Cost-Effectiveness of Product Reformulation in Response to the Health Star Rating Food Labelling System in Australia
Abstract
1. Introduction
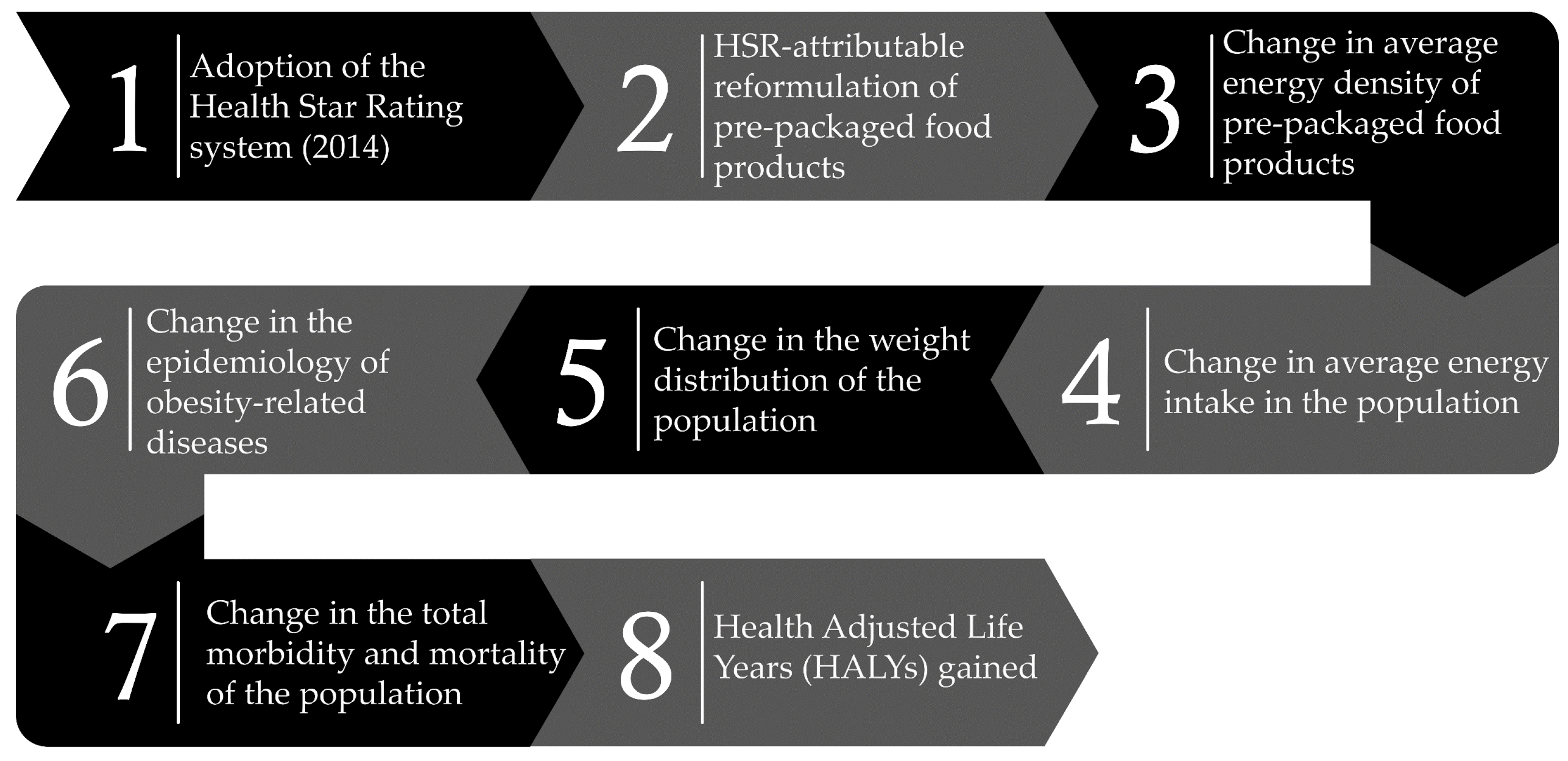
2. Materials and Methods
2.1. Analytical Approach
2.2. Intervention Effect Size
2.3. Health Benefit Modelling
2.4. Intervention Costs
2.5. Cost-Effectiveness Modelling
3. Results
3.1. Intervention Effect Size
3.2. Cost-Effectiveness Modelling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Ziaee, A.; Oveisi, S.; Afaghi, A. A Comparison of Health-Related Quality of Life among Normal-Weight, Overweight and Obese Adults in Qazvin Metabolic Diseases Study (QMDS), Iran: Health-Related Quality of Life among Obese Adults. Glob. J. Health Sci. 2013, 5, 156. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M. What is the economic case for treating obesity? Obes. Res. 1998, 6 (Suppl. 1), 2s–7s. [Google Scholar] [CrossRef] [PubMed]
- Trogdon, J.G.; Finkelstein, E.A.; Hylands, T.; Dellea, P.S.; Kamal-Bahl, S.J. Indirect costs of obesity: A review of the current literature. Obes. Rev. 2008, 9, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Goettler, A.; Grosse, A.; Sonntag, D. Productivity loss due to overweight and obesity: A systematic review of indirect costs. BMJ Open 2017, 7, e014632. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Health Expenditure Australia 2012–13; AIHW: Canberra, Australia, 2014. [Google Scholar]
- Price Waterhouse Coopers. Weighing the Cost of Obesity: A Case for Action; PWC: Canberra, Australia, 2015. [Google Scholar]
- Mustajoki, P. Obesogenic food environment explains most of the obesity epidemic. Duodecim 2015, 131, 1345–1352. [Google Scholar] [PubMed]
- Mejean, C.; Macouillard, P.; Péneau, S.; Hercberg, S.; Castetbon, K. Consumer acceptability and understanding of front-of-pack nutrition labels. J. Hum. Nutr. Diet. 2013, 26, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Mejean, C.; Macouillard, P.; Péneau, S.; Hercberg, S.; Castetbon, K. Perception of front-of-pack labels according to social characteristics, nutritional knowledge and food purchasing habits. Public Health Nutr. 2013, 16, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Feunekes, G.I.; Gortemaker, I.A.; Willems, A.A.; Lion, R.; Van Den Kommer, M. Front-of-pack nutrition labelling: Testing effectiveness of different nutrition labelling formats front-of-pack in four European countries. Appetite 2008, 50, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Crino, M.; Dunford, E.; Gao, A.; Greenland, R.; Li, N.; Ngai, J.; Ni Mhurchu, C.; Pettigrew, S.; Sacks, G.; et al. Effects of Different Types of Front-of-Pack Labelling Information on the Healthiness of Food Purchases-A Randomised Controlled Trial. Nutrients 2017, 9, 1284. [Google Scholar] [CrossRef] [PubMed]
- Hamlin, R.; McNeill, L. Does the Australasian “Health Star Rating” Front of Pack Nutritional Label System Work? Nutrients 2016, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.; Warin, L. Impact of food labelling systems on food choices and eating behaviours: A systematic review and meta-analysis of randomized studies. Obes. Rev. 2016, 17, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Rayner, M.; Wood, A.; Lawrence, M.; Mhurchu, C.N.; Albert, J.; Barquera, S.; Friel, S.; Hawkes, C.; Kelly, B.; Kumanyika, S.; et al. Monitoring the health-related labelling of foods and non-alcoholic beverages in retail settings. Obes. Rev. 2013, 14 (Suppl. 1), 70–81. [Google Scholar] [CrossRef] [PubMed]
- Cowburn, G.; Stockley, L. Consumer understanding and use of nutrition labelling: A systematic review. Public Health Nutr. 2005, 8, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.; Doxey, J.; Hammond, D. Nutrition labels on pre-packaged foods: A systematic review. Public Health Nutr. 2011, 14, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Ni Mhurchu, C.; Volkova, E.; Jiang, Y. Effects of interpretive nutrition labels on consumer food purchases: The Starlight randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 695–704. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Vyth, E.L.; Steenhuis, I.H.; Roodenburg, A.J.; Brug, J.; Seidell, J.C. Front-of-pack nutrition label stimulates healthier product development: A quantitative analysis. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Young, L.R.; Nestle, M. The contribution of expanding portion sizes to the US obesity epidemic. Am. J. Public Health 2002, 92, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Dummer, J. Sodium reduction in Canadian food products with the health check program. Can. J. Diet. Pract. Res. 2012, 73, e227–e232. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.A.; Roe, L.S.; Rolls, B.J. Assessment of satiety depends on the energy density and portion size of the test meal. Obesity 2014, 22, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Health Star Rating Advisory Committee. About Health Star Ratings. 2014. Available online: http://healthstarrating.gov.au/internet/healthstarrating/publishing.nsf/content/About-health-stars (accessed on 18 March 2018).
- Siegrist, M.; Leins-Hess, R.; Keller, C. Which front-of-pack nutrition label is the most efficient one? The results of an eye-tracker study. Food Qual. Prefer. 2015, 39, 183–190. [Google Scholar] [CrossRef]
- Young, L.; Swinburn, B. Impact of the Pick the Tick food information programme on the salt content of food in New Zealand. Health Promot. Int. 2002, 17, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; McMahon, A.; Boustead, R. A case study of sodium reduction in breakfast cereals and the impact of the Pick the Tick food information program in Australia. Health Promot. Int. 2003, 18, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.W.; Bello, N.M.; Sundar, R.P.; Peltier, C.; Bix, L. Front of pack labels enhance attention to nutrition information in novel and commercial brands. Food Policy 2015, 56, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Mhurchu, C.; Eyles, H.; Choi, Y.-H. Effects of a Voluntary Front-of-Pack Nutrition Labelling System on Packaged Food Reformulation: The Health Star Rating System in New Zealand. Nutrients 2017, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Health Star Rating Advisory Committee. Two Year Progress Review Report on the Implementation of the Health Star Rating System—June 2014–June 2016. Available online: http://healthstarrating.gov.au/internet/healthstarrating/publishing.nsf/Content/reviews (accessed on 8 May 2017).
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int. J. Technol. Assess. Health Care 2013, 29, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Ello-Martin, J.A.; Ledikwe, J.H.; Rolls, B.J. The influence of food portion size and energy density on energy intake: Implications for weight management. Am. J. Clin. Nutr. 2005, 82 (Suppl. 1), 236s–241s. [Google Scholar] [CrossRef] [PubMed]
- Roberto, C.A.; Swinburn, B.; Hawkes, C.; Huang, T.T.; Costa, S.A.; Ashe, M.; Zwicker, L.; Cawley, J.H.; Brownell, K.D. Patchy progress on obesity prevention: Emerging examples, entrenched barriers, and new thinking. Lancet 2015, 385, 2400–2409. [Google Scholar] [CrossRef]
- Swinburn, B.; Sacks, G.; Vandevijvere, S.; Kumanyika, S.; Lobstein, T.; Neal, B.; Barquera, S.; Friel, S.; Hawkes, C.; Kelly, B.; et al. INFORMAS (International Network for Food and Obesity/non-communicable diseases Research, Monitoring and Action Support): Overview and key principles. Obes. Rev. 2013, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Dunford, E.; Trevena, H.; Goodsell, C.; Ng, K.H.; Webster, J.; Millis, A.; Goldstein, S.; Hugueniot, O.; Neal, B. FoodSwitch: A Mobile Phone App to Enable Consumers to Make Healthier Food Choices and Crowdsourcing of National Food Composition Data. JMIR mHealth uHealth 2014, 2, e37. [Google Scholar] [CrossRef] [PubMed]
- Dunford, E.; Webster, J.; Metzler, A.B.; Czernichow, S.; Mhurchu, C.N.; Wolmarans, P.; Snowdon, W.; L’Abbe, M.; Li, N.; Maulik, P.K.; et al. International collaborative project to compare and monitor the nutritional composition of processed foods. Eur. J. Prev. Cardiol. 2012, 19, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. Australian Health Survey 2011–12; ABS: Canberra, Australia, 2015.
- Hall, K.D.; Butte, N.F.; Swinburn, B.A.; Chow, C.C. Dynamics of childhood growth and obesity: Development and validation of a quantitative mathematical model. Lancet Diabet. Endocrinol. 2013, 1, 97–105. [Google Scholar] [CrossRef]
- Mantilla Herrera, A.M.; Erskine, H.E.; Ananthapavan, J.; Sacks, G.; Whiteford, H.; Barendregt, J.J.; Lee, Y.Y. Health economic evaluation of obesity interventions: The development and validation of the Centre for Research Excellence in Obesity Policy and Food Systems model (the CRE-Obesity model). 2017. submitted. [Google Scholar]
- Barendregt, J.J.; Van Oortmarssen, G.J.; Van Hout, B.A.; Van Den Bosch, J.M.; Bonneux, L. Coping with multiple morbidity in a life table. Math. Popul. Stud. 1998, 7, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.R.; Stevenson, D.; Fryback, D.G. HALYs and QALYs and DALYs, Oh My: Similarities and Differences in Summary Measures of Population Health. Annu. Rev. Public Health 2002, 23, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Mantilla-Herrera, A.; Veerman, L.; Backholer, K.; Sacks, G.; Moodie, M.; Siahpush, M.; Carter, R.; Peeters, A. Equity and cost-effectiveness of a sugar sweetened beverage tax across socioeconomic groups. PLoS Med. 2017, 14, e1002326. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.; Moodie, M.; Cobiac, L.; Herrera, A.M.; Carter, R. Obesity-related health impacts of fuel excise taxation—An evidence review and cost-effectiveness study. BMC Public Health 2017, 17, 359. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.; Moodie, M.; Herrera, A.M.; Veerman, J.L.; Carter, R. Active transport and obesity prevention—A transportation sector obesity impact scoping review and assessment for Melbourne, Australia. Prev. Med. 2017, 96, 49–66. [Google Scholar] [CrossRef] [PubMed]
- PricewaterhouseCoopers. Health Star Rating System: Cost Benefit Analysis; PWC: Canberra, Australia, 2014. [Google Scholar]
- PricewaterhouseCoopers. Food Standards Australia New Zealand: Cost Schedule for Food Labelling Changes—Final Report; PWC: Canberra, Australia, 2008. [Google Scholar]
- Australian Institute of Health and Welfare. Disease Costs and Impacts Study Data; AIHW: Canberra, Australia, 2001.
- Robertson, K. Independent Study: Why Label Changes Don’t Affect Food Prices. 2013. Available online: http://www.justlabelit.org/wp-content/uploads/2013/09/Kai-Roberston-Food-Labeling-Study-2013.pdf (accessed on 30 April 2018).
- Australian Institute of Health and Welfare. Health Expenditure Australia 2014-15. Health and Welfare Expenditure Series No. 57. Cat. No. HWE 67; AIHW: Canberra, Australia, 2016.
- Harris, A.H.; Hill, S.R.; Chin, G.; Li, J.J.; Walkom, E. The role of value for money in public insurance coverage decisions for drugs in Australia: A retrospective analysis 1994–2004. Med. Decis. Mak. 2008, 28, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.H.; Weinstein, M.C.; Fenwick, E.A.; Karnon, J.; Sculpher, M.J.; Paltiel, A.D. Model parameter estimation and uncertainty: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force-6. Value Health 2012, 15, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.; Sculpher, M.; Claxton, K. Decision Modelling for Health Economic Evaluation; OUP Oxford: Oxford, UK, 2006. [Google Scholar]
- EpiGear International. Ersatz Version 1.3. 2016. Available online: http://www.epigear.com/index_files/ersatz.html (accessed on 10 March 2016).
- Briggs, A.; Fenn, P. Confidence intervals or surfaces? Uncertainty on the cost-effectiveness plane. Health Econ. 1998, 7, 723–740. [Google Scholar] [CrossRef]
- EpiGear International. Ersatz User Guide Version 1.35. Available online: https://www.epigear.com/Products/EpigearXL/epigearxl.html (accessed on 18 March 2018).
- Veerman, J.L.; Barendregt, J.J.; Beeck, E.F.; Seidell, J.C.; Mackenbach, J.P. Stemming the obesity epidemic: A tantalizing prospect. Obesity 2007, 15, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013, 380, 2224–2260. [Google Scholar] [CrossRef]
- Sacks, G.; Veerman, J.L.; Moodie, M.; Swinburn, B. ‘Traffic-light’nutrition labelling and ‘junk-food’tax: A modelled comparison of cost-effectiveness for obesity prevention. Int. J. Obes. 2011, 35, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Crino, M.; Herrera, A.M.M.; Ananthapavan, J.; Wu, J.H.; Neal, B.; Lee, Y.Y.; Zheng, M.; Lal, A.; Sacks, G. Modelled Cost-Effectiveness of a Package Size Cap and a Kilojoule Reduction Intervention to Reduce Energy Intake from Sugar-Sweetened Beverages in Australia. Nutrients 2017, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Vyth, E.L.; Steenhuis, I.H.; Brandt, H.E.; Roodenburg, A.J.; Brug, J.; Seidell, J.C. Methodological quality of front-of-pack labeling studies: A review plus identification of research challenges. Nutr. Rev. 2012, 70, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.H. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics 2000, 17, 479–500. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.A.; Dickie, S.; Woods, J.L. Do Nutrient-Based Front-of-Pack Labelling Schemes Support or Undermine Food-Based Dietary Guideline Recommendations? Lessons from the Australian Health Star Rating System. Nutrients 2018, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- System, H.S.R. Formal Review of the System after Five Years of Implementation (June 2014 to June 2019). Available online: http://healthstarrating.gov.au/internet/healthstarrating/publishing.nsf/Content/formal-review-of-the-system-after-five-years (accessed on 26 March 2018).



| Costing Items | Value in 2010 A$ | |
|---|---|---|
| Voluntary | Mandatory | |
| 1. Cost to industry a | 2.5 m (1.2 m, 3.7 m) | 37.0 m (18.5 m, 55.4 m) |
| 2. Cost to government b | 1.2 m (0.6 m, 1.8 m) | 17.8 m (8.9 m, 26.7 m) |
| 2.1 Cost of legislation c | not applicable | 1.1 m (1.0 m, 1.2 m) |
| Input Parameters | Uncertainty Distribution | Assumptions | Data Sources |
|---|---|---|---|
| Change in weight resulting from the intervention | Normal | The point estimate was the mean obtained from the data source. A standard deviation was assigned to this point estimate that was equal to the mean in the absence of relevant data. | Based on data from the Food Switch database and the Australian Health survey |
| Intervention costs to industry | Pert | The point estimate (obtained from the data source) was assigned a range of likely minimum and maximum values based on expert opinion. | Based on the projected costs from the HSR report [24] |
| Intervention costs to government | Pert | The point estimate (obtained from the data source) was assigned a range of likely minimum and maximum values based on expert opinion [53,56]. | Based on the projected costs from the HSR report [24]. Costs to the government related to passing a legislation were only applied to the mandatory scenario and were modelled using a gamma distribution [43] |
| 2010 Australian population BMI by age and sex | Lognormal | The mean and standard deviation for each population cohort (by age and sex) was obtained from the data source. A lognormal distribution was used to: restrict the occurrence of values between the interval [0, +∞]; and account for the positively skewed BMI distribution observed in the population [57]. | Sourced from the Australian Bureau of Statistics [38] |
| Relative risks of obesity-related diseases per 5-unit increase of BMI | Lognormal | The mean was obtained from the data source and the standard deviation was calculated as the lognormal of the mean. A lognormal distribution was used to restrict the occurrence of values between [0, +∞]. | Sourced from the Global Burden of Disease study [58] |
| Food Category | Average Energy Density (kJ per 100 g) in 2013 | Average Energy Density (kJ per 100 g) in 2016 | Change in Average Energy Density between 2013 and 2016 | % Change in kJ per 100 g (from Baseline) | % Change in kJ per 100 g Attributable to HSR | ||||
|---|---|---|---|---|---|---|---|---|---|
| With HSR | Without HSR | With HSR | Without HSR | With HSR | Without HSR | With HSR | Without HSR | ||
| Bread and bakery products | 1585 | 1588 | 1581 | 1586 | −3.3 | −2.2 | −0.2 | −0.1 | −0.1 |
| Cereal and grain products | 1521 | 1370 | 1513 | 1360 | −7.9 | −10.4 | −0.5 | −0.8 | 0.2 |
| Confectionery | 2070 | 1720 | 2089 | 1724 | 19.7 | 4.0 | 1.0 | 0.2 | 0.7 |
| Convenience foods | 444 | 512 | 433 | 509 | −10.9 | −3.2 | −2.5 | −0.6 | −1.8 |
| Dairy | 608 | 933 | 594 | 932 | −13.4 | −0.9 | −2.2 | −0.1 | −2.1 |
| Edible oils and oil emulsions | 2724 | 3066 | 2706 | 3071 | −18.1 | 5.0 | −0.7 | 0.2 | −0.8 |
| Fish and fish products | 721 | 693 | 720 | 693 | −1.0 | 0.0 | −0.1 | 0.0 | −0.1 |
| Fruit and vegetables | 881 | 998 | 881 | 999 | −0.6 | 0.6 | −0.1 | 0.1 | −0.1 |
| Meat and meat products | 828 | 878 | 824 | 882 | −4.1 | 3.9 | −0.5 | 0.4 | −0.9 |
| Non-alcoholic beverages | 213 | 197 | 208 | 195 | −4.6 | −2.1 | −2.1 | −1.1 | −1.1 |
| Sauces, dressings, spreads and dips | 1046 | 816 | 981 | 810 | −64.7 | −5.5 | −6.2 | −0.7 | −5.5 |
| Snack foods | 2013 | 1883 | 2079 | 1882 | 65.8 | −0.8 | 3.3 | 0.0 | 3.3 |
| Sugars, honey and related products | 1454 | 1404 | 1435 | 1406 | −19.7 | 1.6 | −1.4 | 0.1 | −1.5 |
| Outputs | Voluntary Scenario * (6.7% HSR Uptake) | Mandatory Scenario * (100% HSR Uptake) |
|---|---|---|
| Incremental intervention costs (95% UI) in 2010 A$ millions | 46.1 m (32.0 m to 60.2 m) | 686.4 m (483.5 m to 894.9 m) |
| Cost offsets ** (95% UI) in 2010 A$ millions | −41.6 m (−61.6 m to −22.1 m) | −488.7 m (−722.8 m to −265.9 m) |
| Net incremental costs (95% UI) in 2010 A$ millions | 4.5 m (−21.2 m to 28.2 m) | 197.7 m (−123.2 m to 513.3 m) |
| Incremental HALYs (95% UI) | 4207 (2438 to 6081) | 49,949 (29,291 to 72,153) |
| Mean ICER (95% UI) in 2010 A$ per HALY | 1728 (dominant to 10,445) | 4752 (dominant to 16,236) |
| Outputs | 50% HSR-Attributable Reformulation | 30% HSR-Attributable Reformulation | 10% HSR-Attributable Reformulation |
|---|---|---|---|
| Incremental intervention costs (95% UI) in A$ millions | 46.1 m (32.0 m to 59.7 m) | 46.0 m (31.7 m to 59.9 m) | 46.0 m (32.4 m to 60.0 m) |
| Cost offsets (95% UI) in A$ millions | −20.9 m (−31.4 m to −11.3 m) | −12.5 m (−18.3 m to −6.5 m) | −4.2 m (−6.3 m to −2.2 m) |
| Net incremental costs (95% UI) in A$ millions | 25.3 m (7.9 m to 42.4 m) | 33.5 m (18.0m to 48.5 m) | 41.8 m (28.2 m to 56.2 m) |
| Incremental HALYs (95% UI) | 2101 (1226 to 3116) | 1253 (702 to 1840) | 424 (235 to 618) |
| Mean ICER (95% UI) A$ per HALY | 13,374 (3044 to 31,940) | 29,006 (11,427 to 59,863) | 106,368 (54,072 to 191,145) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantilla Herrera, A.M.; Crino, M.; Erskine, H.E.; Sacks, G.; Ananthapavan, J.; Mhurchu, C.N.; Lee, Y.Y. Cost-Effectiveness of Product Reformulation in Response to the Health Star Rating Food Labelling System in Australia. Nutrients 2018, 10, 614. https://doi.org/10.3390/nu10050614
Mantilla Herrera AM, Crino M, Erskine HE, Sacks G, Ananthapavan J, Mhurchu CN, Lee YY. Cost-Effectiveness of Product Reformulation in Response to the Health Star Rating Food Labelling System in Australia. Nutrients. 2018; 10(5):614. https://doi.org/10.3390/nu10050614
Chicago/Turabian StyleMantilla Herrera, Ana Maria, Michelle Crino, Holly E. Erskine, Gary Sacks, Jaithri Ananthapavan, Cliona Ni Mhurchu, and Yong Yi Lee. 2018. "Cost-Effectiveness of Product Reformulation in Response to the Health Star Rating Food Labelling System in Australia" Nutrients 10, no. 5: 614. https://doi.org/10.3390/nu10050614
APA StyleMantilla Herrera, A. M., Crino, M., Erskine, H. E., Sacks, G., Ananthapavan, J., Mhurchu, C. N., & Lee, Y. Y. (2018). Cost-Effectiveness of Product Reformulation in Response to the Health Star Rating Food Labelling System in Australia. Nutrients, 10(5), 614. https://doi.org/10.3390/nu10050614







