–e330. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Wijarnpreecha, K.; Kittanamongkolchai, W. Psoriasis and risk of celiac disease: A systematic review and meta-analysis. Indian J. Dermatol. 2017, 62, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, B.; Millsop, J.; Debbaneh, M.; Koo, J.; Linos, E.; Liao, W. Diet and psoriasis, part II: Celiac disease and role of a gluten-free diet. Am. Acad. Dermatol. 2014, 71, 350–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieri, M.; Tofani, E.; Defraia, E.; Giuntini, V.; Franchi, L. Enamel defects and aphthous stomatitis in celiac and healthy subjects: Systematic review and meta-analysis of controlled studies. J. Dent. 2017, 65, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Weinstock, L.B.; Thyssen, E.P.; Gislason, G.H.; Thyssen, J.P. Rosacea and gastrointestinal disorders: A population-based cohort study. Br. J. Dermatol. 2017, 176, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Bonciolini, V.; Bianchi, B.; Del Bianco, E.; Verdelli, A.; Caproni, M. Cutaneous manifestations of non-celiac gluten sensitivity: Clinical histological and immunopathological features. Nutrients 2015, 7, 7798–7805. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Principi, M.; Iannone, A.; Amoruso, A.; Ierardi, E.; Di Leo, A.; Barone, M. Extra-intestinal manifestations of non-celiac gluten sensitivity: An expanding paradigm. World J. Gastroenterol. 2018, 24, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Bai, J.C.; Bonaz, B.; Bouma, G.; Calabrò, A.; Carroccio, A.; Castillejo, G.; Ciacci, C.; Cristofori, F.; Dolinsek, J.; et al. Non-Celiac Gluten sensitivity: The new frontier of gluten related disorders. Nutrients 2013, 5, 3839–3853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leffler, D.A.; Green, P.H.; Fasano, A. Extraintestinal manifestations of coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Grünewald, R.A.; Davies-Jones, G.A. Gluten sensitivity as a neurological illness. J. Neurol. Neurosurg. Psychiatry 2002, 72, 560–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, J.; Sollid, L.M. The human intestinal B-cell response. Mucosal Immunol. 2016, 9, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabri, B.; Sollid, L.M. T Cells in Celiac Disease. J. Immunol. 2017, 198, 3005–3014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reunala, T.; Salmi, T.T.; Hervonen, K.; Kaukinen, K.; Collin, P. Dermatitis Herpetiformis: A Common Extraintestinal Manifestation of Coeliac Disease. Nutrients 2018, 10, E602. [Google Scholar] [CrossRef] [PubMed]
- Champion, R.H.; Roberts, S.O.; Carpenter, R.G.; Roger, J. Urticaria and angio-oedema. A review of 554 patients. Br. J. Dermatol. 1969, 81, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Toubi, E.; Kessel, A.; Avshovich, N.; Bamberger, E.; Sabo, E.; Nusem, D.; Panasoff, J. Clinical and laboratory parameters in predicting chronic urticaria duration: A prospective study of 139 patients. Allergy 2004, 59, 869–873. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, B.; Lawlor, F.; Simpson, J.; Morgan, M.; Greaves, M. The impact of chronic urticaria on the quality of life. Br. J. Dermatol. 1997, 136, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Palikhe, N.; Sin, H.; Kim, S.; Sin, H.; Hwang, E.; Ye, Y.; Park, H. Genetic variability of prostaglandin E2 receptor subtype EP4 gene in aspirin-intolerant chronic urticaria. J. Hum. Genet. 2012, 57, 494–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dice, J.P. Physical urticaria. Immunol. Allergy Clin. 2004, 24, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Carr, W. Urticarial vasculitis. Allergy Asthma Proc. 2007, 28, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.; Robertson, L. Chronic urticaria and autoimmunity. Skin Ther. Lett. 2013, 18, 5–9. [Google Scholar]
- Kolkhir, P.; Church, M.; Weller, K.; Metz, M.; Schmetzer, O.; Maurer, M. Autoimmune chronic spontaneous urticaria: What we know and what we do not know. J. Allergy Clin. Immunol. 2017, 139, 1772–1781.e1. [Google Scholar] [CrossRef] [PubMed]
- O′Donnell, B.; O’Neill, C.; Francis, D.; Niimi, N.; Barr, R.; Barlow, R.; Kobza Black, A.; Welsh, K.; Greaves, M. Human leucocyte antigen class II associations in chronic idiopathic urticaria. Br. J. Dermatol. 1999, 140, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Piccini, B.; Vascotto, M.; Serracca, L.; Luddi, A.; Margollicci, M.; Balestri, P.; Vindigni, C.; Bassotti, G.; Villanacci, V. HLA-DQ typing in the diagnostic algorithm of celiac disease. Rev. Esp. Enferm. Dig. 2012, 104, 248–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hautekeete, M.; De Clerck, L.; Stevens, W. Chronic urticaria associated with coeliac disease. Lancet 1987, 329, 157. [Google Scholar] [CrossRef]
- Kolkhir, P.; Borzova, E.; Grattan, C.; Asero, R.; Pogorelov, D.; Maurer, M. Autoimmune comorbidity in chronic spontaneous urticaria: A systematic review. Autoimmun. Rev. 2017, 16, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, L.; Passalacqua, G.; Magazzu, G.; Comisi, F.; Vita, D.; Barberio, G.; Sferlazzas, C.; Pajno, G. Chronic urticaria and associated coeliac disease in children: A case-control study. Pediatr. Allergy Immunol. 2005, 16, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M.W. Chronic idiophatic urticarial. Curr. Opin. Allergy Clin. Immunol. 2003, 4, 363–368. [Google Scholar] [CrossRef]
- Vakharia, P.; Chopra, R.; Sacotte, R.; Patel, K.; Singam, V.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.; et al. Burden of skin pain in atopic dermatitis. Ann. Allergy Asthma Immunol. 2017, 119, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.; Garg, N.; Paller, A.; Fishbein, A.; Zee, P. Sleep Disturbances in Adults with Eczema Are Associated with Impaired Overall Health: A US Population-Based Study. J. Investig. Dermatol. 2015, 135, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Attarian, H.; Zee, P.; Silverberg, J. Burden of Sleep and Fatigue in US Adults With Atopic Dermatitis. Dermatitis 2016, 27, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Silverberg, J. Association between Atopic Dermatitis and Depression in US Adults. J. Investig. Dermatol. 2015, 135, 3183–3186. [Google Scholar] [CrossRef] [PubMed]
- Yaghmaie, P.; Koudelka, C.; Simpson, E. Mental health comorbidity in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2013, 131, 428–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strom, M.; Fishbein, A.; Paller, A.; Silverberg, J. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br. J. Dermatol. 2016, 175, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J. Associations between atopic dermatitis and other disorders. F1000Resarch 2018, 7, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plötz, S.; Wiesender, M.; Todorova, A.; Ring, J. What is new in atopic dermatitis/eczema? Expert Opin. Emerg. Drugs 2014, 19, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Herd, R.M.; Tidman, M.J.; Prescott, R.J.; Hunter, J.A. Prevalence of atopic eczema in the community: The Lothian atopic dermatitis study. Br. J. Dermatol. 1996, 135, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. Atopic Dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Alduraywish, S.; Lodge, C.; Campbell, B.; Allen, K.; Erbas, B.; Lowe, A.; Dharmage, S. The march from early life food sensitization to allergic disease: A systematic review and meta-analyses of birth cohort studies. Allergy 2015, 71, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.; Moran, T.; Floudas, A.; Wurlod, F.; Kaszlikowska, A.; Salimi, M.; Quinn, E.; Oliphant, C.; Núñez, G.; McManus, R.; et al. Spontaneous atopic dermatitis is mediated by innate immunity, with the secondary lung inflammation of the atopic march requiring adaptive immunity. J. Allergy Clin. Immunol. 2016, 137, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, N.; Kim, B.; Jung, M.; Kim, D.; Moniaga, C.; Kabashima, K.; Choi, E. Acidification of stratum corneum prevents the progression from atopic dermatitis to respiratory allergy. Exp. Dermatol. 2017, 26, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Otsuka, A.; Nomura, T. Linking air pollution to atopic dermatitis. Nat. Immunol. 2016, 18, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Hanakawa, S.; Kabashima, K. Classification of inflammatory skin diseases: A proposal based on the disorders of the three-layered defense systems, barrier, innate immunity and acquired immunity. J. Dermatol. Sci. 2014, 76, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiwakura, J.; Okayama, Y.; Furue, M.; Kabashima, K.; Shimada, S.; Ra, C.; Siraganian, R.; Kawakami, Y.; Kawakami, T. Most Highly Cytokinergic IgEs Have Polyreactivity to Autoantigens. Allergy Asthma Immunol. Res. 2012, 4, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rerknimitr, P.; Otsuka, A.; Nakashima, C.; Kabashima, K. The etiopathogenesis of atopic dermatitis: Barrier disruption, immunological derangement, and pruritus. Inflamm. Regen. 2017, 37, 14. [Google Scholar] [CrossRef] [PubMed]
- Ress, K.; Annus, T.; Putnik, U.; Luts, K.; Uibo, R.; Uibo, O. Celiac Disease in Children with Atopic Dermatitis. Pediatr. Dermatol. 2014, 31, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, T.; Schupp, C.; Armstrong, A. Psoriasis prevalence among adults in the United States. J. Am. Acad. Dermatol. 2014, 70, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Christophers, E. Psoriasis—Epidemiology and clinical spectrum. Clin. Exp. Dermatol. 2001, 26, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, J.; Grewal, S.; Langan, S.; Mehta, N.; Ogdie, A.; Van Voorhees, A.; Gelfand, J. Psoriasis and comorbid diseases. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehncke, W.; Boehncke, S. More than skin-deep: The many dimensions of the psoriatic disease. Swiss Med. Wkly. 2014, 144, w13968. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.; Shuster, S. Intestinal malabsorption and the skin. Gut 1971, 12, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; Aguilar Sánchez, J.; Guerriero, C.; Fossati, B.; Capizzi, R.; De Simmone, C.; Migneco, A.; Amerio, P.; Gasbarrini, G.; Gasbarrini, A. High prevalence of celiac disease in psoriasis. Am. J. Gastroenterol. 2003, 98, 2574–2575. [Google Scholar] [CrossRef]
- Michaelsson, G.; Gerden, B.; Ottosson, M.; Parra, A.; Sjoberg, O.; Hjelmquist, G.; Loof, L. Patients with psoriasis often have increased serum levels of IgA antibodies to gliadin. Br. J. Dermatol. 1993, 129, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, S.; Gür, G.; Topal, F.; Senel, E.; Topal, F.; Alli, N.; Saritas, Ü. Coeliac Disease-Associated Antibodies in Psoriasis. Ann. Dermatol. 2013, 25, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagui, N.; El Nabarawy, E.; Mahgoub, D.; Mashaly, H.; Saad, N.; El-Deeb, D. Estimation of (IgA) anti-gliadin, anti-endomysium and tissue transglutaminase in the serum of patients with psoriasis. Clin. Exp. Dermatol. 2011, 36, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Damasiewicz-Bodzek, A.; Wielkoszyński, T. Serologic markers of celiac disease in psoriatic patients. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sonkar, G.; Usha; Singh, S. Celiac disease-associated antibodies in patients with psoriasis and correlation with HLA Cw6. J. Clin. Lab. Anal. 2010, 24, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; De Simone, C.; Aguilar Sanchez, J.; Capizzi, R.; Migneco, A.; Guerriero, C.; Cazzato, A.; Gasbarrini, G.; Amerio, P.; Gasbarrini, A. Malabsorption in psoriatic patients: Cause or consequence? Scand. J. Gastroenterol. 2006, 41, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.; Boer, W.; Haas, F. Is there a relationship between psoriasis and coeliac disease? J. Intern. Med. 1995, 237, 118. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Ahmad, Q.; Sultan, S. Antigliadin antibodies in psoriasis. Australas. J. Dermatol. 2010, 51, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Zamani, F.; Alizadeh, S.; Amiri, A.; Shakeri, R.; Robati, M.; Alimohamadi, S.; Abdi, H.; Malekzadeh, R. Psoriasis and Coeliac Disease; Is There Any Relationship? Acta Derm.-Venereol. 2010, 90, 295–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kia, K.; Nair, R.; Ike, R.; Hiremagalore, R.; Elder, J.; Ellis, C. Prevalence of Antigliadin Antibodies in Patients with Psoriasis is Not Elevated Compared with Controls. Am. J. Clin. Dermatol. 2007, 8, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, C.; Degl’innocenti, D.; Caproni, M.; Fabbri, P. Is the search for serum antibodies to gliadin, endomysium and tissue transglutaminase meaningful in psoriatic patients? Relationship between the pathogenesis of psoriasis and coeliac disease. Br. J. Dermatol. 2002, 147, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Woo, W.; McMillan, S.; Watson, R.; Mccluggage, W.; Sloan, J.; McMillan, J. Coeliac disease-associated antibodies correlate with psoriasis activity. Br. J. Dermatol. 2004, 151, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, U.; Rudsander, Å.; Boström, Å.; Nilsson, B.; Michaëlsson, G. IgA antibodies to gliadin and coeliac disease in psoriatic arthritis. Rheumatology 2002, 41, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrzak, D.; Pietrzak, A.; Krasowska, D.; Borzęcki, A.; Franciszkiewicz-Pietrzak, K.; Polkowska-Pruszyńska, B.; Baranowska, M.; Reich, K. Digestive system in psoriasis: An update. Arch. Dermatol. Res. 2017, 309, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Counsell, C.E.; Taha, A.; Ruddell, W. Coeliac disease and autoimmune thyroid disease. Gut 1994, 35, 844–846. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Hahn, E. Celiac Disease and its Link to Type 1 Diabetes Mellitus. J. Pediatr. Endocrinol. Metab. 2001, 14 (Suppl. S1), 597–605. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, H.; Nikamo, P.; Qi Low, H.; Helms, C.; Seielstad, M.; Liu, J.; Bowcock, A.; Stahle, M.; Liao, W. Association of Cardiovascular and Metabolic Disease Genes with Psoriasis. J. Investig. Dermatol. 2013, 133, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Trynka, G.; Hunt, K.; Bockett, N.; Romanos, J.; Mistry, V.; Szperl, A.; Bakker, S.; Bardella, M.; Bhaw-Rosun, L.; Castillejo, G.; et al. Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nat. Genet. 2011, 43, 1193–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoi, L.; Spain, S.; Knight, J.; Ellinghaus, E.; Stuart, P.; Capon, F.; Ding, J.; Li, Y.; Tejasvi, T.; Gudjonsson, J.; et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat. Genet. 2012, 44, 1341–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkenfeld, S.; Dreiher, J.; Weitzman, D.; Cohen, A. Coeliac disease associated with psoriasis. Br. J. Dermatol. 2009, 161, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.; Magazzù, G.; Greco, L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. Gastroenterology 1999, 117, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Holick, M. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef] [PubMed]
- De Bastiani, R.; Gabrielli, M.; Lora, L.; Napoli, L.; Tosetti, C.; Pirrotta, E.; Ubaldi, E.; Bertolusso, L.; Zamparella, M.; De Polo, M.; et al. Association between Coeliac Disease and Psoriasis: Italian Primary Care Multicentre Study. Dermatology 2015, 230, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Kolchak, N.; Tetarnikova, M.; Theodoropoulou, M.; Michalopoulou, A.; Theodoropoulos, D. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J. Multidiscip. Healthc. 2017, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Pastore, L.; Carroccio, A.; Compilato, D.; Panzarella, V.; Serpico, R.; Muzio, L. Oral Manifestations of Celiac Disease. J. Clin. Gastroenterol. 2008, 42, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Chavan, M.; Jain, H.; Diwan, N.; Khedkar, S.; Shete, A.; Durkar, S. Recurrent aphthous stomatitis: A review. J. Oral Pathol. Med. 2012, 41, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Lahteenoja, H.; Toivanen, A.; Viander, M.; Maki, M.; Irjala, K.; Raiha, I.; Syrjanen, S. Oral mucosal changes in coeliac patients on a gluten-free diet. Eur. J. Oral Sci. 1998, 106, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Bucci, P.; Carile, F.; Sangianantoni, A.; D’Angiò, F.; Santarelli, A.; Muzio, L. Oral aphthous ulcers and dental enamel defects in children with coeliac disease. Acta Paediatr. 2007, 95, 203–207. [Google Scholar] [CrossRef]
- Macho, V.; Coelho, A.; Veloso e Silva, D.M.; Andrade, D. Oral Manifestations in Pediatric Patients with Coeliac Disease—A Review Article. Open Dent. J. 2017, 11, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Zarkadas, M.; Anca, A.; Limeback, H. Oral manifestations of celiac disease: A clinical guide for dentists. J. Can. Dent. Assoc. 2011, 77, b39. [Google Scholar] [PubMed]
- Ferguson, R.; Basu, M.; Asquith, P.; Cooke, W. Jejunal mucosal abnormalities in patients with recurrent aphthous ulceration. BMJ 1976, 1, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Wray, D.; Carmichael, H.; Russell, R.; Lee, F. Coeliac disease associated with recurrent aphthae. Gut 1980, 21, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Wray, D. Gluten-sensitive recurrent aphthous stomatitis. Dig. Dis. Sci. 1981, 26, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Two, A.M.; Wu, W.; Gallo, R.L.; Hata, T.R. Rosacea: Part I. Introduction, categorization, histology, pathogenesis, and risk factors. J. Am. Acad. Dermatol. 2015, 72, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.D.; Steinhoff, M. Integrative concepts of rosacea pathophysiology, clinical presentation and new therapeutics. Exp. Dermatol. 2017, 26, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.L.S.; Raber, I.; Xu, J.; Li, R.; Spitale, R.; Chen, J.; Kiefer, A.K.; Tian, C.; Eriksson, N.K.; Hinds, D.A.; et al. Assessment of the genetic basis of rosacea by genome-wide association study. J. Investig. Dermatol. 2015, 135, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Hansen, P.; Gislason, G.; Thyssen, J. Clustering of autoimmune diseases in patients with rosacea. J. Am. Acad. Dermatol. 2016, 74, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Darwin, E.; Hirt, P.A.; Fertig, R.; Doliner, B.; Delcanto, G.; Jimenez, J.J. Alopecia Areata: Review of Epidemiology, Clinical Features, Pathogenesis, and New Treatment Options. Int. J. Trichol. 2018, 10, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Strazzulla, L.; Wang, E.H.C.; Avila, L.; Lo Sicco, K.; Brinster, N.; Christiano, A.M.; Shapiro, J. Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J. Am. Acad. Dermatol. 2018, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, V.; Tosun, M.; Erdem, T. Screening of celiac disease in children with alopecia areata. Indian J. Dermatol. 2014, 59, 317. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.R.; Andreani, M.L.; Venturo, N.; Bernardi, M.; Tosti, A.; Gasbarrini, G. Celiac disease and alopecia areata: Report of a new association. Gastroenterology 1995, 109, 1333–1337. [Google Scholar] [CrossRef]
- Volta, U.; Bardazzi, F.; Zauli, D.; Franceschi, L.; Tosti, A.; Mounaro, N.; Ghetti, S.; Tetta, C.; Grassi, A.; Bianchi, F. Serological screening for coeliac disease in vitiligo and alopecia areata. Br. J. Dermatol. 1997, 136, 801–802. [Google Scholar] [CrossRef] [PubMed]
- Hallaji, Z.; Akhyani, M.; Ehsani, A.H.; Noormohammadpour, P.; Gholamali, F.; Bagheri, M.; Jahromi, J. Prevalence of anti-gliadin antibody in patients with alopecia areata: A case-control study. Tehran Univ. Med. J. 2011, 68, 738–742. [Google Scholar]
- Collin, P.; Reunala, T. Recognition and Management of the Cutaneous Manifestations of Celiac Disease. Am. J. Clin. Dermatol. 2003, 4, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, F.; Panjehpour, T.; Naeini, F.; Hosseini, S.; Nilforoushzadeh, M.; Matin, M. The frequency distribution of celiac autoantibodies in alopecia areata. Int. J. Prev. Med. 2016, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Naveh, Y.; Rosenthal, E.; Ben-Arieh, Y.; Etzioni, A. Celiac disease-associated alopecia in childhood. J. Pediatr. 1999, 134, 362–364. [Google Scholar] [CrossRef]
- Baigrie, D.; Crane, J.S. Leukocytoclastic Vasculitis (Hypersensitivity Vasculitis); StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Pulido-Pérez, A.; Avilés-Izquierdo, J.; Suárez-Fernández, R. Cutaneous vasculitis. Actas Dermo-Sifiliogr. 2012, 103, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Holdstock, D.; Oleesky, S. Vasculitis in coeliac diseases. BMJ 1970, 4, 369. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.A. The skin: A mirror of the gut. Geriatrics 1973, 28, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Similä, S.; Kokkonen, J.; Kallioinen, M. Cutaneous vasculitis as a manifestation of coeliac disease. Acta Paediatr. Scand. 1982, 71, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Meyers, S.; Dikman, S.; Spiera, H.; Schultz, N.; Janowitz, H. Cutaneous vasculitis complicating coeliac disease. Gut 1981, 22, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Alegre, V.; Winkelmann, R.; Diez-Martin, J.; Banks, P. Adult celiac disease, small and medium vessel cutaneous necrotizing vasculitis, and T cell lymphoma. J. Am. Acad. Dermatol. 1988, 19, 973–978. [Google Scholar] [CrossRef]
- Menzies, I.; Pounder, R.; Heyer, S.; Laker, M.; Bull, J.; Wheeler, P.; Creamer, B. Abnormal intestinal permeability to sugars in villous atrophy. Lancet 1979, 314, 1107–1109. [Google Scholar] [CrossRef]
- Buderus, S.; Wagner, N.; Lentze, M. Concurrence of Celiac Disease and Juvenile Dermatomyositis: Result of a Specific Immunogenetic Susceptibility? J. Pediatr. Gastroenterol. Nutr. 1997, 25, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Farber, D.; Bitton, A.; Jass, J.; Singer, M.; Karpati, G. Dermatomyositis Associated with Celiac Disease: Response to a Gluten-Free Diet. Can. J. Gastroenterol. 2006, 20, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Evron, E.; Abarbanel, J.M.; Branski, D.; Sthoeger, Z.M. Polymyositis, arthritis, and proteinuria in a patient with adult celiac disease. J. Rheumatol. 1996, 23, 782–783. [Google Scholar] [PubMed]
- Hadjivassiliou, M.; Chattopadhyay, A.; Grünewald, R.; Jarratt, J.; Kandler, R.; Rao, D.; Sanders, D.; Wharton, S.; Davies-Jones, G. Myopathy associated with gluten sensitivity. Muscle Nerve 2007, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Shahmoradi, Z.; Najafian, J.; Naeini, F.F.; Fahimipour, F. Vitiligo and autoantibodies of celiac disease. Int. J. Prev. Med. 2013, 4, 200–203. [Google Scholar] [PubMed]
- Rodríguez-García, C.; González-Hernández, S.; Pérez-Robayna, N.; Guimerá, F.; Fagundo, E.; Sánchez, R. Repigmentation of Vitiligo Lesions in a Child with Celiac Disease after a Gluten-Free Diet. Pediatr. Dermatol. 2011, 28, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Mirza, N.; Bonilla, E.; Phillips, P. Celiac disease in a patient with systemic lupus erythematosus: A case report and review of literature. Clin. Rheumatol. 2006, 26, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Adult celiac disease followed by onset of systemic lupus erythematosus. J. Clin. Gastroenterol. 2008, 42, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Jamal, A.; Memon, I.; Yasmeen, S.; Tresa, V.; Shaikh, S. Multiple autoimmune syndrome: Hashimoto’s thyroiditis, Coeliac disease and systemic lupus erythematosus (SLE). J. Pak. Med. Assoc. 2010, 60, 863–865. [Google Scholar] [PubMed]
- Cigic, L.; Gavic, L.; Simunic, M.; Ardalic, Z.; Biocina-Lukenda, D. Increased prevalence of celiac disease in patients with oral lichen planus. Clin. Oral Investig. 2015, 19, 627–635. [Google Scholar] [CrossRef] [PubMed]
- De, D. Eruptive lichen planus in a child with celiac disease. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Villaverde, R.; Blasco Melguizo, J.; Menéndez García Estrada, A.; Díez García, F. Erosive mucosal lichen associated to hyper IgE syndrome and coeliac disease. An. Pediatr. 2004, 60, 281–282. [Google Scholar] [CrossRef]
- Scully, C.; Porter, S.R.; Eveson, J.W. Oral lichen planus and coeliac disease. Lancet 1993, 341, 1660. [Google Scholar] [CrossRef]
- Fortune, F.; Buchanan, J. Oral lichen planus and coeliac disease. Lancet 1993, 341, 1154–1155. [Google Scholar] [CrossRef]
- Compilato, D.; Carroccio, A.; Campisi, G. Hidden coeliac disease in patients suffering from oral lichen planus. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 390–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karadag, A.; Kavala, M.; Ozlu, E.; Zindancı, İ.; Ozkanlı, S.; Turkoglu, Z.; Zemheri, E. The co-occurrence of lichen sclerosus et atrophicus and celiac disease. Indian Dermatol. Online J. 2014, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.; Gilliam, A.; Khavari, N.; Bass, D. Association between Lichen Sclerosus and Celiac Disease: A Report of Three Pediatric Cases. Pediatr. Dermatol. 2014, 31, e128–e131. [Google Scholar] [CrossRef] [PubMed]
- Essoussi, A.S.; Jomaa, B.; El Fehaiel, A. Association of celiac disease with sclero-atrophic lichen in a child with the HLA-B8 group. Tunis. Med. 1981, 59, 506–507. [Google Scholar] [PubMed]
- Daoud, W.; El Euch, D.; Mokni, M.; Cherif, F.; Ben Tekaya, N.; Azaiz, M.I.; Ben Osman-Dhahri, A. Linear IgA bullous dermatosis associated with celiac disease. Ann. Dermatol. Venereol. 2006, 133, 588–589. [Google Scholar] [CrossRef]
- Vaz, S.; Franco, C.; Santos, P.; Amaral, R. Skin and coeliac disease, a lot to think about: A case series. BMJ Case Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bonciolini, V.; Antiga, E.; Fabbri, P.; Caproni, M. Skin manifestations of celiac disease: Not always dermatitis herpetiformis. Int. J. Dermatol. 2014, 53, e352–e353. [Google Scholar] [CrossRef] [PubMed]
- Randle, H.W.; Winkelmann, R.K. Pityriasis rubra pilaris and celiac sprue with malabsorption. Cutis 1980, 25, 626–627. [Google Scholar] [PubMed]
- Tasanen, K.; Raudasoja, R.; Kallioinen, M.; Ranki, A. Erythema elevatum diutinum in association with coeliac disease. Br. J. Dermatol. 1997, 136, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Collin, P.; Korpela, M.; Hällström, O.; Viander, M.; Keyriläinen, O.; Mäki, M. Rheumatic Complaints as a Presenting Symptom in Patients with Coeliac Disease. Scand. J. Rheumatol. 1992, 21, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Serna, M.; Fortea, J.; Perez, A.; Febrer, I.; Ribes, C.; Aliaga, A. Erythema elevatum diutinum associated with celiac disease: Response to a gluten-free diet. Pediatr. Dermatol. 1993, 10, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Goodenberger, D.M.; Lawley, T.J.; Strober, W.; Wyatt, L.; Sangree, M.H., Jr.; Sherwin, R.; Rosenbaum, H.; Braverman, I.; Katz, S.I. Necrolytic Migratory Erythema Without Glucagonoma. Arch. Dermatol. 1979, 115, 1429–1432. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Johnston, C.; Nolan, N.; Keeling, P.; Weir, D. Necrolytic Migratory Erythema with Elevated Plasma Enteroglucagon in Celiac Disease. Gastroenterology 1989, 96, 1350–1353. [Google Scholar] [CrossRef]
- Thorisdottir, K.; Camisa, C.; Tomecki, K.; Bergfeld, W. Necrolytic migratory erythema: A report of three cases. J. Am. Acad. Dermatol. 1994, 30, 324–329. [Google Scholar] [CrossRef]
- Cribier, B.; Caille, A.; Heid, E.; Grosshans, E. Erythema nodosum and associated diseases. A study of 129 cases. Int. J. Dermatol. 1998, 37, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.; Lefevre, P.; Weiller, C. Erythema nodosum and coeliac disease. Br. J. Dermatol. 1991, 125, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Bartyik, K.; Varkonyi, A.; Kirschner, A.; Endreffy, E.; Turi, S.; Karg, E. Erythema Nodosum in Association with Celiac Disease. Pediatr. Dermatol. 2004, 21, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Twaddle, S.; Wassif, W.S.; Deacon, A.C.; Peters, T.J. Celiac disease in patients with variegate porphyria. Dig. Dis. Sci. 2001, 46, 1506–1508. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.; Disler, P. Drug sensitive diseases-I-acute porphyrias. Advers. Drug React. Bull. 1988, 129, 484–487. [Google Scholar] [CrossRef]
- Katsikas, G.; Maragou, M.; Rontogianni, D.; Gouma, P.; Koutsouvelis, I.; Kappou-Rigatou, I. Secondary cutaneous nodular AA amyloidosis in a patient with primary Sjögren syndrome and celiac disease. J. Clin. Rheumatol. 2008, 14, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.; Lewis-Jones, S.; Gipson, M. Acquired cutis laxa with dermatitis herpetiformis and sarcoidosis. J. Am. Acad. Dermatol. 1993, 29, 846–848. [Google Scholar] [CrossRef]
- García-Patos, V.; Pujol, R.; Barnadas, M.; Pérez, M.; Moreno, A.; Condomines, J.; Gelpi, C.; Rodríguez, J.; De Moragas, J. Generalized acquired cutis laxa associated with coeliac disease: Evidence of immunoglobulin A deposits on the dermal elastic fibres. Br. J. Dermatol. 1996, 135, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.; Floridis, J. Hypertrichosis Lanuginosa acquisita: A rare dermatological disorder. Lancet 2016, 387, 2035. [Google Scholar] [CrossRef]
- Menni, S.; Boccardi, D.; Brusasco, A. Ichthyosis revealing coeliac disease. Eur. J. Dermatol. 2000, 10, 398–399. [Google Scholar] [PubMed]
- O’Mahony, D.; O’Mahony, S.; Whelton, M.; McKiernan, J. Partial lipodystrophy in coeliac disease. Gut 1990, 31, 717–718. [Google Scholar] [CrossRef] [PubMed]
- Foti, C.; Cassano, N.; Palmieri, V.O.; Portincasa, P.; Conserva, A.; Lamuraglia, M.; Palasciano, G.; Vena, G.A. Transverse leukonychia in severe hypocalcemia. Eur. J. Dermatol. 2004, 14, 67–68. [Google Scholar] [PubMed]
- Montalto, M.; Diociaiuti, A.; Alvaro, G.; Manna, R.; Amerio, P.L.; Gasbarrini, G. Atypical mole syndrome and congenital giant naevus in a patient with celiac disease. Panminerva Med. 2003, 45, 219–221. [Google Scholar] [PubMed]
- Lebwohl, B.; Eriksson, H.; Hansson, J.; Green, P.; Ludvigsson, J. Risk of cutaneous malignant melanoma in patients with celiac disease: A population-based study. J. Am. Acad. Dermatol. 2014, 71, 245–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastore, L.; De Benedittis, M.; Petruzzi, M.; Tatò, D.; Napoli, C.; Montagna, M.T.; Catassi, C.; Serpico, R. Importance of oral signs in the diagnosis of atypical forms of celiac disease. Recenti Prog. Med. 2004, 95, 482–490. [Google Scholar] [PubMed]
- Lahteenoja, H. Oral Mucosa Is Frequently Affected in Patients with Dermatitis Herpetiformis. Arch. Dermatol. 1998, 134, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, E.; Cicciù, M.; Matacena, G.; Costa, S.; Magazzù, G. Clinical Evaluation of Specific Oral Manifestations in Pediatric Patients with Ascertained versus Potential Coeliac Disease: A Cross-Sectional Study. Gastroenterol. Res. Pract. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Clinical presentation of celiac disease in the pediatric population. Gastroenterology 2005, 128, S68–S73. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Newnham, E.D. Coeliac disease in the 21st century: Paradigm shifts in the modern age. J. Gastroenterol. Hepatol. 2017, 32, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Rostami Nejad, M.; Hogg-Kollars, S.; Ishaq, S.; Rostami, K. Subclinical celiac disease and gluten sensitivity. Gastroenterol. Hepatol. Bed Bench 2011, 4, 102–108. [Google Scholar] [PubMed]
- Tonutti, E.; Bizzaro, N. Diagnosis and classification of celiac disease and gluten sensitivity. Autoimmun. Rev. 2014, 13, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Gatti, S.; Pulvirenti, A.; Catassi, C. Celiac disease from a global perspective. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Card, T.R.; Kaukinen, K.; Bai, J.; Zingone, F.; Sanders, D.S.; Murray, J.A. Screening for celiac disease in the general population and in high-risk groups. United Eur. Gastroenterol. J. 2015, 3, 106–120. [Google Scholar] [CrossRef] [PubMed]

| Type of Mechanism | Diseases Found in Association with Celiac Disease | Relative Risk in Celiac Disease Compared to the General or Control Population [Reference] | Fortuitous Association (Sporadic Cases) |
|---|---|---|---|
| Allergic | Urticaria Chronic urticaria Atopic dermatitis | HR: 1.51 (CI = 1.36–1.68) [12] HR: 1.92 (CI = 1.48–2.48) [12] OR:3.17 (CI = 1.02–9.82) [13] | Prurigo nodularis |
| Inflammatory | Pityriasis rubra pilaris Erythroderma Erythema elevatum diutinum Necrolytic migratory erythema Pityriasis lichenoides Erythema nodosum | ||
| Immune-mediated | Psoriasis | HR: 1.72 (CI = 1.54–1.92) [14] OR: 1.44 (CI = 1.40–1.92) [15] OR: 3.09 (CI = 1.92–4.97) [16] IgA anti-gliadin: OR: 2.36 (CI = 1.15–4.83) [17] | |
| Autoimmune | Alopecia areata Cutaneous vasculitis Ig A linear dermatosis Dermatomyositis Vitiligo Lupus erythematous Lichen sclerosus | ||
| Miscellaneous | Aphthous stomatitis Rosacea | OR: 3.79 (CI = 2.67–5.39) [18] HR: 1.46 (CI = 1.11–1.93) [19] | Cutaneous amyloidosis Annular erythema Partial lipodystrophy Generalized acquired cutis laxa Icthyosis Transverse leukonychia Porphyria Hypertricosis lanuginosa |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo, L.; Beteta-Gorriti, V.; Alvarez, N.; Gómez de Castro, C.; De Dios, A.; Palacios, L.; Santos-Juanes, J. Cutaneous and Mucosal Manifestations Associated with Celiac Disease. Nutrients 2018, 10, 800. https://doi.org/10.3390/nu10070800
Rodrigo L, Beteta-Gorriti V, Alvarez N, Gómez de Castro C, De Dios A, Palacios L, Santos-Juanes J. Cutaneous and Mucosal Manifestations Associated with Celiac Disease. Nutrients. 2018; 10(7):800. https://doi.org/10.3390/nu10070800
Chicago/Turabian StyleRodrigo, Luis, Valia Beteta-Gorriti, Nuria Alvarez, Celia Gómez de Castro, Alvaro De Dios, Laura Palacios, and Jorge Santos-Juanes. 2018. "Cutaneous and Mucosal Manifestations Associated with Celiac Disease" Nutrients 10, no. 7: 800. https://doi.org/10.3390/nu10070800
APA StyleRodrigo, L., Beteta-Gorriti, V., Alvarez, N., Gómez de Castro, C., De Dios, A., Palacios, L., & Santos-Juanes, J. (2018). Cutaneous and Mucosal Manifestations Associated with Celiac Disease. Nutrients, 10(7), 800. https://doi.org/10.3390/nu10070800







