Growth Responses of Preterm Pigs Fed Formulas with Different Protein Levels and Supplemented with Leucine or β-Hydroxyl β-Methylbutyrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Nutrition Support
2.2.1. Experiment 1
2.2.2. Experiment 2
2.3. Assessment of Responses
2.4. Statistics
3. Results
3.1. General Observations and Results
3.2. Growth during EN
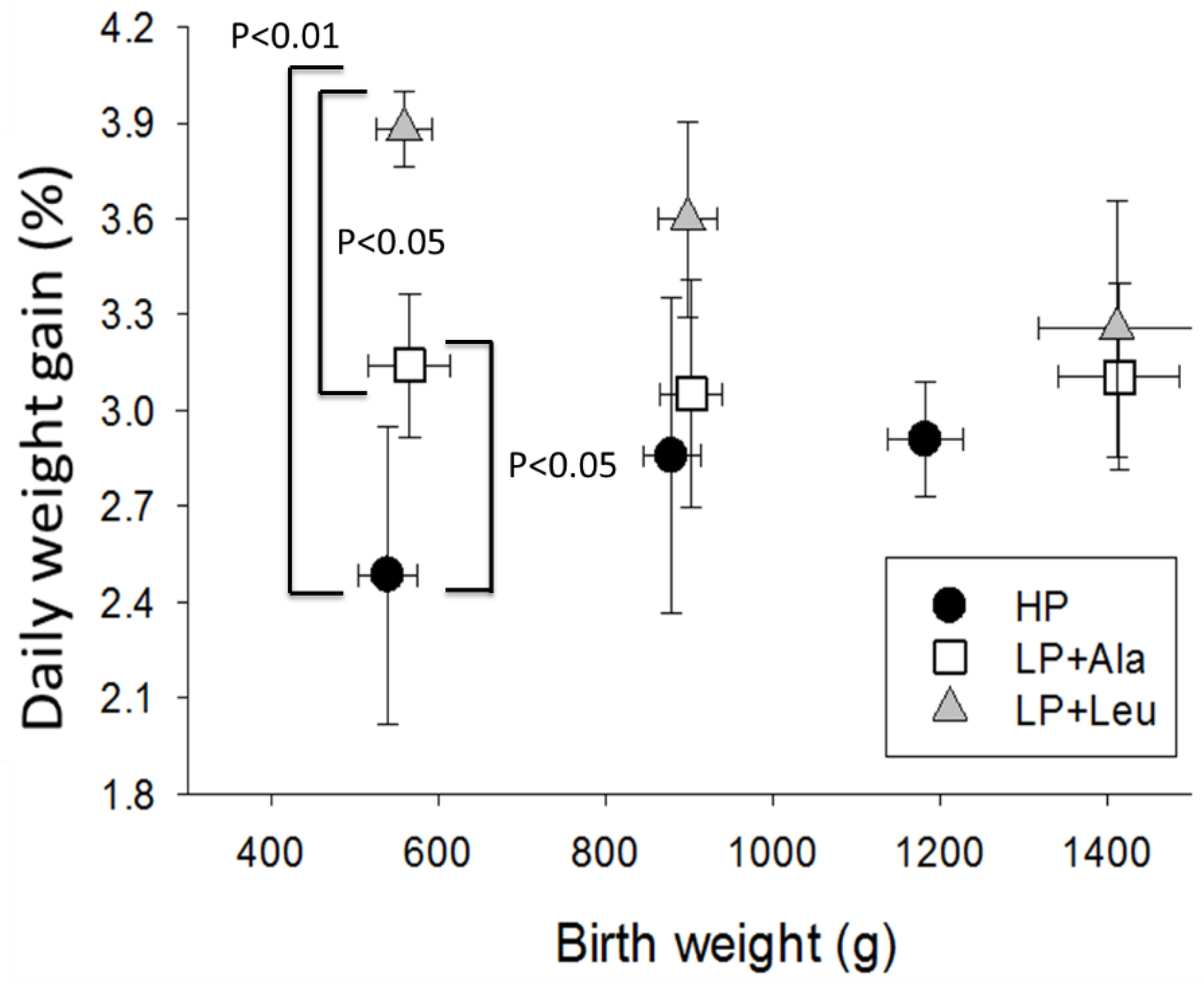
3.2.1. Experiment 1
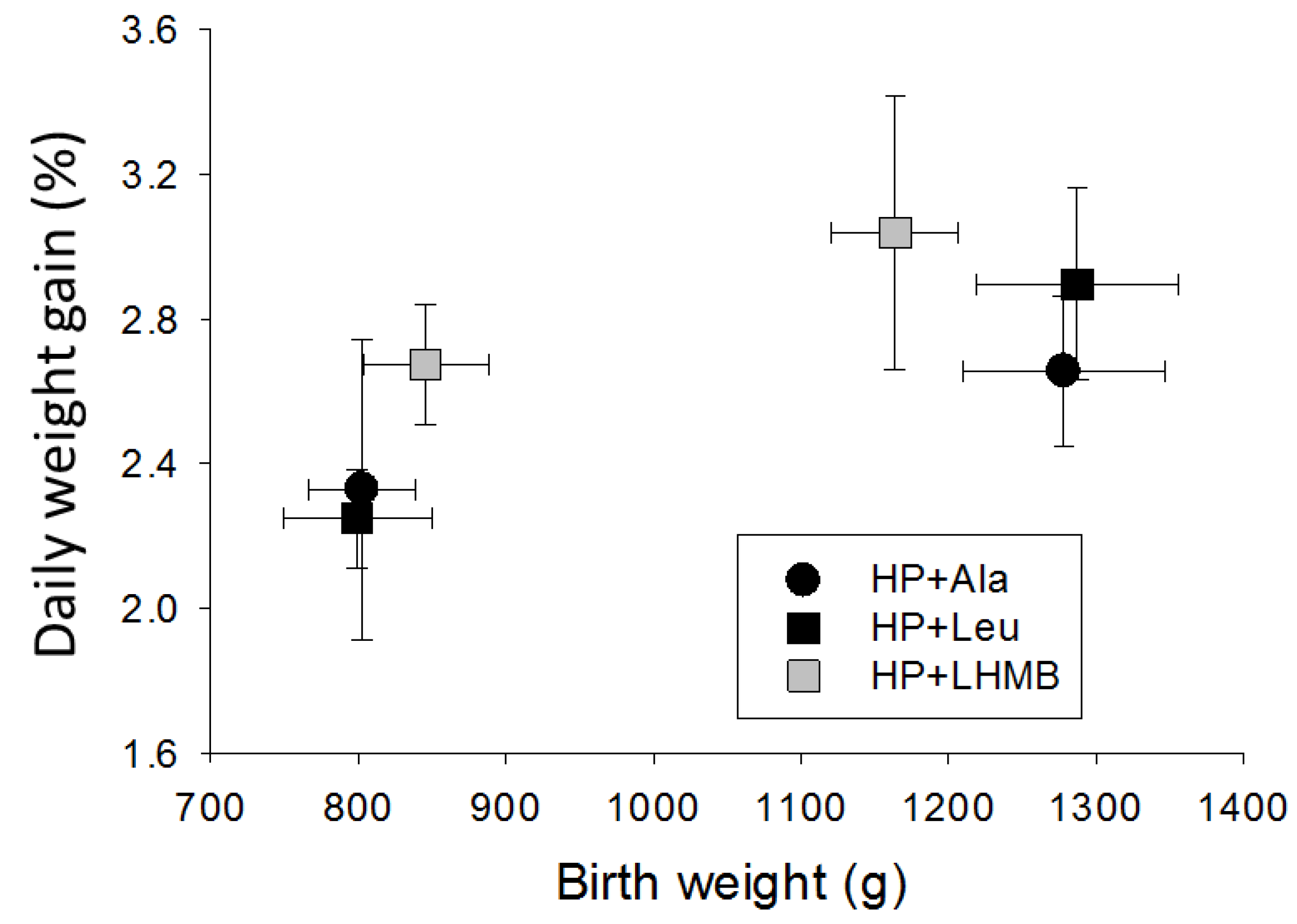
3.2.2. Experiment 2
3.3. Hematology and Blood Chemistries
3.3.1. Experiment 1
3.3.2. Experiment 2
4. Discussion
4.1. Growth Responses to Different Levels of Protein
4.2. Responses to Leucine and HMB
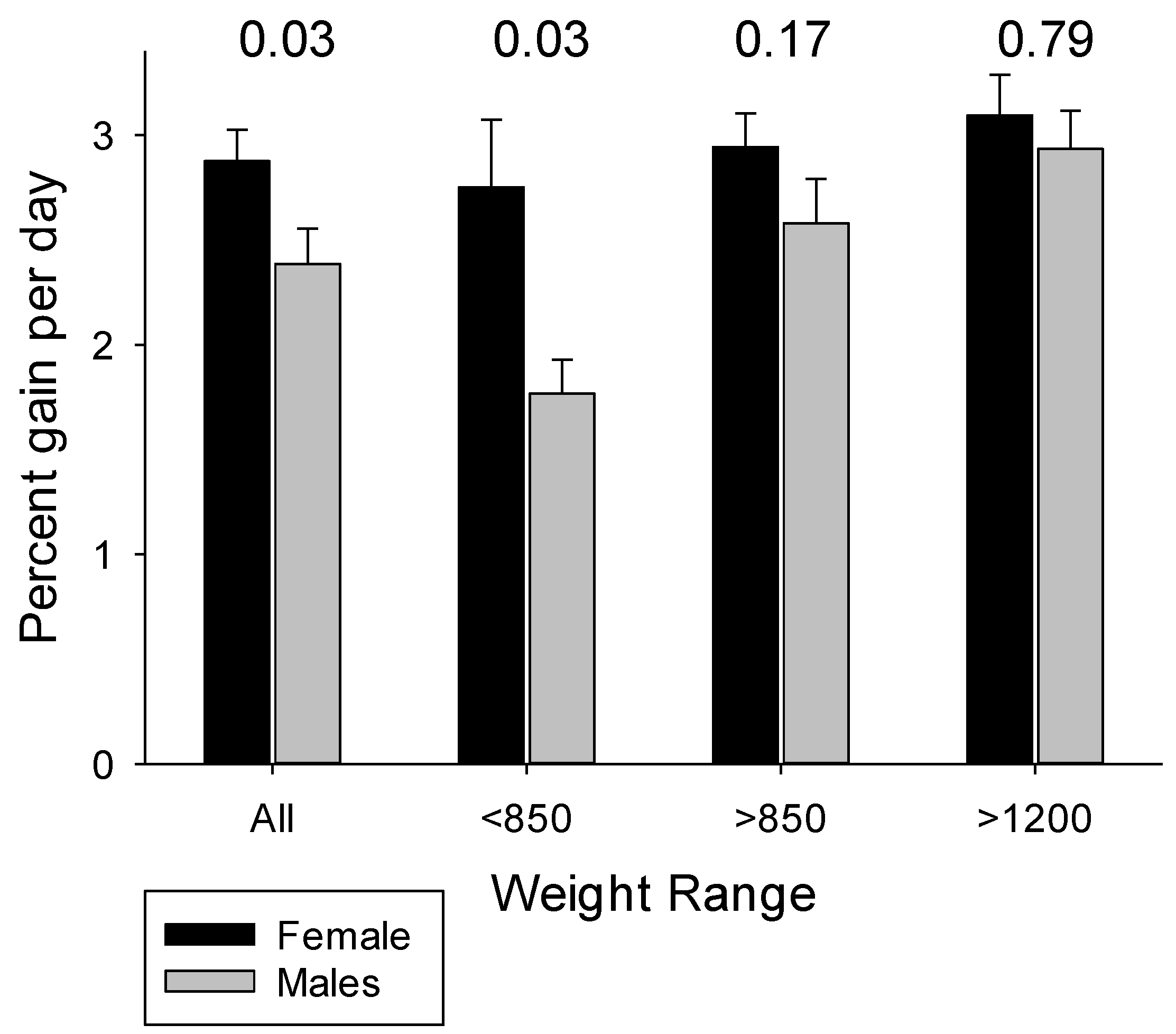
4.3. The Influences of Birth Weight and Sex
4.4. Hematology and Clinical Chemistries
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Pampanini, V.; Boiani, A.; De Marchis, C.; Giacomozzi, C.; Navas, R.; Agostino, R.; Dini, F.; Ghirri, P.; Cianfarani, S. Preterm infants with severe extrauterine growth retardation (EUGR) are at high risk of growth impairment during childhood. Eur. J. Pediatr. 2015, 174, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fanaro, S. Which is the ideal target for preterm growth? Miner. Pediatr. 2010, 62, 77–82. [Google Scholar]
- Fenton, T.R.; Nasser, R.; Eliasziw, M.; Kim, J.H.; Bilan, D.; Sauve, R. Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr. 2013, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, E. Protein Needs of Preterm Infants: Why Are They So Difficult to Meet? Nestlé Nutr. Inst. Workshop Ser. 2016, 86, 121–128. [Google Scholar] [PubMed]
- Hay, W.W.; Hendrickson, K.C. Preterm formula use in the preterm very low birth weight infant. Semin. Fetal Neonatal Med. 2017, 22, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Yumani, D.F.; Lafeber, H.N.; van Weissenbruch, M.M. Dietary proteins and IGF I levels in preterm infants: Determinants of growth, body composition, and neurodevelopment. Pediatr. Res. 2014, 77, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Denne, S.C.; Poindexter, B.B. Evidence supporting early nutritional support with parenteral amino acid infusion. Semin. Perinatol. 2007, 31, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Premji, S.S.; Al-Wassia, H.; Sauve, R.S. Higher versus lower protein intake in formula-fed low birth weight infants. Cochrane Database Syst. Rev. 2014, CD003959. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, I. Effects of High Protein Intakes. Nestlé Nutr. Workshop Ser. Pediatr. Program 2006, 58, 121–131. [Google Scholar] [PubMed]
- Premji, S.; Fenton, T.; Sauve, R. Does amount of protein in formula matter for low-birthweight infants? A Cochrane systematic review. J. Parenter. Enter. Nutr. 2006, 30, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Beyer, J.; Brands, B.; Demmelmair, H.; Grote, V.; Haile, G.; Gruszfeld, D.; Rzehak, P.; Socha, P.; Weber, M. Early influences of nutrition on postnatal growth. Nestlé Nutr. Inst. Workshop Ser. 2013, 71, 11–27. [Google Scholar] [PubMed]
- Wilkinson, D.J.; Hossain, T.; Hill, D.; Phillips, B.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.; Breen, L.; Phillips, S. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Suryawan, A.; Torrazza, R.M.; Gazzaneo, M.C.; Orellana, R.A.; Fiorotto, M.L.; El-Kadi, S.W.; Srivastava, N.; Nguyen, H.V.; Davis, T.A. Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr. Res. 2012, 71, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, Z.; Deng, X.; Xu, X.; Feng, C.; Li, Y.; Cui, C.; Su, W.; Zhao, H.; Yu, D. Involvement of insulin in early development of mouse one-cell stage embryos. Sci. China Ser. C Life Sci. 2008, 51, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Kim, J.; Song, G.; Ka, H.; Tekwe, C.D.; Wu, G. Select nutrients, progesterone, and interferon tau affect conceptus metabolism and development. Ann. N. Y. Acad. Sci. 2012, 1271, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, S.M.; El-Kadi, S.W.; Suryawan, A.; Boutry, C.; Orellana, R.A.; Nguyen, H.V.; Davis, S.R.; Davis, T.A. Protein synthesis in skeletal muscle of neonatal pigs is enhanced by administration of β-hydroxy-β-methylbutyrate. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E91–E99. [Google Scholar] [CrossRef] [PubMed]
- Manjarín, R.; Columbus, D.A.; Suryawan, A.; Nguyen, H.V.; Hernandez-García, A.D.; Hoang, N.-M.; Fiorotto, M.L.; Davis, T. Leucine supplementation of a chronically restricted protein and energy diet enhances mTOR pathway activation but not muscle protein synthesis in neonatal pigs. Amino Acids 2016, 48, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Zhu, J.; Su, G.; Liu, Y.; Hua, L.; Hu, L.; Wu, C.; Zhang, R.; Zhou, P.; Shen, Y. Dietary supplementation with β-hydroxy-β-methylbutyrate calcium during the early postnatal period accelerates skeletal muscle fibre growth and maturity in intra-uterine growth-retarded and normal-birth-weight piglets. Br. J. Nutr. 2016, 115, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Bai, K.; He, J.; Su, W.; Dong, L.; Zhang, L.; Wang, T. Leucine improves growth performance of intrauterine growth retardation piglets by modifying gene and protein expression related to protein synthesis. Nutrition 2016, 32, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Sangild, P.T.; Thymann, T.; Schmidt, M.; Stoll, B.; Burrin, D.G.; Buddington, R.K. Invited review: The preterm pig as a model in pediatric gastroenterology. J. Anim. Sci. 2013, 91, 4713–4729. [Google Scholar] [CrossRef] [PubMed]
- Foxcroft, G.; Dixon, W.; Dyck, M.; Novak, S.; Harding, J.; Almeida, F. Prenatal programming of postnatal development in the pig. Soc. Reprod. Fertil. Suppl. 2009, 66, 213. [Google Scholar] [PubMed]
- Buddington, R.K.; Davis, S.L.; Buddington, K.K. The risk of necrotizing enterocolitis differs among preterm pigs fed formulas with either lactose or maltodextrin. J. Pediatr. Gastroenterol. Nutr. 2018, 66, e61–e66. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Strathe, A.; Kebreab, E.; France, J.; Theil, P. Predicting milk yield and composition in lactating sows: A Bayesian approach. J. Anim. Sci. 2012, 90, 2285–2298. [Google Scholar] [CrossRef] [PubMed]
- Declerck, I.; Dewulf, J.; Piepers, S.; Decaluwé, R.; Maes, D. Sow and litter factors influencing colostrum yield and nutritional composition. J. Anim. Sci. 2015, 93, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Heo, S.; Jin, Z.; Yun, J.; Choi, J.; Yoon, S.; Park, M.; Yang, B.; Chae, B. Effects of lysine intake during late gestation and lactation on blood metabolites, hormones, milk composition and reproductive performance in primiparous and multiparous sows. Anim. Reprod. Sci. 2009, 112, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Mahan, D.; Watts, M.; St-Pierre, N. Macro-and micromineral composition of fetal pigs and their accretion rates during fetal development. J. Anim. Sci. 2009, 87, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.; Ji, F.; Wu, G.; Blanton, J.; Kim, S. Growth and compositional changes of fetal tissues in pigs. J. Anim. Sci. 2004, 82, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Wangsness, P.J.; Soroka, G.H. Effect of energy concentration of milk on voluntary intake of lean and obese piglets. J. Nutr. 1978, 108, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Columbus, D.A.; Steinhoff-Wagner, J.; Suryawan, A.; Nguyen, H.V.; Hernandez-Garcia, A.; Fiorotto, M.L.; Davis, T.A. Impact of prolonged leucine supplementation on protein synthesis and lean growth in neonatal pigs. Am. J. Physiol.-Endocrinol. Metab. 2015, 309, E601–E610. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.W.; Escobar, J.; Suryawan, A.; Kimball, S.R.; Nguyen, H.V.; Jefferson, L.S.; Davis, T.A. Protein synthesis and translation initiation factor activation in neonatal pigs fed increasing levels of dietary protein. J. Nutr. 2005, 135, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.; Edwards, S.; Kyriazakis, I. Management strategies to improve the performance of low birth weight pigs to weaning and their long-term consequences. J. Anim. Sci. 2014, 92, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Buddington, R.; Sangild, P.T.; Hance, B.; Huang, E.; Black, D. Prenatal gastrointestinal development in the pig and responses after preterm birth. J. Anim. Sci. 2012, 90, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.C.; Babayan, V.K. Medium-chain triglycerides: An update. Am. J. Clin. Nutr. 1982, 36, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Yao, K.; Liu, Z.; Gong, M.; Ruan, Z.; Deng, D.; Tan, B.; Liu, Z.; Wu, G. Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 2010, 39, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Kao, M.; Columbus, D.A.; Suryawan, A.; Steinhoff-Wagner, J.; Hernandez-Garcia, A.; Nguyen, H.V.; Fiorotto, M.L.; Davis, T.A. Enteral β-hydroxy-β-methylbutyrate supplementation increases protein synthesis in skeletal muscle of neonatal pigs. Am. J. Physiol.-Endocrinol. Metab. 2016, 310, E1072–E1084. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Coscia, A.; Mombrò, M.; Boni, L.; Rossetti, G.; Fabris, C.; Spada, E.; Milani, S. Postnatal weight increase and growth velocity of very low birthweight infants. Arch. Dis. Child.-Fetal Neonatal Ed. 2006, 91, F349–F356. [Google Scholar] [CrossRef] [PubMed]
- Euser, A.; De Wit, C.; Finken, M.; Rijken, M.; Wit, J. Growth of preterm born children. Hormone Res. Paediatr. 2008, 70, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Giannì, M.L.; Roggero, P.; Garbarino, F.; Bracco, B.; Fumagalli, M.; Agosti, M.; Mosca, F. Nutrition and growth in infants born preterm from birth to adulthood. Early Hum. Dev. 2013, 89, S41–S44. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liu, Y.; Yan, C.; Peng, X.; Xu, Q.; Xuan, Y.; Han, F.; Tian, G.; Fang, Z.; Lin, Y. Postnatal nutritional restriction affects growth and immune function of piglets with intra-uterine growth restriction. Br. J. Nutr. 2015, 114, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ferenc, K.; Pietrzak, P.; Godlewski, M.M.; Piwowarski, J.; Kilianczyk, R.; Guilloteau, P.; Zabielski, R. Intrauterine growth retarded piglet as a model for humans–Studies on the perinatal development of the gut structure and function. Reprod. Biol. 2014, 14, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Walter, B.; Brust, P.; Füchtner, F.; Zwiener, U. Impact of asymmetric intrauterine growth restriction on organ function in newborn piglets. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, S40–S49. [Google Scholar] [CrossRef]
- Matthews, T.; MacDorman, M.F.; Thoma, M.E. Infant mortality statistics from the 2013 period linked birth/infant death data set. Natl. Vital Stat. Rep. 2015, 64, 1–30. [Google Scholar] [PubMed]
- Glass, H.C.; Costarino, A.T.; Stayer, S.A.; Brett, C.; Cladis, F.; Davis, P.J. Outcomes for extremely premature infants. Anesth. Analg. 2015, 120, 1337. [Google Scholar] [CrossRef] [PubMed]
- Proytcheva, M.A. Issues in neonatal cellular analysis. Am. J. Clin. Pathol. 2009, 131, 560–573. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, E.; Cetintaş, S.; Ceylan, T.; ÖğÜş, E.; Haberal, A.; Gürakan, B.; Özbek, N. Complete blood count parameters for healthy, small-for-gestational-age, full-term newborns. Int. J. Lab. Hematol. 2006, 28, 97–104. [Google Scholar]
- Morgan, C. The potential risks and benefits of insulin treatment in hyperglycaemic preterm neonates. Early Hum. Dev. 2015, 91, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.; Blanco, V.; Barnes, M.; Green, R. Impact of renal function and protein intake on blood urea nitrogen in preterm infants in the first 3 weeks of life. J. Perinatol. 2015, 35, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Newport, M.J.; Henschel, M.J. Evaluation of the neonatal pig as a model for infant nutrition: Effects of different proportions of casein and whey protein in milk on nitrogen metabolism and composition of digesta in the stomach. Pediatr. Res. 1984, 18, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Lonnerdal, B.; Chen, C. Effects of formula protein level and ratio on infant growth, plasma amino acids and serum trace elements. Acta Paediatr. Scand. 1990, 79, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.; Ziegler, E. Adjustable fortification of human milk fed to preterm infants: Does it make a difference? J. Perinatol. 2006, 26, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Escobar, J.; Frank, J.W.; Suryawan, A.; Nguyen, H.V.; Davis, T.A. Amino acid availability and age affect the leucine stimulation of protein synthesis and eIF4F formation in muscle. Am. J. Physiol.-Endocrinol. Metab. 2007, 293, E1615–E1621. [Google Scholar] [CrossRef] [PubMed]
| HP | LP+Ala | LP+Leu | |
|---|---|---|---|
| Ingredient (g/L) | |||
| Protein | 100 | 50 | 50 |
| Lipid | 47.5 | 47.5 | 47.5 |
| Medium Chain Triglyceride oil | 0 | 22.5 | 22.5 |
| Lactose | 50 | 50 | 50 |
| Alanine | 0 | 6.9 (76 mM) | 0 |
| Leucine | 0 | 0 | 10 (76 mM) |
| Vitamins and Minerals | 5 | 5 | 5 |
| LEC/STAR487/MO B | 4.4 | 4.4 | 4.4 |
| HP+Ala | HP+Leu | HP+HMB | |
|---|---|---|---|
| Component | g/L | ||
| Alanine | 6.8 (76 mmol) | 0 | 0 |
| Leucine | 0 | 10 (76 mmol) | 0 |
| HMB | 0 | 0 | 1.1 (4 mmol) |
| Ca-Acetate | 0.6 (6.1 mmol) | 0.6 (6.1 mmol) | 0 (5.5 mmol) |
| Experiment 1 | |||
|---|---|---|---|
| Group | HP | LP+Ala | LP+Leu |
| Birth Weight (g) | 795 ± 80 (47) | 1053 ± 73 (41) | 997 ± 70 (61) |
| Experiment 2 | |||
| Group | HP+Ala | HP+Leu | HP+HMB |
| Birth Weight (g) | 1147 ± 77 (67) | 1122 ± 66 (63) | 1054 ± 63 (36) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buddington, R.K.; Howard, S.C.; Lee, H.W.; Buddington, K.K. Growth Responses of Preterm Pigs Fed Formulas with Different Protein Levels and Supplemented with Leucine or β-Hydroxyl β-Methylbutyrate. Nutrients 2018, 10, 636. https://doi.org/10.3390/nu10050636
Buddington RK, Howard SC, Lee HW, Buddington KK. Growth Responses of Preterm Pigs Fed Formulas with Different Protein Levels and Supplemented with Leucine or β-Hydroxyl β-Methylbutyrate. Nutrients. 2018; 10(5):636. https://doi.org/10.3390/nu10050636
Chicago/Turabian StyleBuddington, Randal K., Scott C. Howard, Harold W. Lee, and Karyl K. Buddington. 2018. "Growth Responses of Preterm Pigs Fed Formulas with Different Protein Levels and Supplemented with Leucine or β-Hydroxyl β-Methylbutyrate" Nutrients 10, no. 5: 636. https://doi.org/10.3390/nu10050636
APA StyleBuddington, R. K., Howard, S. C., Lee, H. W., & Buddington, K. K. (2018). Growth Responses of Preterm Pigs Fed Formulas with Different Protein Levels and Supplemented with Leucine or β-Hydroxyl β-Methylbutyrate. Nutrients, 10(5), 636. https://doi.org/10.3390/nu10050636





