Protective Effects of Salvianolic Acid A against Dextran Sodium Sulfate-Induced Acute Colitis in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Treatments
2.3. Disease Activity Index (DAI) Evaluations
2.4. Histologic Analysis
2.5. Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
2.6. Microbiota Analysis
2.7. Statistical Analyses
3. Results
3.1. Effect of Salvianolic Acid A on DSS-Induced Colitis Symptoms
3.2. Salvianolic Acid A Decreased Inflammatory Gene Expression in the Colonic Mucosa of Rats after DSS-Induced Colitis
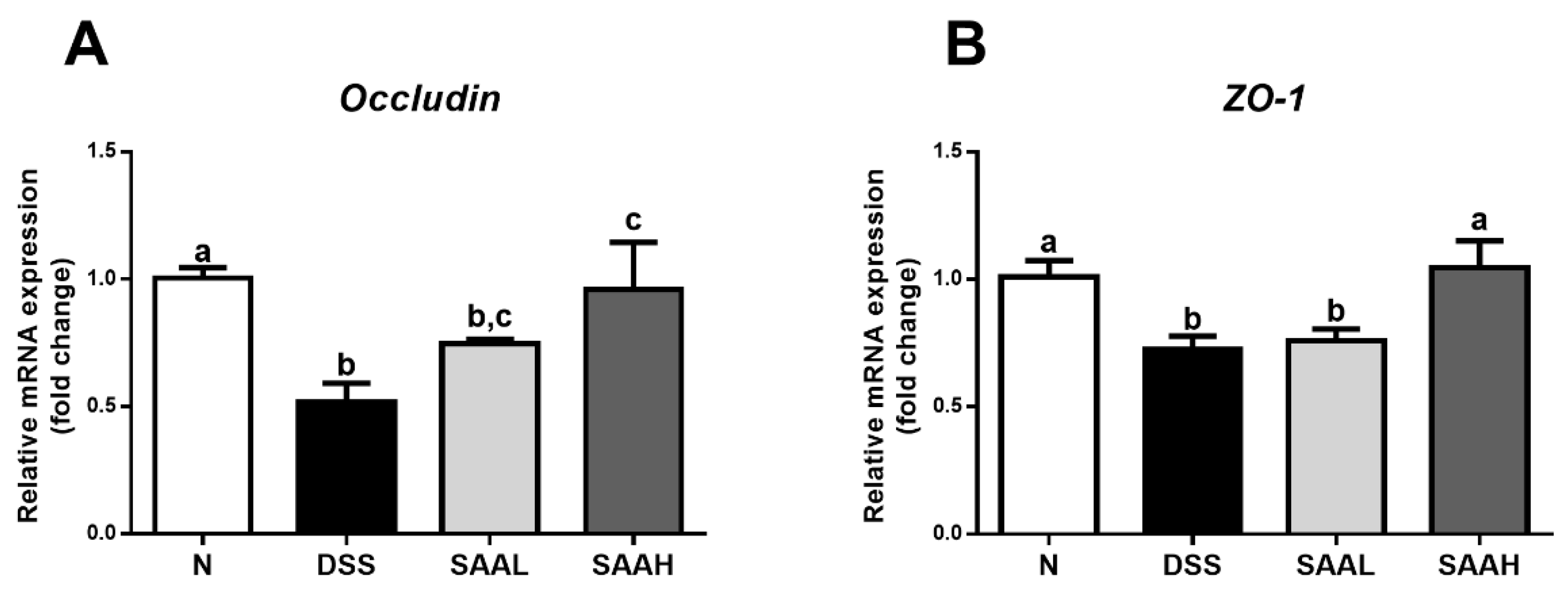
3.3. SAA Proteced against DSS-Induced Damage to Tight Junctions in the Colon of Rats
3.4. Effects of SAA on Gut Microbial Composition in DSS-Induced-Colitis Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiou, Y.S.; Lee, P.S.; Pan, M.H. Food Bioactives and Their Effects on Obesity-Accelerated Inflammatory Bowel Disease. J. Agric. Food Chem. 2018, 66, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.C.; Stappenbeck, T.S. Genetics and pathogenesis of inflammatory bowel disease. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Lin, J.; Li, W.; Wu, Z.; He, Z.; Huang, G. Huangqin-tang ameliorates dextran sodium sulphate-induced colitis by regulating intestinal epithelial cell homeostasis, inflammation and immune response. Sci. Rep. 2016, 6, 39299. [Google Scholar] [CrossRef] [PubMed]
- Muluye, R.A.; Bian, Y.; Alemu, P.N. Anti-inflammatory and antimicrobial effects of heat-clearing Chinese herbs: A current review. J. Tradit. Complement. Med. 2014, 4, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Kim, D.H.; Lim, B.O. Natural products as a source of anti-inflammatory agents associated with inflammatory bowel disease. Molecules 2013, 18, 7253–7270. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Q. Salvia miltiorrhiza: Chemical and pharmacological review of a medicinal plant. J. Med. Plants Res. 2010, 4, 2813–2820. [Google Scholar] [CrossRef]
- Dai, H.; Xiao, C.; Liu, H.; Hao, F.; Tang, H. Combined NMR and LC-DAD-MS analysis reveals comprehensive metabonomic variations for three phenotypic cultivars of Salvia miltiorrhiza Bunge. J. Proteome Res. 2010, 9, 1565–1578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pan, Y.; Huang, X.; Feng, G.; Ma, M.; Ni, J. Effects of dexamethasone and Salvia miltiorrhizae on the small intestine and immune organs of rats with severe acute pancreatitis. Inflammation 2010, 33, 259–266. [Google Scholar] [CrossRef]
- Wen, X.D.; Wang, C.Z.; Yu, C.H.; Zhang, Z.Y.; Tyler, C.; Wang, Y.W. Salvia miltiorrhiza (dan shen) significantly ameliorates colon inflammation in dextran sulfate sodium induced colitis. Am. J. Chin. Med. 2013, 41, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.Y.; Jiang, Q.; Li, K.R.; Zhao, Y.X.; Cao, C. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic. Biol. Med. 2014, 69, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tian, S.; Yang, F.H.; Yang, X.; Du, G.H. Cardioprotective effect of salvianolic acid A on isoproterenol-induced myocardial infarction in rats. Eur. J. Pharmacol. 2009, 615, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.S.; Oh, B.K.; Mun, J.; Seo, H.W.; Lee, B.H. Salvianolic acid A suppress lipopolysaccharide-induced NF-κB signaling pathway by targeting IKKβ. Int. Immunopharmacol. 2011, 11, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Qiang, G.; Yang, X.; Shi, L.; Zhang, H.; Chen, B.; Zhao, Y. Antidiabetic effect of salvianolic acid A on diabetic animal models via AMPK activation and mitochondrial regulation. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 36, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Fu, H.; Kong, Q.; Xiao, Y.; Shou, Q.; Chen, H. Prevention of pulmonary fibrosis with salvianolic acid a by inducing fibroblast cell cycle arrest and promoting apoptosis. J. Ethnopharmacol. 2014, 155, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Narula, A.; Jobin, C. Salvia miltiorrhiza water-soluble extract, but not its constituent salvianolic acid B, abrogates LPS-induced NF-κB signalling in intestinal epithelial cells. Clin. Exp. Immunol. 2005, 141, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, X.; You, M.; Tian, W.; Leu, R.K.L.; Topping, D.L. Dietary Propolis Ameliorates Dextran Sulfate Sodium-Induced Colitis and Modulates the Gut Microbiota in Rats Fed a Western Diet. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Mascaraque, C.; González, R.; Suárez, M.D.; Zarzuelo, A.; Medina, F.S.; Martínez-Augustin, O. Intestinal anti-inflammatory activity of apigenin K in two rat colitis models induced by trinitrobenzenesulfonic acid and dextran sulphate sodium. Br. J. Nutr. 2015, 113, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Valatas, V.; Bamias, G.; Kolios, G. Experimental colitis models: Insights into the pathogenesis of inflammatory bowel disease and translational issues. Eur. J. Pharmacol. 2015, 759, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Cytokines, IBD, and colitis-associated cancer. Inflamm. Bowel Dis. 2015, 21, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, T.; Du, Y.; Pan, D.; Wu, W.; Zhu, H. Salvianolic acid A attenuates cell apoptosis, oxidative stress, Akt and NF-κB activation in angiotensin-II induced murine peritoneal macrophages. Curr. Pharm. Biotechnol. 2016, 17, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Li, B. Salvianolic Acid A Suppresses CCL-20 Expression in TNF-α–treated Macrophages and ApoE-deficient Mice. J. Cardiovasc. Pharmacol. 2014, 64, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, H.; Kim, S.J.; Hahm, K.B. Connection between inflammation and carcinogenesis in gastrointestinal tract: Focus on TGF-β signaling. World J. Gastroenterol. 2010, 16, 2080–2093. [Google Scholar] [CrossRef] [PubMed]
- Qiang, G.; Yang, X.; Xuan, Q.; Shi, L.; Zhang, H.; Chen, B. Salvianolic acid A prevents the pathological progression of hepatic fibrosis in high-fat diet-fed and streptozotocin-induced diabetic rats. Am. J. Chin. Med. 2014, 42, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Su, S.; Guo, J.; Zhu, Y.; Zhao, M.; Duan, J. The aerial parts of Salvia miltiorrhiza Bge. strengthen intestinal barrier and modulate gut microbiota imbalance in streptozocin-induced diabetic mice. J. Funct. Foods 2017, 36, 362–374. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G. The gut microbiota and host health: A new clinical frontier. Gut 2015, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lian, F.; Zhao, L.; Zhao, Y.; Chen, X.; Zhang, X. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. J. ISME 2015, 9, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, M.; Zhao, A.; Jia, W. Traditional Chinese medicine: Balancing the gut ecosystem. Phytother. Res. 2009, 23, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, H.B.; Li, S.L. Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Med. Res. Rev. 2017, 37, 1140–1185. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Belzer, C.; De Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef]
- Kang, C.-S.; Ban, M.; Choi, E.-J.; Moon, H.-G.; Jeon, J.-S.; Kim, D.-K.; Park, S.-K.; Jeon, S.G.; Roh, T.-Y.; Myung, S.-J. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Normal | DSS | SAA (4 mg/kg) | SAA (8 mg/kg) |
|---|---|---|---|---|
| Body weight (g) | 321.14 ± 9.06 a | 298.63 ± 12.13 b | 305.21 ± 17.1 a,b | 315.9 ± 12.02 a |
| Liver weight (mg/g) | 40.67 ± 4.62 a | 43.58 ± 6.93 b | 39.66 ± 2.77 a | 36.66 ± 3.27 c |
| Spleen weight (mg/g) | 2.32 ± 0.44 | 2.6 ± 0.51 | 2.93 ± 0.65 | 2.94 ± 0.41 |
| Kidney weight (mg/g) | 8.09 ± 0.71 | 8.68 ± 0.59 | 7.95 ± 1.71 | 8.08 ± 0.33 |
| Epididymal adipose tissue (mg/g) | 7.2 ± 1.64 | 7.45 ± 1.62 | 6.84 ± 3.23 | 8.38 ± 2.17 |
| Retroperitoneal adipose tissue (mg/g) | 6.29 ± 2.22 | 7.49 ± 2.52 | 7.1 ± 2.06 | 7.39 ± 2.35 |
| Groups | Effective Tags | Good’s Coverage | ACE Estimators | Chao1 Estimators | Simpson Indices | Shannon Indices | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 38,053 | ± | 3143 | 0.996 | ± | 0.001 | 675 | ± | 20a | 691 | ± | 18a | 0.0608 | ± | 0.0070 a | 4.1 | ± | 0.1 a |
| DSS | 36,479 | ± | 2008 | 0.996 | ± | 0.001 | 521 | ± | 21b | 537 | ± | 19c | 0.1526 | ± | 0.0215 b | 3.3 | ± | 0.1 b |
| SAA (8 mg/kg) | 32,925 | ± | 1770 | 0.997 | ± | 0.001 | 629 | ± | 10a | 639 | ± | 13b | 0.0471 | ± | 0.0052 a | 4.2 | ± | 0.1 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Yang, Q.; Ma, Q.; Wang, B.; Wan, Z.; Chen, M.; Wu, L. Protective Effects of Salvianolic Acid A against Dextran Sodium Sulfate-Induced Acute Colitis in Rats. Nutrients 2018, 10, 791. https://doi.org/10.3390/nu10060791
Wang K, Yang Q, Ma Q, Wang B, Wan Z, Chen M, Wu L. Protective Effects of Salvianolic Acid A against Dextran Sodium Sulfate-Induced Acute Colitis in Rats. Nutrients. 2018; 10(6):791. https://doi.org/10.3390/nu10060791
Chicago/Turabian StyleWang, Kai, Qinqin Yang, Quanxin Ma, Bei Wang, Zhengrui Wan, Minli Chen, and Liming Wu. 2018. "Protective Effects of Salvianolic Acid A against Dextran Sodium Sulfate-Induced Acute Colitis in Rats" Nutrients 10, no. 6: 791. https://doi.org/10.3390/nu10060791
APA StyleWang, K., Yang, Q., Ma, Q., Wang, B., Wan, Z., Chen, M., & Wu, L. (2018). Protective Effects of Salvianolic Acid A against Dextran Sodium Sulfate-Induced Acute Colitis in Rats. Nutrients, 10(6), 791. https://doi.org/10.3390/nu10060791






