Abstract
Celiac disease (CD) is an immune-mediated, gluten-induced enteropathy that affects predisposed individuals of all ages. Many patients with CD do not report gastrointestinal symptoms making it difficult to reach an early diagnosis. On the other hand, CD is related to a wide spectrum of extra-intestinal manifestations, with dermatitis herpetiformis (DH) being the best characterized. These associated conditions may be the clue to reaching the diagnosis of CD. Over the last few years, there have been multiple reports of the association between CD and several cutaneous manifestations that may improve with a gluten-free diet (GFD). The presence of some of these skin diseases, even in the absence of gastrointestinal symptoms, should give rise to an appropriate screening method for CD. The aim of this paper is to describe the different cutaneous manifestations that have been associated with CD and the possible mechanisms involved.
1. Introduction
Celiac disease (CD) is a chronic autoimmune systemic disease associated with an enteropathy triggered by gluten intake which affects genetically predisposed individuals of both sexes and can develop at any age. Gluten and its major protein fractions, gliadin and glutenin, are present in wheat, rye, barley, oats, related species and hybrids, and processed foods [1]. Almost all patients with CD present the human leukocyte antigen (HLA) DQ2 (>90%) or HLA DQ8 (5–10%); nevertheless, up to 40% of people in the Americas, Europe, and Southeast Asia also carry these alleles, indicating that these genes are necessary but not sufficient for CD development [2]. The findings of inflammatory changes in intestinal biopsies, ranging from lymphocytic enteritis to various degrees of villous atrophy, are the gold standard for CD diagnosis, even in the presence of a negative serology for CD. IgA anti-tissue transglutaminases are the most sensitive and cost-effective antibodies for the diagnosis of CD, although deamidated gliadin peptide IgG antibodies might be useful in seronegative patients with innate IgA deficiency. A life-long gluten-free diet (GFD) is mandatory, achieving clinical and histological recovery in most patients [1].
In past decades, CD was considered to be an uncommon disease affecting mainly children and limited to individuals of European ancestry. Currently, we know that this disorder may be detected at any age and is regarded as one of the most common chronic diseases encountered worldwide with a prevalence of about 1–2% [3]. The mean age of adult CD diagnosis is 45 years, although up to 20% of patients are diagnosed at the age of 60 years or above. CD is probably an under-diagnosed entity in adulthood partly because many patients in this age group lack the classical symptoms, such as diarrhea or signs of malabsorption. In fact, in most adult patients, gastrointestinal symptoms are subtle or even absent, and clinical suspicion arises from extra-intestinal manifestations (non-classic or atypical CD), such as anemia, cutaneous disorders, neurological disease, osteoporosis, and abnormal liver function tests [2,4]. We emphasize the importance of considering non-typical symptoms to diagnose adult CD and actively searching extra-intestinal associated manifestations in order to start an early GFD and prevent the onset of long-term complications.
CD patients are more frequently affected by other immune-mediated disorders (ID) compared to the general population, as reported in previous studies, mainly thyroid and skin diseases and type 1 diabetes mellitus [5,6]. This observation may be partially explained by a possible spread of the adaptive immune response, initially triggered in the gastrointestinal tract, to other tissues [4,6]. Hashimoto’s thyroiditis is the most frequently associated ID, followed by several skin disorders, such as psoriasis, atopic dermatitis (AD), vitiligo, systemic lupus erythematosus (SLE), alopecia areata (AA), and oral lichen planus (OLP) [6]. Interestingly, 60% of CD patients with associated thyroid disease that develop a third ID are skin related. These data suggest a relationship among the immunological systems of the thyroid, skin, and small bowel, which seem to be more susceptible to developing aberrant immunological responses against auto-antigens. [6,7,8,9,10,11,12].
Cutaneous manifestations associated with CD, other than dermatitis herpetiformis (DH), are poorly known. It is currently recognized that DH is an undoubted extra-intestinal manifestation of CD. In addition, there is growing evidence that supports the link between CD and several skin disorders. In 2006, Humbert et al. proposed a classification of skin diseases associated with CD, dividing them into four categories: autoimmune, allergic, inflammatory, and miscellaneous (Table 1) [9,10,11,12,13,14,15,16,17,18,19]. Recently, Bonciolini et al. described 17 patients affected by non-celiac gluten sensitivity with skin manifestations similar to eczema, psoriasis, and DH who did not show a specific histological pattern [20]. The only common findings in most of these patients were severe itching, the presence of C3 at the dermo–epidermal junction and rapid resolution after adopting a GFD. The authors emphasized the importance of close collaboration between gastroenterologists and dermatologists due to the multiple associations between gastrointestinal and skin disorders. In the present paper, we aim to describe the multiple skin disorders associated with CD and the possible mechanisms involved.

Table 1.
Strength of evidence for the association between celiac disease and skin diseases.
2. Immunopathogenesis of Skin and Oral Lesions Associated with CD
The immune responses in CD are very wide. A probable explanation lies in the presence of an increase in intestinal permeability in both groups of patients, in relation to the direct toxic effect of gliadin on the surface of the intestinal epithelium [21,22]. This enables the passage of gluten peptides and other related peptides to the bloodstream, provoking the appearance of different inflammatory or autoimmune processes that may affect any organ or tissue, which can be the result of aberrant immune responses [21,23,24]. As Hadjivassiliou stated more than 15 years ago, “that gluten sensitivity is regarded as principally a disease of the small bowel is a historical misconception.” [22,25].
In the submucosa of the small intestine, starting with the the action of tissue transglutaminase type 2 which unfolds gluten, a cascade of events occurs, causing a Th1 response that stimulates B lymphocytes that release IgE and other immunoglobulins [26] which play a important roles in the appearance of urticaria and AD, and a stimulation of Th2 mediated by T-lymphocytes which produces the release of pro-inflammatory cytokines, such as TNF-α and interferon gamma (IFNγ), among others [27], and that play important roles in several types of immune-mediated dermatitis, such as psoriasis. In addition, these immunological responses can also cause the production of circulating immunocomplexes due to antigen–antibody interactions which predominate in vasculitic lesions.
3. Dermatitis Herpetiformis
DH is a common extra-intestinal manifestation of CD. A special review article on this disease was recently published in the May 2018 special issue in Nutrients [28]. In summary, DH presents with itchy vesicles and papules, mainly on the elbows, knees, and buttocks. Overt gastrointestinal symptoms are rare. Although in duodenal biopsies, up to 75% of patients with DH with varying degrees of villous atrophy are observed, predominantly mild to moderate, it should be taking into account that in the remaining 25%, only inflammatory changes of changes of lymphocytic enteritis are observed, in the absence of villous atrophy. A diagnosis of DH is easily confirmed by biopsies showing pathognomonic granular immunoglobulin A (IgA) deposits in the papillary dermis by direct immunofluorescence. A valid hypothesis for the immune pathogenesis of DH is that it starts from latent or manifested CD in the gut and evolves into an immune complex deposition of high avidity IgA epidermal transglutaminase (TG3) antibodies, together with the TG3 enzyme, in the papillary dermis. The DH to CD prevalence ratio is 1:8 in Finland and the United Kingdom (UK). The annual DH incidence rate, currently 2.7 per 100,000 in Finland and 0.8 per 100,000 in the UK, is decreasing, whereas the reverse is true for CD. The long-term prognosis of DH patients on a GFD is excellent, with the mortality rate being even lower than for the general population [28].
4. Urticaria
Urticaria is characterized by the onset of wheals, angioedema, or both (Figure 1) [29]. Urticaria is a common disorder, occurring in 15–25% of individuals at some point in life [29,30]. Chronic urticaria (CU) (duration ≥6 weeks) is seen in about 0.5–1% of the general population [31,32]. CU is associated with a substantial decrease in quality of life [31]. The etiopathogenesis of CU is thought to be associated with autoimmune mechanisms [33,34,35,36]. CU has been shown to have a genetic association with the human leukocyte antigen HLA-DQ8 alleles [37]. Interestingly, HLADQ8 has an association with CD [37,38].
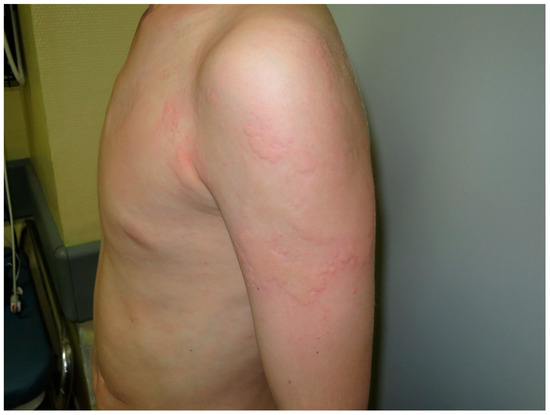
Figure 1.
Urticaria. Pale to red, well-demarcated, transient swellings, involving the dermis, mainly at the thorax and the left arm.
In 1987, Hauteke et al. first described the association between CD and chronic urticaria [39], although the relationship between these two diseases is not fully clear. Recently, Kolkhir et al. stated that chronic spontaneous urticaria is strongly linked with various autoimmune diseases, including Hashimoto’s thyroiditis, pernicious anemia, vitiligo, diabetes mellitus type 1, Grave’s disease, rheumatoid arthritis, and CD [40]. In a large population study, 453 patients with CD and no previous diagnosis of urticaria developed urticaria, and 79 of these 453 patients had chronic urticaria [12]. The corresponding hazard ratios were 1.51 for any urticaria (95%CI = 1.36–1.68) and 1.92 for chronic urticaria (95%CI = 1.48–2.48). These data support an increased prevalence of urticaria and chronic urticaria in patients with CD [12].
In some cases of CU, the adoption of a GFD has proven its effectiveness in controlling skin flares [41,42], further sustaining that CU may be a cutaneous manifestation of CD and not only a fortuitous association [11].
5. Atopic Dermatitis
AD is a chronic inflammatory skin disease that is associated with a heterogeneous group of symptoms and signs. The cutaneous signs of AD include erythema, lichenification, scaling, and prurigo nodules (Figure 2). The symptoms of AD include cutaneous itch and pain [43], sleep disturbance and fatigue [44,45], and mental health symptoms [46,47,48]. All of these manifestations contribute to diminish the quality of life, limiting the ability to perform activities of daily life and causing psychosocial distress and stigma [49]. AD affects 40 million individuals worldwide [50], and its prevalence is still increasing. Notably, AD appears to be more prevalent among children under five years of age, and its prevalence decreases with advancing age [51]. The onset of AD occurs primarily in childhood and is thought to precede allergic disorders mediated by immunoglobulin E (IgE) sensitization to environmental antigens, namely AD, asthma, and allergic rhino-conjunctivitis, the so-called atopic triade [52,53,54,55]. Though extensive recent studies have shed light on the understanding of AD, the exact pathogenesis of the disease is still unknown. The complex interaction between genetics, environmental factors, microbiota, skin barrier deficiency, immunological derangement, and possibly autoimmunity contributes to the development of the disease [56,57,58,59].
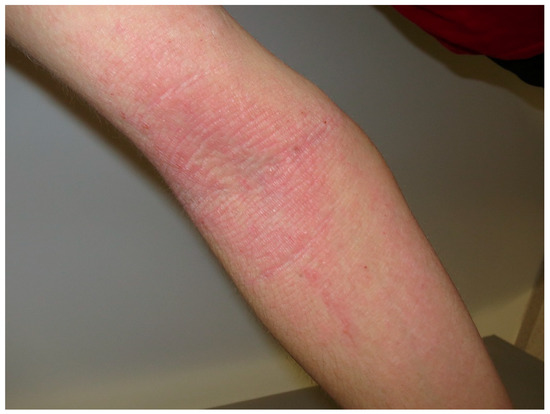
Figure 2.
Atopic dermatitis. Excoriated bilateral erythematous scaling papules and plaques on the right flexor elbow surface.
AD has also been linked with CD. Ress et al. analyzed the prevalence of CD in 351 children with AD compared with a general pediatric population and showed a four-fold greater risk of developing CD in patients with AD (OR, 4.18; 95% CI, 1.12–15.64) [60]. This study also emphasizes the need to evaluate the cost-effectiveness of screening patients with AD for CD in time to prevent long-term complications. Moreover, Ciacci et al. conducted a case control study involving 4114 adult patients, with and without CD, and observed that AD was three-fold more frequent in patients with CD and two-fold more frequent in their relatives than in their spouses (OR, 3.17; 95% CI, 1.02–9.82) [13].
6. Psoriasis
Psoriasis is an autoimmune chronic inflammatory skin disease with an estimated prevalence of 2–4% in the adult population [61,62]. It affects over 7.5 million people in the United States and approximately 125 million people worldwide [54]. Psoriasis is considered to be a multifactorial disease, in which the genetic background interacts with environmental factors to define an individual’s risk [62,63,64]. The classical clinical manifestations of psoriasis consist of the presence of red, infiltrated plaques, covered with a coarse, silvery scaling (Figure 3 and Figure 4). Predilection sites include the elbows and knees, scalp, and periumbilical and lumbar regions, although any anatomical site might be affected [65]. The clinical course of psoriasis is marked by frequent relapses with fluctuating rates [62].
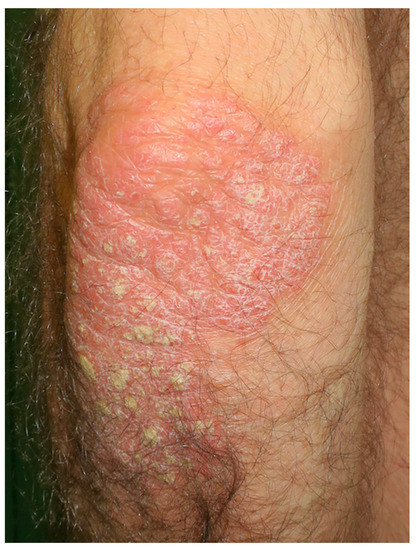
Figure 3.
Extense plaque of psoriasis at the left elbow extensor side.
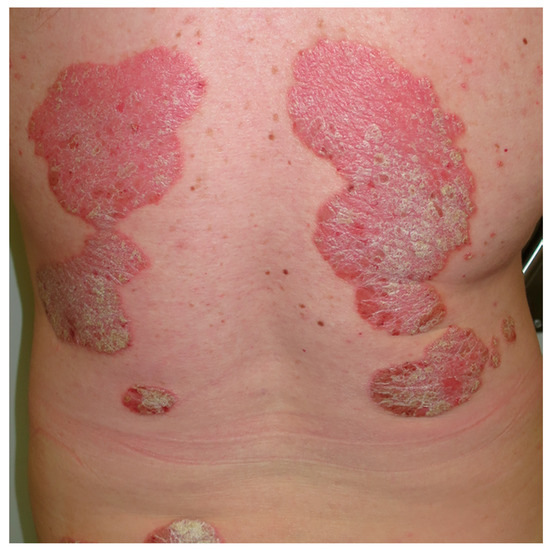
Figure 4.
Psoriasis. Well demarcated, erythematous, scaly plaques that are relatively symmetrical on the back.
Psoriasis is known to be associated with an increased risk of several comorbidities, including inflammatory arthritis, metabolic syndrome, and atherosclerotic disease [63]. The association between psoriasis and CD has been of recent interest, but its first recognition was in 1971 by Marks and Shuster [66]. They described, for the first time, a “psoriatic enteropathy” in a small group of patients with severe psoriasis. For many years, the relationship between psoriasis and CD has remained controversial since the few available data were inconclusive. A recent meta-analysis demonstrated a significantly higher risk of CD among patients with psoriasis compared with participants without psoriasis with the OR of 3.09 (95%CI = 1.92–4.97) [16]. Furthermore, seven studies have reported a positive association between psoriasis and CD markers [66,67,68,69,70,71,72]. In contrast, other studies did not find evidence of an association between psoriasis and CD markers. However, these studies were of smaller size and some did not employ control groups [73,74,75,76,77]. To resume the evidence for CD antibody positivity in psoriasis, Bhatia et al. performed a meta-analysis of nine studies that reported the frequency of IgA anti-gliadin antibody (IgA AGA) positivity in psoriasis cases and controls [17]. They found a statistically significant higher relative risk of having positive IgA AGA in patients with psoriasis compared to controls (OR = 2.36, 95% CI 1.15–4.83). Other two studies suggested that levels of CD antibodies correlate with psoriasis or psoriatic arthritis severity [78,79]. The use of AGA determination for the diagnosis of CD has low sensitivity, and its use in clinical practice is being abandoned, being replaced by other types of antibodies, such as anti-transglutaminase and deaminated peptides of gliadin [6,21].
The pathophysiologic mechanisms behind the increased risk of CD among patients with psoriasis are not known, but there are different hypotheses that try to explain them [16,80]. The association between CD and several autoimmune diseases, such as type I diabetes mellitus and autoimmune thyroid disease, is well-documented [81,82]. It is believed that shared genes (at-risk HLA haplotypes) might be responsible for this association. The shared genes might play similar roles in the association between psoriasis and CD. Genetic-wide association studies of these two conditions identified genetic susceptibility loci at eight genes that regulate innate and adaptive immune responses: TNFAIP3, RUNX3, ELMO1, ZMIZ1, ETS1, SH2B3, SOCS1 and UBE2L3 [80,83,84,85]. Another possible explanation is that the increased proliferation rate of keratinocytes found in patients with psoriasis is known to produce an excessive amount of interleukin (IL)-1 and IL-18, the essential signals for the induction of Th1 response. Interestingly, mucosal inflammation in patients with CD is also caused by the activation of Th1 in response to dietary gluten [86]. Therefore, it is possible that these ILs might predispose patients to CD. On the other hand, it is possible that intestinal barrier dysfunction associated with undiagnosed or untreated CD may allow the increased passage of immune triggers resulting in an increased risk of autoimmune diseases, including psoriasis [86,87]. Finally, CD-related malabsorption may affect psoriasis by causing a vitamin D deficiency status [9,88]. It is well known that low levels of vitamin D predispose individuals to psoriasis and that exposure to sunlight and topical administration of vitamin D analogues improves psoriatic lesions, probably due to its immunoregulatory properties [88].
Although available data regarding the coexistence of CD and psoriasis are still inconclusive, there is a considerable amount of evidence that suggests that psoriatic patients with concomitant CD may benefit from a GFD [17,21,80,89]. Furthermore, the prevalence of the anti-gliadin IgA antibody is significant higher among patients with psoriasis without a diagnosis of gluten-related disorders. For this reason, anti-gliadin IgA testing can identify patients who are likely to benefit from GFDs [90].
To summarize, the relatively frequent coexistence of CD and psoriasis justifies monitoring of patients with either condition for clinical evidence of the other. This is especially important in the case of psoriasis, as it could be the only manifestation of an undiagnosed CD, even in the absence of obvious digestive symptoms. It is advisable to perform the entire protocol to actively search for CD, including duodenal biopsies, even when serological markers are negative. In the case of negative CD findings, performing a trial with a GFD is currently the recognized diagnostic method [23].
7. Aphthous Stomatitis
Numerous authors have described a wide variety of oral cavity disorders in patients with CD, and some of these manifestations may be considered diagnostic clues in silent, atypical forms of CD [91].
Recurrent aphthous stomatitis (RAS) is a common clinical condition that produces painful ulcerations in the oral cavity. RAS is characterized by multiple recurrent small, round, or ovoid ulcers with circumscribed margins, erythematous haloes, and yellow or gray floors, typically first presenting in childhood or adolescence [92,93] (Figure 5). RAS has been recognized for many years as a symptom of CD (CD) [93,94,95,96]. A recent meta-analysis showed that celiac patients have greater frequency of RAS (OR = 3.79, 95%CI = 2.67–5.39). When only the children were considered, the OR was 4.31 (95%CI = 3.03–6.13), while in the adults, the OR of only one study was 47.90 (95%CI 6.29–364.57) [18]. RAS patients should be considered at-risk subjects, even in the absence of any gastrointestinal symptoms and should therefore undergo a diagnostic procedure for CD [97]. RAS may also be present in patients with DH [98]. A study reported non-specific mucosal ulcers in up to 40% of patients with DH [99]. The etiopathology of RAS is obscure; it is not known whether RAS lesions are directly influenced by the gluten sensitivity disorder, or if these are related to hematinic deficiency with low levels of serum iron, folic acid, and vitamin B12 or trace element deficiencies due to malabsorption in patients with untreated CD [96].
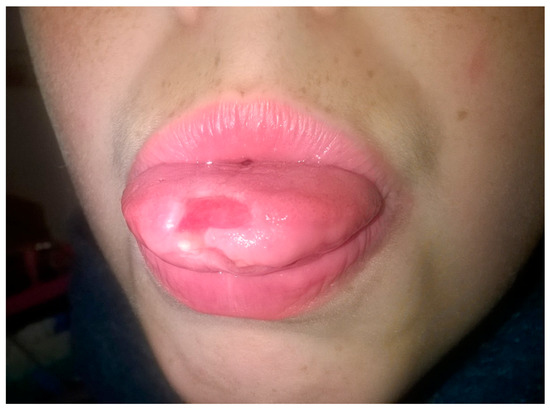
Figure 5.
Aphthous lesion on the tip of the tongue, on the upper side.
8. Rosacea
Rosacea is an inflammatory skin condition characterized primarily by persistent or recurrent episodes of centrofacial erythema, with women being more affected than men [100] (Figure 6). The pathophysiology is not completely understood, but dysregulation of the immune system as well as changes in the nervous and vascular systems have been identified [101]. Rosacea can seriously affect a patient’s quality of life, and this should prompt clinicians to diagnose it early and start treatment [100]. Rosacea shares genetic risk loci with autoimmune diseases, such as type 1 diabetes mellitus and CD [102]. One study showed that women with rosacea had a significantly increased risk of CD (OR = 2.03, 95%CI 1.35–3.07) [103]. In a nationwide cohort study, the prevalence of CD was higher among patients with rosacea when compared to control subjects (HR = 1.46, 95%CI = 1.11–1.93) [19]. In this study, rosacea was associated with an increased prevalence of Crohn’s disease, ulcerative colitis, irritable bowel disease, small intestinal bacterial overgrowth, and Helicobacter pylori infection.
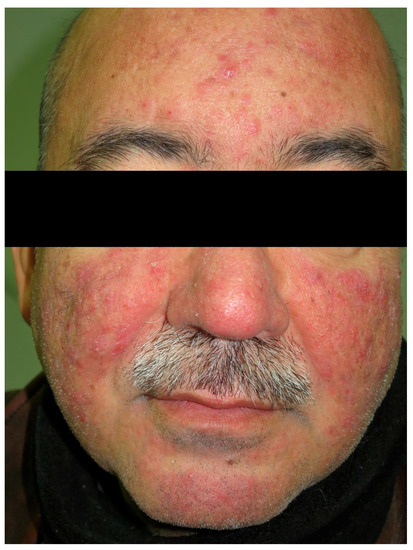
Figure 6.
Rosacea. Papule-pustular lesions on the face.
9. Alopecia Areata
AA is an autoimmune disease that presents as a non-scarring type of hair loss. AA affects both sexes equally, affects patients of all ages, and is found in approximately 2% of the general population [104]. Clinical presentation of AA is very heterogeneous, ranging from small and well-circumscribed patches of hair loss to a complete absence of body and scalp hair (Figure 7). Exclamation point hairs, dystrophic hairs, and yellow dots are features of AA that can be identified with trichoscopy. Nail abnormalities, such as pitting, brittleness, or striations are seen in 10% to 20% of patients. The main factors affecting prognosis include age at onset and disease extent; younger age at initial presentation and severity at onset are the most important prognostic indicators [105]. The etiology of AA remains unclear, though it is believed to result from a loss of immune privilege in the hair follicle, autoimmune-mediated hair follicle destruction, and the upregulation of inflammatory pathways [105].
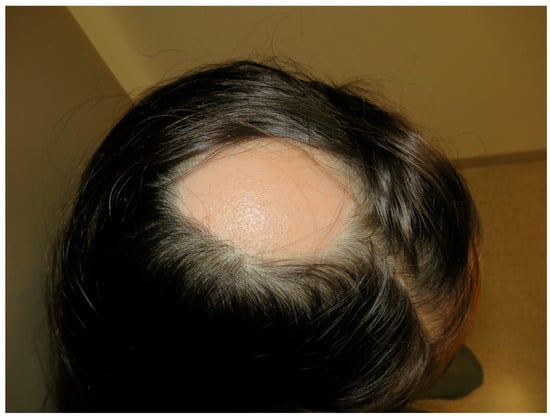
Figure 7.
Alopecia areata. Patchy head hair loss.
AA is associated with other autoimmune disorders, such as Addison’s disease, autoimmune thyroiditis, atrophic gastritis, systemic lupus erythematous, rheumatoid arthritis, myasthenia gravis, and vitiligo [106]. In 1995, Corazza et al. described, for the first time, the association between AA and CD [107]. Since then, there have been other reports of this association. The estimated prevalence rate of CD in patients with AA is from 1:85 to 1:116 [108,109], similar to that found in the general population, so it could be considered to be a random association. However, due to the fact that alopecia improves and even disappears with a GFD, its presence should indicate the possible existence of an undiagnosed CD [11,108,109,110]. In addition, the prevalence of anti-gliadin antibodies in patients with AA was 18:100 in a study conducted in 2011, occurring more often in severe variants of AA, in particular, alopecia universalis [109]. An active search for CD using serological screening tests has been recommended to diagnose the numerous cases of subclinical CD [9], but a recent study stated that the biological tests to search for CD do not bring enough information and proof to disclose gluten intolerance in AA patients [111].
The positive effects of a GFD on the pattern of autoimmune conditions associated with CD, such as AA, have been attributed to the normalization of the immune response [109]. Although remission and recurrence may be observed during the clinical course of AA, many patients on a GFD have shown complete regrowth of the scalp and other body hair and no further recurrence of AA at follow-up [112].
10. Cutaneous Vasculitis
Leukocytoclastic vasculitis, also known as “hypersensitivity vasculitis”, is a histopathologic diagnosis given to cutaneous, small vessel vasculitis, characterized by the inflammation of the walls of postcapillary venules [113]. The clinical features of leukocitoclastic vasculitis include palpable purpura, nodules, hemorrhagic vesicles, bullae, and livedo reticularis, mainly distributed in the lower extremities (Figure 8) [114]. Extracutaneous involvement is seen in approximately 30% of patients. Systemic vasculitis shows a predilection for certain organs, such as the kidneys and lungs. In most cases, leucocytoclastic vasculitis is mediated by immunocomplex deposition, with the antigen being either exogenous or endogenous [115,116,117,118].
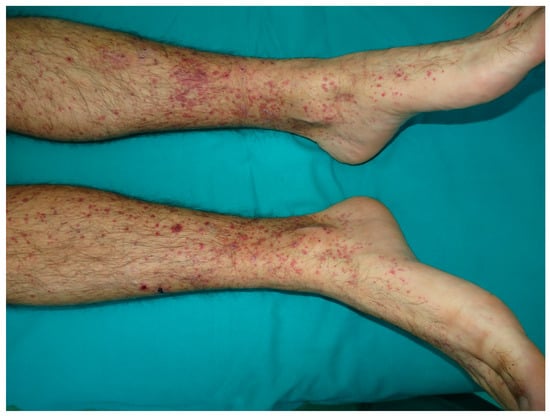
Figure 8.
Vasculitis. Palpable purpuric papules on the lower extremities.
When leukocytoclastic vasculitis is suspected, a biopsy should be performed, preferably in the first 24 to 48 h of lesion onset. Additionally, direct immunofluorescence should be performed to evaluate for the presence of immunoglobulins. If no systemic symptoms are present, laboratory testing, including a complete blood count, measurement of the erythrocyte sedimentation rate, basic metabolic panel, liver function tests, and urinalysis should be done as well. If there is concern for systemic involvement, more extensive workup can be fulfilled. Around 90% of leukocytoclastic vasculitis cases are self-limited, showing spontaneous resolution within weeks to months. The treatment depends on the severity of the disease and can range from an oral corticosteroid course to various steroid-sparing agents [113,114].
There are sporadic reports about the association between CD and cutaneous vasculitis (CV) [115,116,117,118,119]. The coexistence of these two entities might be related to increased intestinal permeability [120], and immune complexes, originating from exogenous or endogenous antigens, might circulate because of the impaired phagocytic function of the reticular endothelium system and be deposited in the skin. As seen in inflammatory bowel disease (IBD), exogenous antigens may permeate the damaged CD mucous in larger quantities than normal. This is reflected by significant serum milk and gluten fraction antibody titers. Moreover, autoimmune sensitization may result because of the release of endogenous antigens from the damaged small bowel mucosa. Meyers et al. [118] described a case of CV associated with CD and the remission of skin lesions after the treatment with a strict GFD. Treatment with a GFD may improve CV lesions in cases associated with CD [9,10,11].
11. Other Skin Conditions Found in Patients with CD
As reported by Humbert et al. and Caproni et al., in addition to skin diseases with proven association with CD and those improved by a GFD and/or with positivity of celiac serological markers, there are also fortuitous associations with other skin conditions [9,10,11]. Some of these associations are more common than others.
Juvenile dermatomyositis and dermatomyositis have been reported in association with CD in adult patients. In particular, when patients are newly diagnosed with these conditions, even in the absence of gastrointestinal symptoms, screening for CD should be performed. Clinical manifestations of dermatomyositis may respond to a GFD [121,122,123,124].
The possible association between CD and vitiligo is controversial. There are few cases that have reported the improvement of vitiligo in patients that started a GFD. A common basic autoimmune mechanism has been hypothesized [125,126].
Many similarities exist between the pathogeneses of CD and SLE, but it is still unknown whether there is a true association or not [127,128,129].
Other reported and less frequent associations include lichen planus and lichen sclerosus [130,131,132,133,134,135,136,137,138], linear IgA bullous dermatosis [139,140], prurigo nodularis [141], pityriasis rubra pilaris and erythroderma [142], erythema elevatum diutinum [143,144,145], necrolytic migratory erythema [146,147,148], pityriasis lichenoides [140], erythema nodosum [140,149,150,151], porphyria [152,153], cutaneous amyloidosis [154], generalized acquired cutis laxa [155,156], acquired hypertrichosis lanuginosa [157], ichthyosis [158], partial lipodystrophy [159], transverse leukonychia [160], atypical mole syndrome, and congenital giant nevus [161]. Finally, we want to mention that earlier studies reported an increased risk of malignant melanoma in patients with CD, but a recent study refuted this relation [162].
12. Other Oral Cavity Disorders
Other oral cavity manifestations among patients with CD have also been described [18,97,98,99,110,163,164]. Rashid et al. described oral and dental manifestations of CD, consisting of enamel defects, delayed eruption, recurrent aphthous ulcers, cheilitis, and atrophic glossitis [96]. Bramanti et al. found atrophic glossitis, angular cheilitis, and burning tongue to be more frequent in CD patients than in control patients [165].
13. Conclusions
CD is a systemic process of autoimmune nature that affects genetically predisposed people in relation to a permanent intolerance to gluten and related proteins. It has a worldwide distribution, a slight female predominance, and can appear at any age, with a variable clinical course, ranging from subclinical or asymptomatic cases to very severe ones. In the physical examination of these patients, it is very important to recognize the presence of several types of dermatitis which are found in association with CD, such as urticaria (HR: 1.51, CI: 1.36–1.68), chronic urticaria (HR: 1.92, CI: 1.48–2.48), atopic dermatitis (OR: 3.17, CI: 1.02–9.82), psoriasis (HR: 1.72, CI = 1.54–1.92), aphthous stomatitis (OR: 3.79, CI = 2.67–5.39) and rosacea (HR: 1.46, CI = 1.11–1.93), and other skin affectation processes that are not so clearly related to CD. All of these diseases may occur in the form of outbreaks, accompanied generally by pruritus, which negatively affects their quality of life. Their relationship with gluten is through allergic, inflammatory, immunological, and mixed processes. The recognition of their probable relationship facilitates the diagnosis of CD, and the establishment of a GFD improves the evolution of cutaneous lesions and in some cases, full recovery is achieved.
It is very important to emphasize that the classic presentations of CD with associated malabsorption syndrome are currently considered to be exceptional, especially from the age of two years, and the predominant forms are those with mild, fluctuating, or even absent digestive symptoms and a wide range of extra-intestinal manifestations [166,167,168,169,170]. Many undiagnosed celiac patients underestimate their multiple and frequent discomfort from digestive and more general causes because they have grown accustomed to living with a state of chronic poor health as though it is normal. They are only able to recognize that they really did have symptoms related to the consumption of gluten when they start the GFD and the improvement becomes obvious [171,172].
Author Contributions
L.R., V.-B.G., and N.A. designed the study and wrote the abstract, the introduction and the Dermatitis herpetiformis description. C.G.d.C. wrote the Alopecia areata, urticaria and cutaneous vasculitis descriptions, A.d.D. and L.P. contributed to the description of atopic dermatitis and psoriasis. J.S.-J. wrote the sections about the oral mucosal and other CD-associated skin conditions. J.S.-J., V.-B.G. and A.d.D. made all the selected figures in this review. All the authors reviewed and approved the final version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bai, J.; Fried, M.; Corazza, G.; Schuppan, D.; Farthing, M.; Catassi, C.; Greco, L.; Cohen, H.; Ciacci, C.; Eliakim, R.; et al. World Gastroenterology Organisation Global Guidelines on celiac disease. J. Clin. Gastroenterol. 2013, 47, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- Collin, P.; Vilppula, A.; Luostarinen, L.; Holmes, G.K.T.; Kaukinen, K. Review article: Coeliac disease in later life must not be missed. Aliment. Pharmacol. Ther. 2018, 47, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, L. Celiac disease. World J. Gastroenterol. 2006, 12, 6585–6593. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Endocrine manifestations in celiac disease. World J. Gastroenterol. 2016, 22, 8472–8479. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Bonura, A.; Garavaglia, D.; Rulli, E.; Floriani, I.; Tagliabue, G.; Contiero, P.; Bardella, M. Immunological comorbity in coeliac disease: Associations, risk factors and clinical implications. J. Clin. Immunol. 2012, 32, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; Kruzliak, P.; Cangemi, G.; Pohanka, M.; Betti, E.; Lauret, E.; Rodrigo, L. The spectrum of differences between childhood and adulthood celiac disease. Nutrients 2015, 7, 8733–8751. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.; Cellier, C. Celiac disease. N. Engl. J. Med. 2007, 357, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Proietti, I.; Leggio, L.; Ferrulli, A.; Vonghia, L.; Capizzi, R.; Rotoli, M.; Amerio, P.L.; Gasbarrini, G.; Addolorato, G. Cutaneous manifestations in celiac disease. World J. Gastroenterol. 2006, 12, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Humbert, P.; Pelletier, F.; Dreno, B.; Puzenat, E.; Aubin, F. Gluten intolerance and skin diseases. Eur. J. Dermatol. 2006, 16, 4–11. [Google Scholar] [PubMed]
- Caproni, M.; Bonciolini, V.; D’Errico, A.; Antiga, E.; Fabbri, P. Celiac disease and dermatologic manifestations: Many skin clue to unfold gluten-sensitive enteropathy. Gastroenterol. Res. Pract. 2012, 2012, 952753. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Lindelöf, B.; Rashtak, S.; Rubio-Tapia, A.; Murray, J.A. Does urticaria risk increase in patients with celiac disease? A large population-based cohort study. Eur. J. Dermatol. 2013, 23, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, C.; Cavallaro, R.; Iovino, P.; Sabbatini, F.; Palumbo, A.; Amoruso, D.; Tortora, R.; Mazzacca, G. Allergy prevalence in adult celiac disease. J. Allergy Clin. Immunol. 2004, 113, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Lindelöf, B.; Zingone, F.; Ciacci, C. Psoriasis in a nationwide cohort study of patients with celiac disease. J. Investig. Dermatol. 2011, 131, 2010–2016. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Griffiths, C.E.M.; Mallbris, L.; Gislason, G.H.; Skov, L. The association between psoriasis and coeliac disease. Br. J. Dermatol. 2017, 177, e329–e330. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Wijarnpreecha, K.; Kittanamongkolchai, W. Psoriasis and risk of celiac disease: A systematic review and meta-analysis. Indian J. Dermatol. 2017, 62, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, B.; Millsop, J.; Debbaneh, M.; Koo, J.; Linos, E.; Liao, W. Diet and psoriasis, part II: Celiac disease and role of a gluten-free diet. Am. Acad. Dermatol. 2014, 71, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Nieri, M.; Tofani, E.; Defraia, E.; Giuntini, V.; Franchi, L. Enamel defects and aphthous stomatitis in celiac and healthy subjects: Systematic review and meta-analysis of controlled studies. J. Dent. 2017, 65, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Weinstock, L.B.; Thyssen, E.P.; Gislason, G.H.; Thyssen, J.P. Rosacea and gastrointestinal disorders: A population-based cohort study. Br. J. Dermatol. 2017, 176, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Bonciolini, V.; Bianchi, B.; Del Bianco, E.; Verdelli, A.; Caproni, M. Cutaneous manifestations of non-celiac gluten sensitivity: Clinical histological and immunopathological features. Nutrients 2015, 7, 7798–7805. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Principi, M.; Iannone, A.; Amoruso, A.; Ierardi, E.; Di Leo, A.; Barone, M. Extra-intestinal manifestations of non-celiac gluten sensitivity: An expanding paradigm. World J. Gastroenterol. 2018, 24, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Bai, J.C.; Bonaz, B.; Bouma, G.; Calabrò, A.; Carroccio, A.; Castillejo, G.; Ciacci, C.; Cristofori, F.; Dolinsek, J.; et al. Non-Celiac Gluten sensitivity: The new frontier of gluten related disorders. Nutrients 2013, 5, 3839–3853. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Green, P.H.; Fasano, A. Extraintestinal manifestations of coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Grünewald, R.A.; Davies-Jones, G.A. Gluten sensitivity as a neurological illness. J. Neurol. Neurosurg. Psychiatry 2002, 72, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.; Sollid, L.M. The human intestinal B-cell response. Mucosal Immunol. 2016, 9, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Jabri, B.; Sollid, L.M. T Cells in Celiac Disease. J. Immunol. 2017, 198, 3005–3014. [Google Scholar] [CrossRef] [PubMed]
- Reunala, T.; Salmi, T.T.; Hervonen, K.; Kaukinen, K.; Collin, P. Dermatitis Herpetiformis: A Common Extraintestinal Manifestation of Coeliac Disease. Nutrients 2018, 10, E602. [Google Scholar] [CrossRef] [PubMed]
- Champion, R.H.; Roberts, S.O.; Carpenter, R.G.; Roger, J. Urticaria and angio-oedema. A review of 554 patients. Br. J. Dermatol. 1969, 81, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Toubi, E.; Kessel, A.; Avshovich, N.; Bamberger, E.; Sabo, E.; Nusem, D.; Panasoff, J. Clinical and laboratory parameters in predicting chronic urticaria duration: A prospective study of 139 patients. Allergy 2004, 59, 869–873. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, B.; Lawlor, F.; Simpson, J.; Morgan, M.; Greaves, M. The impact of chronic urticaria on the quality of life. Br. J. Dermatol. 1997, 136, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Palikhe, N.; Sin, H.; Kim, S.; Sin, H.; Hwang, E.; Ye, Y.; Park, H. Genetic variability of prostaglandin E2 receptor subtype EP4 gene in aspirin-intolerant chronic urticaria. J. Hum. Genet. 2012, 57, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Dice, J.P. Physical urticaria. Immunol. Allergy Clin. 2004, 24, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Carr, W. Urticarial vasculitis. Allergy Asthma Proc. 2007, 28, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.; Robertson, L. Chronic urticaria and autoimmunity. Skin Ther. Lett. 2013, 18, 5–9. [Google Scholar]
- Kolkhir, P.; Church, M.; Weller, K.; Metz, M.; Schmetzer, O.; Maurer, M. Autoimmune chronic spontaneous urticaria: What we know and what we do not know. J. Allergy Clin. Immunol. 2017, 139, 1772–1781.e1. [Google Scholar] [CrossRef] [PubMed]
- O′Donnell, B.; O’Neill, C.; Francis, D.; Niimi, N.; Barr, R.; Barlow, R.; Kobza Black, A.; Welsh, K.; Greaves, M. Human leucocyte antigen class II associations in chronic idiopathic urticaria. Br. J. Dermatol. 1999, 140, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Piccini, B.; Vascotto, M.; Serracca, L.; Luddi, A.; Margollicci, M.; Balestri, P.; Vindigni, C.; Bassotti, G.; Villanacci, V. HLA-DQ typing in the diagnostic algorithm of celiac disease. Rev. Esp. Enferm. Dig. 2012, 104, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hautekeete, M.; De Clerck, L.; Stevens, W. Chronic urticaria associated with coeliac disease. Lancet 1987, 329, 157. [Google Scholar] [CrossRef]
- Kolkhir, P.; Borzova, E.; Grattan, C.; Asero, R.; Pogorelov, D.; Maurer, M. Autoimmune comorbidity in chronic spontaneous urticaria: A systematic review. Autoimmun. Rev. 2017, 16, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, L.; Passalacqua, G.; Magazzu, G.; Comisi, F.; Vita, D.; Barberio, G.; Sferlazzas, C.; Pajno, G. Chronic urticaria and associated coeliac disease in children: A case-control study. Pediatr. Allergy Immunol. 2005, 16, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M.W. Chronic idiophatic urticarial. Curr. Opin. Allergy Clin. Immunol. 2003, 4, 363–368. [Google Scholar] [CrossRef]
- Vakharia, P.; Chopra, R.; Sacotte, R.; Patel, K.; Singam, V.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.; et al. Burden of skin pain in atopic dermatitis. Ann. Allergy Asthma Immunol. 2017, 119, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.; Garg, N.; Paller, A.; Fishbein, A.; Zee, P. Sleep Disturbances in Adults with Eczema Are Associated with Impaired Overall Health: A US Population-Based Study. J. Investig. Dermatol. 2015, 135, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Attarian, H.; Zee, P.; Silverberg, J. Burden of Sleep and Fatigue in US Adults With Atopic Dermatitis. Dermatitis 2016, 27, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Silverberg, J. Association between Atopic Dermatitis and Depression in US Adults. J. Investig. Dermatol. 2015, 135, 3183–3186. [Google Scholar] [CrossRef] [PubMed]
- Yaghmaie, P.; Koudelka, C.; Simpson, E. Mental health comorbidity in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2013, 131, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Strom, M.; Fishbein, A.; Paller, A.; Silverberg, J. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br. J. Dermatol. 2016, 175, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J. Associations between atopic dermatitis and other disorders. F1000Resarch 2018, 7, 303. [Google Scholar] [CrossRef] [PubMed]
- Plötz, S.; Wiesender, M.; Todorova, A.; Ring, J. What is new in atopic dermatitis/eczema? Expert Opin. Emerg. Drugs 2014, 19, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Herd, R.M.; Tidman, M.J.; Prescott, R.J.; Hunter, J.A. Prevalence of atopic eczema in the community: The Lothian atopic dermatitis study. Br. J. Dermatol. 1996, 135, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. Atopic Dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Alduraywish, S.; Lodge, C.; Campbell, B.; Allen, K.; Erbas, B.; Lowe, A.; Dharmage, S. The march from early life food sensitization to allergic disease: A systematic review and meta-analyses of birth cohort studies. Allergy 2015, 71, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.; Moran, T.; Floudas, A.; Wurlod, F.; Kaszlikowska, A.; Salimi, M.; Quinn, E.; Oliphant, C.; Núñez, G.; McManus, R.; et al. Spontaneous atopic dermatitis is mediated by innate immunity, with the secondary lung inflammation of the atopic march requiring adaptive immunity. J. Allergy Clin. Immunol. 2016, 137, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, N.; Kim, B.; Jung, M.; Kim, D.; Moniaga, C.; Kabashima, K.; Choi, E. Acidification of stratum corneum prevents the progression from atopic dermatitis to respiratory allergy. Exp. Dermatol. 2017, 26, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Otsuka, A.; Nomura, T. Linking air pollution to atopic dermatitis. Nat. Immunol. 2016, 18, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Dainichi, T.; Hanakawa, S.; Kabashima, K. Classification of inflammatory skin diseases: A proposal based on the disorders of the three-layered defense systems, barrier, innate immunity and acquired immunity. J. Dermatol. Sci. 2014, 76, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Kashiwakura, J.; Okayama, Y.; Furue, M.; Kabashima, K.; Shimada, S.; Ra, C.; Siraganian, R.; Kawakami, Y.; Kawakami, T. Most Highly Cytokinergic IgEs Have Polyreactivity to Autoantigens. Allergy Asthma Immunol. Res. 2012, 4, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Rerknimitr, P.; Otsuka, A.; Nakashima, C.; Kabashima, K. The etiopathogenesis of atopic dermatitis: Barrier disruption, immunological derangement, and pruritus. Inflamm. Regen. 2017, 37, 14. [Google Scholar] [CrossRef] [PubMed]
- Ress, K.; Annus, T.; Putnik, U.; Luts, K.; Uibo, R.; Uibo, O. Celiac Disease in Children with Atopic Dermatitis. Pediatr. Dermatol. 2014, 31, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, T.; Schupp, C.; Armstrong, A. Psoriasis prevalence among adults in the United States. J. Am. Acad. Dermatol. 2014, 70, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Christophers, E. Psoriasis—Epidemiology and clinical spectrum. Clin. Exp. Dermatol. 2001, 26, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, J.; Grewal, S.; Langan, S.; Mehta, N.; Ogdie, A.; Van Voorhees, A.; Gelfand, J. Psoriasis and comorbid diseases. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.; Boehncke, S. More than skin-deep: The many dimensions of the psoriatic disease. Swiss Med. Wkly. 2014, 144, w13968. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.; Shuster, S. Intestinal malabsorption and the skin. Gut 1971, 12, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; Aguilar Sánchez, J.; Guerriero, C.; Fossati, B.; Capizzi, R.; De Simmone, C.; Migneco, A.; Amerio, P.; Gasbarrini, G.; Gasbarrini, A. High prevalence of celiac disease in psoriasis. Am. J. Gastroenterol. 2003, 98, 2574–2575. [Google Scholar] [CrossRef]
- Michaelsson, G.; Gerden, B.; Ottosson, M.; Parra, A.; Sjoberg, O.; Hjelmquist, G.; Loof, L. Patients with psoriasis often have increased serum levels of IgA antibodies to gliadin. Br. J. Dermatol. 1993, 129, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, S.; Gür, G.; Topal, F.; Senel, E.; Topal, F.; Alli, N.; Saritas, Ü. Coeliac Disease-Associated Antibodies in Psoriasis. Ann. Dermatol. 2013, 25, 298. [Google Scholar] [CrossRef] [PubMed]
- Nagui, N.; El Nabarawy, E.; Mahgoub, D.; Mashaly, H.; Saad, N.; El-Deeb, D. Estimation of (IgA) anti-gliadin, anti-endomysium and tissue transglutaminase in the serum of patients with psoriasis. Clin. Exp. Dermatol. 2011, 36, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Damasiewicz-Bodzek, A.; Wielkoszyński, T. Serologic markers of celiac disease in psoriatic patients. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sonkar, G.; Usha; Singh, S. Celiac disease-associated antibodies in patients with psoriasis and correlation with HLA Cw6. J. Clin. Lab. Anal. 2010, 24, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; De Simone, C.; Aguilar Sanchez, J.; Capizzi, R.; Migneco, A.; Guerriero, C.; Cazzato, A.; Gasbarrini, G.; Amerio, P.; Gasbarrini, A. Malabsorption in psoriatic patients: Cause or consequence? Scand. J. Gastroenterol. 2006, 41, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.; Boer, W.; Haas, F. Is there a relationship between psoriasis and coeliac disease? J. Intern. Med. 1995, 237, 118. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Ahmad, Q.; Sultan, S. Antigliadin antibodies in psoriasis. Australas. J. Dermatol. 2010, 51, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Zamani, F.; Alizadeh, S.; Amiri, A.; Shakeri, R.; Robati, M.; Alimohamadi, S.; Abdi, H.; Malekzadeh, R. Psoriasis and Coeliac Disease; Is There Any Relationship? Acta Derm.-Venereol. 2010, 90, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Kia, K.; Nair, R.; Ike, R.; Hiremagalore, R.; Elder, J.; Ellis, C. Prevalence of Antigliadin Antibodies in Patients with Psoriasis is Not Elevated Compared with Controls. Am. J. Clin. Dermatol. 2007, 8, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, C.; Degl’innocenti, D.; Caproni, M.; Fabbri, P. Is the search for serum antibodies to gliadin, endomysium and tissue transglutaminase meaningful in psoriatic patients? Relationship between the pathogenesis of psoriasis and coeliac disease. Br. J. Dermatol. 2002, 147, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Woo, W.; McMillan, S.; Watson, R.; Mccluggage, W.; Sloan, J.; McMillan, J. Coeliac disease-associated antibodies correlate with psoriasis activity. Br. J. Dermatol. 2004, 151, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, U.; Rudsander, Å.; Boström, Å.; Nilsson, B.; Michaëlsson, G. IgA antibodies to gliadin and coeliac disease in psoriatic arthritis. Rheumatology 2002, 41, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, D.; Pietrzak, A.; Krasowska, D.; Borzęcki, A.; Franciszkiewicz-Pietrzak, K.; Polkowska-Pruszyńska, B.; Baranowska, M.; Reich, K. Digestive system in psoriasis: An update. Arch. Dermatol. Res. 2017, 309, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Counsell, C.E.; Taha, A.; Ruddell, W. Coeliac disease and autoimmune thyroid disease. Gut 1994, 35, 844–846. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Hahn, E. Celiac Disease and its Link to Type 1 Diabetes Mellitus. J. Pediatr. Endocrinol. Metab. 2001, 14 (Suppl. S1), 597–605. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, H.; Nikamo, P.; Qi Low, H.; Helms, C.; Seielstad, M.; Liu, J.; Bowcock, A.; Stahle, M.; Liao, W. Association of Cardiovascular and Metabolic Disease Genes with Psoriasis. J. Investig. Dermatol. 2013, 133, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Trynka, G.; Hunt, K.; Bockett, N.; Romanos, J.; Mistry, V.; Szperl, A.; Bakker, S.; Bardella, M.; Bhaw-Rosun, L.; Castillejo, G.; et al. Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nat. Genet. 2011, 43, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, L.; Spain, S.; Knight, J.; Ellinghaus, E.; Stuart, P.; Capon, F.; Ding, J.; Li, Y.; Tejasvi, T.; Gudjonsson, J.; et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat. Genet. 2012, 44, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Birkenfeld, S.; Dreiher, J.; Weitzman, D.; Cohen, A. Coeliac disease associated with psoriasis. Br. J. Dermatol. 2009, 161, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.; Magazzù, G.; Greco, L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. Gastroenterology 1999, 117, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Holick, M. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef] [PubMed]
- De Bastiani, R.; Gabrielli, M.; Lora, L.; Napoli, L.; Tosetti, C.; Pirrotta, E.; Ubaldi, E.; Bertolusso, L.; Zamparella, M.; De Polo, M.; et al. Association between Coeliac Disease and Psoriasis: Italian Primary Care Multicentre Study. Dermatology 2015, 230, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Kolchak, N.; Tetarnikova, M.; Theodoropoulou, M.; Michalopoulou, A.; Theodoropoulos, D. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J. Multidiscip. Healthc. 2017, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Pastore, L.; Carroccio, A.; Compilato, D.; Panzarella, V.; Serpico, R.; Muzio, L. Oral Manifestations of Celiac Disease. J. Clin. Gastroenterol. 2008, 42, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Chavan, M.; Jain, H.; Diwan, N.; Khedkar, S.; Shete, A.; Durkar, S. Recurrent aphthous stomatitis: A review. J. Oral Pathol. Med. 2012, 41, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Lahteenoja, H.; Toivanen, A.; Viander, M.; Maki, M.; Irjala, K.; Raiha, I.; Syrjanen, S. Oral mucosal changes in coeliac patients on a gluten-free diet. Eur. J. Oral Sci. 1998, 106, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Bucci, P.; Carile, F.; Sangianantoni, A.; D’Angiò, F.; Santarelli, A.; Muzio, L. Oral aphthous ulcers and dental enamel defects in children with coeliac disease. Acta Paediatr. 2007, 95, 203–207. [Google Scholar] [CrossRef]
- Macho, V.; Coelho, A.; Veloso e Silva, D.M.; Andrade, D. Oral Manifestations in Pediatric Patients with Coeliac Disease—A Review Article. Open Dent. J. 2017, 11, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Zarkadas, M.; Anca, A.; Limeback, H. Oral manifestations of celiac disease: A clinical guide for dentists. J. Can. Dent. Assoc. 2011, 77, b39. [Google Scholar] [PubMed]
- Ferguson, R.; Basu, M.; Asquith, P.; Cooke, W. Jejunal mucosal abnormalities in patients with recurrent aphthous ulceration. BMJ 1976, 1, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Wray, D.; Carmichael, H.; Russell, R.; Lee, F. Coeliac disease associated with recurrent aphthae. Gut 1980, 21, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Wray, D. Gluten-sensitive recurrent aphthous stomatitis. Dig. Dis. Sci. 1981, 26, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Two, A.M.; Wu, W.; Gallo, R.L.; Hata, T.R. Rosacea: Part I. Introduction, categorization, histology, pathogenesis, and risk factors. J. Am. Acad. Dermatol. 2015, 72, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.D.; Steinhoff, M. Integrative concepts of rosacea pathophysiology, clinical presentation and new therapeutics. Exp. Dermatol. 2017, 26, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.L.S.; Raber, I.; Xu, J.; Li, R.; Spitale, R.; Chen, J.; Kiefer, A.K.; Tian, C.; Eriksson, N.K.; Hinds, D.A.; et al. Assessment of the genetic basis of rosacea by genome-wide association study. J. Investig. Dermatol. 2015, 135, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Hansen, P.; Gislason, G.; Thyssen, J. Clustering of autoimmune diseases in patients with rosacea. J. Am. Acad. Dermatol. 2016, 74, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Darwin, E.; Hirt, P.A.; Fertig, R.; Doliner, B.; Delcanto, G.; Jimenez, J.J. Alopecia Areata: Review of Epidemiology, Clinical Features, Pathogenesis, and New Treatment Options. Int. J. Trichol. 2018, 10, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Strazzulla, L.; Wang, E.H.C.; Avila, L.; Lo Sicco, K.; Brinster, N.; Christiano, A.M.; Shapiro, J. Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J. Am. Acad. Dermatol. 2018, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, V.; Tosun, M.; Erdem, T. Screening of celiac disease in children with alopecia areata. Indian J. Dermatol. 2014, 59, 317. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.R.; Andreani, M.L.; Venturo, N.; Bernardi, M.; Tosti, A.; Gasbarrini, G. Celiac disease and alopecia areata: Report of a new association. Gastroenterology 1995, 109, 1333–1337. [Google Scholar] [CrossRef]
- Volta, U.; Bardazzi, F.; Zauli, D.; Franceschi, L.; Tosti, A.; Mounaro, N.; Ghetti, S.; Tetta, C.; Grassi, A.; Bianchi, F. Serological screening for coeliac disease in vitiligo and alopecia areata. Br. J. Dermatol. 1997, 136, 801–802. [Google Scholar] [CrossRef] [PubMed]
- Hallaji, Z.; Akhyani, M.; Ehsani, A.H.; Noormohammadpour, P.; Gholamali, F.; Bagheri, M.; Jahromi, J. Prevalence of anti-gliadin antibody in patients with alopecia areata: A case-control study. Tehran Univ. Med. J. 2011, 68, 738–742. [Google Scholar]
- Collin, P.; Reunala, T. Recognition and Management of the Cutaneous Manifestations of Celiac Disease. Am. J. Clin. Dermatol. 2003, 4, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, F.; Panjehpour, T.; Naeini, F.; Hosseini, S.; Nilforoushzadeh, M.; Matin, M. The frequency distribution of celiac autoantibodies in alopecia areata. Int. J. Prev. Med. 2016, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Naveh, Y.; Rosenthal, E.; Ben-Arieh, Y.; Etzioni, A. Celiac disease-associated alopecia in childhood. J. Pediatr. 1999, 134, 362–364. [Google Scholar] [CrossRef]
- Baigrie, D.; Crane, J.S. Leukocytoclastic Vasculitis (Hypersensitivity Vasculitis); StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Pulido-Pérez, A.; Avilés-Izquierdo, J.; Suárez-Fernández, R. Cutaneous vasculitis. Actas Dermo-Sifiliogr. 2012, 103, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Holdstock, D.; Oleesky, S. Vasculitis in coeliac diseases. BMJ 1970, 4, 369. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.A. The skin: A mirror of the gut. Geriatrics 1973, 28, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Similä, S.; Kokkonen, J.; Kallioinen, M. Cutaneous vasculitis as a manifestation of coeliac disease. Acta Paediatr. Scand. 1982, 71, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Meyers, S.; Dikman, S.; Spiera, H.; Schultz, N.; Janowitz, H. Cutaneous vasculitis complicating coeliac disease. Gut 1981, 22, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Alegre, V.; Winkelmann, R.; Diez-Martin, J.; Banks, P. Adult celiac disease, small and medium vessel cutaneous necrotizing vasculitis, and T cell lymphoma. J. Am. Acad. Dermatol. 1988, 19, 973–978. [Google Scholar] [CrossRef]
- Menzies, I.; Pounder, R.; Heyer, S.; Laker, M.; Bull, J.; Wheeler, P.; Creamer, B. Abnormal intestinal permeability to sugars in villous atrophy. Lancet 1979, 314, 1107–1109. [Google Scholar] [CrossRef]
- Buderus, S.; Wagner, N.; Lentze, M. Concurrence of Celiac Disease and Juvenile Dermatomyositis: Result of a Specific Immunogenetic Susceptibility? J. Pediatr. Gastroenterol. Nutr. 1997, 25, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Farber, D.; Bitton, A.; Jass, J.; Singer, M.; Karpati, G. Dermatomyositis Associated with Celiac Disease: Response to a Gluten-Free Diet. Can. J. Gastroenterol. 2006, 20, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Evron, E.; Abarbanel, J.M.; Branski, D.; Sthoeger, Z.M. Polymyositis, arthritis, and proteinuria in a patient with adult celiac disease. J. Rheumatol. 1996, 23, 782–783. [Google Scholar] [PubMed]
- Hadjivassiliou, M.; Chattopadhyay, A.; Grünewald, R.; Jarratt, J.; Kandler, R.; Rao, D.; Sanders, D.; Wharton, S.; Davies-Jones, G. Myopathy associated with gluten sensitivity. Muscle Nerve 2007, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Shahmoradi, Z.; Najafian, J.; Naeini, F.F.; Fahimipour, F. Vitiligo and autoantibodies of celiac disease. Int. J. Prev. Med. 2013, 4, 200–203. [Google Scholar] [PubMed]
- Rodríguez-García, C.; González-Hernández, S.; Pérez-Robayna, N.; Guimerá, F.; Fagundo, E.; Sánchez, R. Repigmentation of Vitiligo Lesions in a Child with Celiac Disease after a Gluten-Free Diet. Pediatr. Dermatol. 2011, 28, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Mirza, N.; Bonilla, E.; Phillips, P. Celiac disease in a patient with systemic lupus erythematosus: A case report and review of literature. Clin. Rheumatol. 2006, 26, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Adult celiac disease followed by onset of systemic lupus erythematosus. J. Clin. Gastroenterol. 2008, 42, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Jamal, A.; Memon, I.; Yasmeen, S.; Tresa, V.; Shaikh, S. Multiple autoimmune syndrome: Hashimoto’s thyroiditis, Coeliac disease and systemic lupus erythematosus (SLE). J. Pak. Med. Assoc. 2010, 60, 863–865. [Google Scholar] [PubMed]
- Cigic, L.; Gavic, L.; Simunic, M.; Ardalic, Z.; Biocina-Lukenda, D. Increased prevalence of celiac disease in patients with oral lichen planus. Clin. Oral Investig. 2015, 19, 627–635. [Google Scholar] [CrossRef] [PubMed]
- De, D. Eruptive lichen planus in a child with celiac disease. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Villaverde, R.; Blasco Melguizo, J.; Menéndez García Estrada, A.; Díez García, F. Erosive mucosal lichen associated to hyper IgE syndrome and coeliac disease. An. Pediatr. 2004, 60, 281–282. [Google Scholar] [CrossRef]
- Scully, C.; Porter, S.R.; Eveson, J.W. Oral lichen planus and coeliac disease. Lancet 1993, 341, 1660. [Google Scholar] [CrossRef]
- Fortune, F.; Buchanan, J. Oral lichen planus and coeliac disease. Lancet 1993, 341, 1154–1155. [Google Scholar] [CrossRef]
- Compilato, D.; Carroccio, A.; Campisi, G. Hidden coeliac disease in patients suffering from oral lichen planus. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.; Kavala, M.; Ozlu, E.; Zindancı, İ.; Ozkanlı, S.; Turkoglu, Z.; Zemheri, E. The co-occurrence of lichen sclerosus et atrophicus and celiac disease. Indian Dermatol. Online J. 2014, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.; Gilliam, A.; Khavari, N.; Bass, D. Association between Lichen Sclerosus and Celiac Disease: A Report of Three Pediatric Cases. Pediatr. Dermatol. 2014, 31, e128–e131. [Google Scholar] [CrossRef] [PubMed]
- Essoussi, A.S.; Jomaa, B.; El Fehaiel, A. Association of celiac disease with sclero-atrophic lichen in a child with the HLA-B8 group. Tunis. Med. 1981, 59, 506–507. [Google Scholar] [PubMed]
- Daoud, W.; El Euch, D.; Mokni, M.; Cherif, F.; Ben Tekaya, N.; Azaiz, M.I.; Ben Osman-Dhahri, A. Linear IgA bullous dermatosis associated with celiac disease. Ann. Dermatol. Venereol. 2006, 133, 588–589. [Google Scholar] [CrossRef]
- Vaz, S.; Franco, C.; Santos, P.; Amaral, R. Skin and coeliac disease, a lot to think about: A case series. BMJ Case Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bonciolini, V.; Antiga, E.; Fabbri, P.; Caproni, M. Skin manifestations of celiac disease: Not always dermatitis herpetiformis. Int. J. Dermatol. 2014, 53, e352–e353. [Google Scholar] [CrossRef] [PubMed]
- Randle, H.W.; Winkelmann, R.K. Pityriasis rubra pilaris and celiac sprue with malabsorption. Cutis 1980, 25, 626–627. [Google Scholar] [PubMed]
- Tasanen, K.; Raudasoja, R.; Kallioinen, M.; Ranki, A. Erythema elevatum diutinum in association with coeliac disease. Br. J. Dermatol. 1997, 136, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Collin, P.; Korpela, M.; Hällström, O.; Viander, M.; Keyriläinen, O.; Mäki, M. Rheumatic Complaints as a Presenting Symptom in Patients with Coeliac Disease. Scand. J. Rheumatol. 1992, 21, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Serna, M.; Fortea, J.; Perez, A.; Febrer, I.; Ribes, C.; Aliaga, A. Erythema elevatum diutinum associated with celiac disease: Response to a gluten-free diet. Pediatr. Dermatol. 1993, 10, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Goodenberger, D.M.; Lawley, T.J.; Strober, W.; Wyatt, L.; Sangree, M.H., Jr.; Sherwin, R.; Rosenbaum, H.; Braverman, I.; Katz, S.I. Necrolytic Migratory Erythema Without Glucagonoma. Arch. Dermatol. 1979, 115, 1429–1432. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Johnston, C.; Nolan, N.; Keeling, P.; Weir, D. Necrolytic Migratory Erythema with Elevated Plasma Enteroglucagon in Celiac Disease. Gastroenterology 1989, 96, 1350–1353. [Google Scholar] [CrossRef]
- Thorisdottir, K.; Camisa, C.; Tomecki, K.; Bergfeld, W. Necrolytic migratory erythema: A report of three cases. J. Am. Acad. Dermatol. 1994, 30, 324–329. [Google Scholar] [CrossRef]
- Cribier, B.; Caille, A.; Heid, E.; Grosshans, E. Erythema nodosum and associated diseases. A study of 129 cases. Int. J. Dermatol. 1998, 37, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.; Lefevre, P.; Weiller, C. Erythema nodosum and coeliac disease. Br. J. Dermatol. 1991, 125, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Bartyik, K.; Varkonyi, A.; Kirschner, A.; Endreffy, E.; Turi, S.; Karg, E. Erythema Nodosum in Association with Celiac Disease. Pediatr. Dermatol. 2004, 21, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Twaddle, S.; Wassif, W.S.; Deacon, A.C.; Peters, T.J. Celiac disease in patients with variegate porphyria. Dig. Dis. Sci. 2001, 46, 1506–1508. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.; Disler, P. Drug sensitive diseases-I-acute porphyrias. Advers. Drug React. Bull. 1988, 129, 484–487. [Google Scholar] [CrossRef]
- Katsikas, G.; Maragou, M.; Rontogianni, D.; Gouma, P.; Koutsouvelis, I.; Kappou-Rigatou, I. Secondary cutaneous nodular AA amyloidosis in a patient with primary Sjögren syndrome and celiac disease. J. Clin. Rheumatol. 2008, 14, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.; Lewis-Jones, S.; Gipson, M. Acquired cutis laxa with dermatitis herpetiformis and sarcoidosis. J. Am. Acad. Dermatol. 1993, 29, 846–848. [Google Scholar] [CrossRef]
- García-Patos, V.; Pujol, R.; Barnadas, M.; Pérez, M.; Moreno, A.; Condomines, J.; Gelpi, C.; Rodríguez, J.; De Moragas, J. Generalized acquired cutis laxa associated with coeliac disease: Evidence of immunoglobulin A deposits on the dermal elastic fibres. Br. J. Dermatol. 1996, 135, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.; Floridis, J. Hypertrichosis Lanuginosa acquisita: A rare dermatological disorder. Lancet 2016, 387, 2035. [Google Scholar] [CrossRef]
- Menni, S.; Boccardi, D.; Brusasco, A. Ichthyosis revealing coeliac disease. Eur. J. Dermatol. 2000, 10, 398–399. [Google Scholar] [PubMed]
- O’Mahony, D.; O’Mahony, S.; Whelton, M.; McKiernan, J. Partial lipodystrophy in coeliac disease. Gut 1990, 31, 717–718. [Google Scholar] [CrossRef] [PubMed]
- Foti, C.; Cassano, N.; Palmieri, V.O.; Portincasa, P.; Conserva, A.; Lamuraglia, M.; Palasciano, G.; Vena, G.A. Transverse leukonychia in severe hypocalcemia. Eur. J. Dermatol. 2004, 14, 67–68. [Google Scholar] [PubMed]
- Montalto, M.; Diociaiuti, A.; Alvaro, G.; Manna, R.; Amerio, P.L.; Gasbarrini, G. Atypical mole syndrome and congenital giant naevus in a patient with celiac disease. Panminerva Med. 2003, 45, 219–221. [Google Scholar] [PubMed]
- Lebwohl, B.; Eriksson, H.; Hansson, J.; Green, P.; Ludvigsson, J. Risk of cutaneous malignant melanoma in patients with celiac disease: A population-based study. J. Am. Acad. Dermatol. 2014, 71, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Pastore, L.; De Benedittis, M.; Petruzzi, M.; Tatò, D.; Napoli, C.; Montagna, M.T.; Catassi, C.; Serpico, R. Importance of oral signs in the diagnosis of atypical forms of celiac disease. Recenti Prog. Med. 2004, 95, 482–490. [Google Scholar] [PubMed]
- Lahteenoja, H. Oral Mucosa Is Frequently Affected in Patients with Dermatitis Herpetiformis. Arch. Dermatol. 1998, 134, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, E.; Cicciù, M.; Matacena, G.; Costa, S.; Magazzù, G. Clinical Evaluation of Specific Oral Manifestations in Pediatric Patients with Ascertained versus Potential Coeliac Disease: A Cross-Sectional Study. Gastroenterol. Res. Pract. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Clinical presentation of celiac disease in the pediatric population. Gastroenterology 2005, 128, S68–S73. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Newnham, E.D. Coeliac disease in the 21st century: Paradigm shifts in the modern age. J. Gastroenterol. Hepatol. 2017, 32, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Rostami Nejad, M.; Hogg-Kollars, S.; Ishaq, S.; Rostami, K. Subclinical celiac disease and gluten sensitivity. Gastroenterol. Hepatol. Bed Bench 2011, 4, 102–108. [Google Scholar] [PubMed]
- Tonutti, E.; Bizzaro, N. Diagnosis and classification of celiac disease and gluten sensitivity. Autoimmun. Rev. 2014, 13, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Gatti, S.; Pulvirenti, A.; Catassi, C. Celiac disease from a global perspective. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Card, T.R.; Kaukinen, K.; Bai, J.; Zingone, F.; Sanders, D.S.; Murray, J.A. Screening for celiac disease in the general population and in high-risk groups. United Eur. Gastroenterol. J. 2015, 3, 106–120. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).