The Impact of Caloric and Non-Caloric Sweeteners on Food Intake and Brain Responses to Food: A Randomized Crossover Controlled Trial in Healthy Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
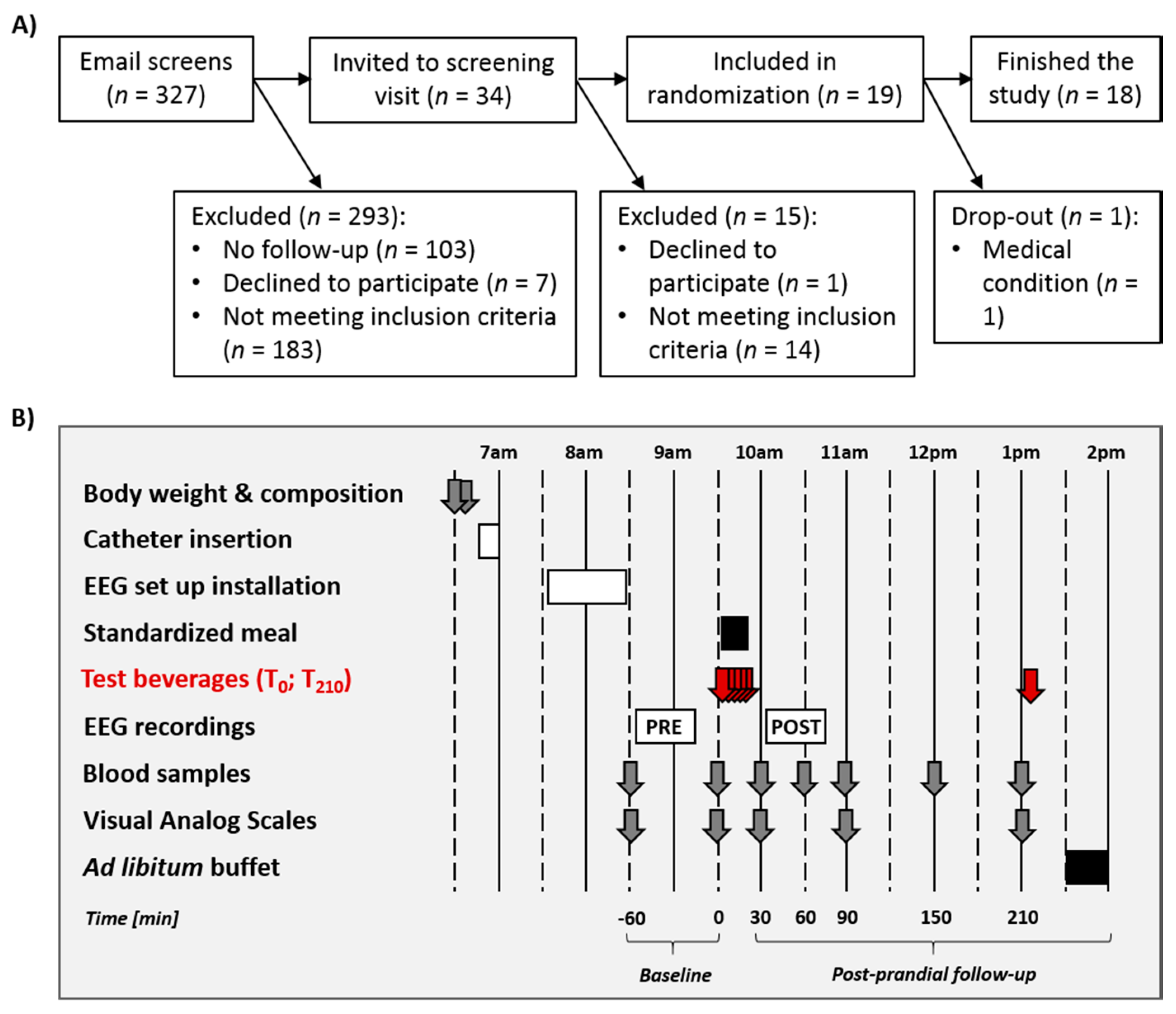
2.2. General Procedure
2.3. Meal and Test Beverage Composition
2.4. Qualitative and Quantitative Assessments of Spontaneous Food Intake
2.5. Analytical Procedures for Plasma Samples
2.6. Behavioral Ratings
2.7. Statistical Analyses of Food Intake, Behavioral Ratings and Plasmatic Parameters
2.8. Electroencephalography (EEG) Stimuli Presentation Procedure, EEG Acquisition and Preprocessing
2.9. EEG Analyses and Estimations of Neural Source Activity
3. Results
3.1. Spontaneous Food Intake at the Ad Libitum Buffet
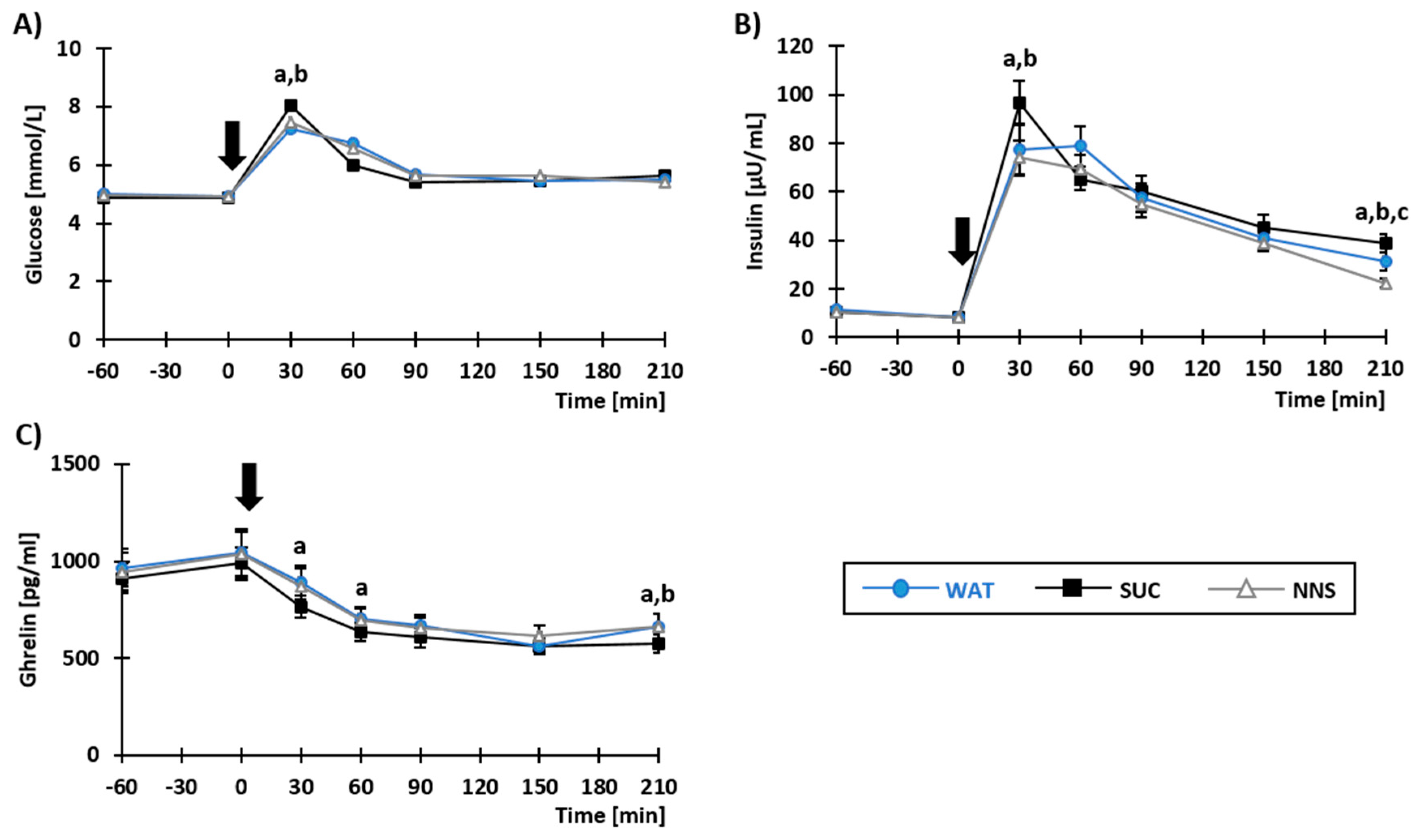
3.2. Plasma Concentrations of Metabolites and Gastro-Intestinal Hormones
3.3. Results of Behavioral Ratings
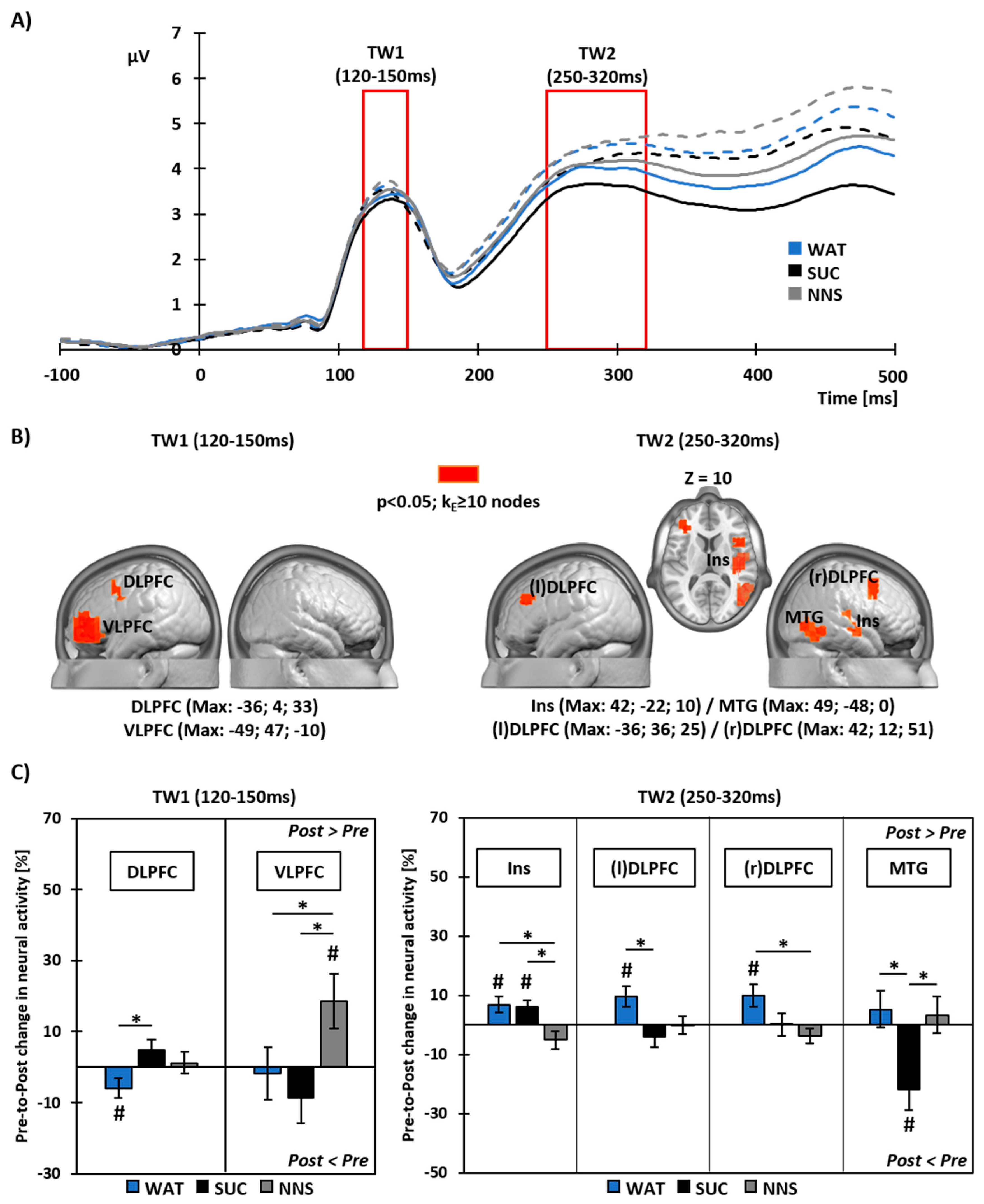
3.4. Pre- to Post-Prandial Changes in Neural Source Activity To Food Viewing
4. Discussion
4.1. Brain Responses to Food Viewing Following Water or Sucrose Consumption
4.2. Brain Responses to Food Viewing Following NNS Consumption
4.3. Integration of Postprandial Brain Responses to Food Viewing with Gastro-Intestinal Hormone Secretion and Food Intake Behavior
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DiMeglio, D.P.; Mattes, R.D. Liquid versus solid carbohydrate: Effects on food intake and body weight. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Hu, F.B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Sullivan, L.; Jacques, P.F.; Wang, T.J.; Fox, C.S.; Meigs, J.B.; D’Agostino, R.B.; Gaziano, J.M.; Vasan, R.S. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007, 116, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.P.; Williams, K.; Resendez, R.G.; Hunt, K.J.; Hazuda, H.P.; Stern, M.P. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity 2008, 16, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Stellman, S.D.; Garfinkel, L. Patterns of artificial sweetener use and weight change in an american cancer society prospective study. Appetite 1988, 11 (Suppl. 1), 85–91. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R. Metabolic and hedonic drives in the neural control of appetite: Who is the boss? Curr. Opin. Neurobiol. 2011, 21, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Laffitte, A.; Neiers, F.; Briand, L. Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Gillis-Smith, S.; Jin, H.; Tränkner, D.; Ryba, N.J.; Zuker, C.S. Sweet and bitter taste in the brain of awake behaving animals. Nature 2015, 527, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Schloegl, H.; Percik, R.; Horstmann, A.; Villringer, A.; Stumvoll, M. Peptide hormones regulating appetite--focus on neuroimaging studies in humans. Diabetes Metab. Res. Rev. 2011, 27, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.V.; Small, D.M. Physiological mechanisms by which non-nutritive sweeteners may impact body weight and metabolism. Physiol. Behav. 2015, 152, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Swithers, S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. 2013, 24, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.L.; Martin, A.A.; Clark, K.; Swithers, S.E. Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: Implications for the learned control of energy and body weight regulation. Q. J. Exp. Psychol. 2011, 64, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.P.; Lin, Y.Q.; Zhang, L.; Wilson, Y.A.; Oyston, L.J.; Cotterell, J.; Qi, Y.; Khuong, T.M.; Bakhshi, N.; Planchenault, Y.; et al. Sucralose promotes food intake through npy and a neuronal fasting response. Cell Metab. 2016, 24, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, O.; Ahmed, S.H.; Atlan, C.; Belegaud, J.; Bortolotti, M.; Canivenc-Lavier, M.C.; Charrière, S.; Girardet, J.P.; Houdart, S.; Kalonji, E.; et al. Review of the nutritional benefits and risks related to intense sweeteners. Arch. Public Health 2015, 73, 41. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, O.; Ahmed, S.H.; Atlan, C.; Belegaud, J.; Bortolotti, M.; Canivenc-Lavier, M.C.; Charrière, S.; Girardet, J.P.; Houdart, S.; Kalonji, E.; et al. Erratum to: Review of the nutritional benefits and risks related to intense sweeteners. Arch. Public Health 2015, 73, 49. [Google Scholar] [CrossRef] [PubMed]
- Renwick, A.G.; Molinary, S.V. Sweet-taste receptors, low-energy sweeteners, glucose absorption and insulin release. Br. J. Nutr. 2010, 104, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.; Ahuja, S.; Sriram, K. Non-nutritive sweeteners: Review and update. Nutrition 2013, 29, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.; Oberndorfer, T.A.; Simmons, A.N.; Paulus, M.P.; Fudge, J.L.; Yang, T.T.; Kaye, W.H. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage 2008, 39, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, L.A.; Coveleskie, K.; Connolly, L.; Labus, J.S.; Ebrat, B.; Stains, J.; Jiang, Z.; Suyenobu, B.Y.; Raybould, H.E.; Tillisch, K.; et al. Influence of sucrose ingestion on brainstem and hypothalamic intrinsic oscillations in lean and obese women. Gastroenterology 2014, 146, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Ginieis, R.; Franz, E.A.; Oey, I.; Peng, M. The “Sweet” Effect: Comparative assessments of dietary sugars on cognitive performance. Physiol. Behav. 2018, 184, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Dagher, A. Functional brain imaging of appetite. Trends Endocrinol. Metab. 2012, 23, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Van der Laan, L.N.; de Ridder, D.T.; Viergever, M.A.; Smeets, P.A. The first taste is always with the eyes: A meta-analysis on the neural correlates of processing visual food cues. Neuroimage 2011, 55, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Crézé, C.; Notter-Bielser, M.L.; Knebel, J.F.; Campos, V.; Tappy, L.; Murray, M.; Toepel, U. The impact of replacing sugar- by artificially-sweetened beverages on brain and behavioral responses to food viewing—An exploratory study. Appetite 2018, 123, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Ordoñez, E.Y.; Rodil, R.; Quintana, J.B.; Cela, R. Determination of artificial sweeteners in beverages with green mobile phases and high temperature liquid chromatography-tandem mass spectrometry. Food Chem. 2015, 169, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Lietti, C.V.; Murray, M.M.; Hudry, J.; le Coutre, J.; Toepel, U. The role of energetic value in dynamic brain response adaptation during repeated food image viewing. Appetite 2012, 58, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Toepel, U.; Knebel, J.F.; Hudry, J.; le Coutre, J.; Murray, M.M. The brain tracks the energetic value in food images. Neuroimage 2009, 44, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Toepel, U.; Ohla, K.; Hudry, J.; le Coutre, J.; Murray, M.M. Verbal labels selectively bias brain responses to high-energy foods. Neuroimage 2014, 87, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Knebel, J.F.; Toepel, U.; Hudry, J.; le Coutre, J.; Murray, M.M. Generating controlled image sets in cognitive neuroscience research. Brain Topogr. 2008, 20, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.; Pernier, J.; Bertrand, O.; Giard, M.H.; Echallier, J.F. Mapping of scalp potentials by surface spline interpolation. Electroencephalogr. Clin. Neurophysiol. 1987, 66, 75–81. [Google Scholar] [CrossRef]
- Murray, M.M.; Brunet, D.; Michel, C.M. Topographic erp analyses: A step-by-step tutorial review. Brain Topogr. 2008, 20, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.; Skrandies, W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr. Clin. Neurophysiol. 1980, 48, 609–621. [Google Scholar] [CrossRef]
- Toepel, U.; Bielser, M.L.; Forde, C.; Martin, N.; Voirin, A.; le Coutre, J.; Murray, M.M.; Hudry, J. Brain dynamics of meal size selection in humans. Neuroimage 2015, 113, 133–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Michel, C.M.; Murray, M.M.; Lantz, G.; Gonzalez, S.; Spinelli, L.; Grave de Peralta, R. Eeg source imaging. Clin. Neurophysiol. 2004, 115, 2195–2222. [Google Scholar] [CrossRef] [PubMed]
- Tataranni, P.A.; Gautier, J.F.; Chen, K.; Uecker, A.; Bandy, D.; Salbe, A.D.; Pratley, R.E.; Lawson, M.; Reiman, E.M.; Ravussin, E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc. Natl. Acad. Sci. USA 1999, 96, 4569–4574. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Sinha, R.; Arora, J.; Giannini, C.; Kubat, J.; Malik, S.; Van Name, M.A.; Santoro, N.; Savoye, M.; Duran, E.J.; et al. Altered brain response to drinking glucose and fructose in obese adolescents. Diabetes 2016, 65, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Weygandt, M.; Mai, K.; Dommes, E.; Leupelt, V.; Hackmack, K.; Kahnt, T.; Rothemund, Y.; Spranger, J.; Haynes, J.D. The role of neural impulse control mechanisms for dietary success in obesity. Neuroimage 2013, 83, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Lavagnino, L.; Arnone, D.; Cao, B.; Soares, J.C.; Selvaraj, S. Inhibitory control in obesity and binge eating disorder: A systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neurosci. Biobehav. Rev. 2016, 68, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Camus, M.; Halelamien, N.; Plassmann, H.; Shimojo, S.; O’Doherty, J.; Camerer, C.; Rangel, A. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex decreases valuations during food choices. Eur. J. Neurosci. 2009, 30, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Hare, T.; Rangel, A. Temporally dissociable mechanisms of self-control: Early attentional filtering versus late value modulation. J. Neurosci. 2013, 33, 18917–18931. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Wiens, S.; Rotshtein, P.; Ohman, A.; Dolan, R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004, 7, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; ffytche, D.H.; Rosenthal, J.M.; Zelaya, F.O.; Barker, G.J.; Withers, D.J.; Williams, S.C. Pyy modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 2007, 450, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Bridge, M.W.; Jones, D.A. Carbohydrate sensing in the human mouth: Effects on exercise performance and brain activity. J. Physiol. 2009, 587, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Connolly, L.; Coveleskie, K.; Kilpatrick, L.A.; Labus, J.S.; Ebrat, B.; Stains, J.; Jiang, Z.; Tillisch, K.; Raybould, H.E.; Mayer, E.A. Differences in brain responses between lean and obese women to a sweetened drink. Neurogastroenterol. Motil. 2013, 25, 579-e460. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M. Flavor is in the brain. Physiol. Behav. 2012, 107, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Hopfinger, J.B.; Buonocore, M.H.; Mangun, G.R. The neural mechanisms of top-down attentional control. Nat. Neurosci. 2000, 3, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D.; Young, A.D.; Femia, L.A.; Bogorodzki, P.; Rogowska, J.; Yurgelun-Todd, D.A. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage 2003, 19, 1381–1394. [Google Scholar] [CrossRef]
- Führer, D.; Zysset, S.; Stumvoll, M. Brain activity in hunger and satiety: An exploratory visually stimulated fmri study. Obesity 2008, 16, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Alonso, M.; Pascual-Leone, A. The right brain hypothesis for obesity. JAMA 2007, 297, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Smeets, P.A.; Weijzen, P.; de Graaf, C.; Viergever, M.A. Consumption of caloric and non-caloric versions of a soft drink differentially affects brain activation during tasting. Neuroimage 2011, 54, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R. The neurobiology of food intake in an obesogenic environment. Proc. Nutr. Soc. 2012, 71, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C. ‘liking’ and ‘wanting’ food rewards: Brain substrates and roles in eating disorders. Physiol. Behav. 2009, 97, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Kringelbach, M.L. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat. Rev. Neurosci. 2005, 6, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.G. The nexus between decision making and emotion regulation: A review of convergent neurocognitive substrates. Behav. Brain Res. 2011, 217, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Green, E.; Murphy, C. Altered processing of sweet taste in the brain of diet soda drinkers. Physiol. Behav. 2012, 107, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Rudenga, K.J.; Small, D.M. Amygdala response to sucrose consumption is inversely related to artificial sweetener use. Appetite 2012, 58, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, J.V. The neurocognitive bases of human multimodal food perception: Consciousness. Brain Res. Rev. 2007, 53, 271–286. [Google Scholar] [CrossRef] [PubMed]
| Beverage Condition | One-Way ANOVA | ||||
|---|---|---|---|---|---|
| WAT | SUC | NNS | F-Value | p-Value | |
| Body weight [kg] | 66.6 ± 1.2 | 66.5 ± 1.2 | 66.5 ± 1.1 | 0.39 | 0.68 |
| BMI [kg/m2] | 21.4 ± 0.4 | 21.4 ± 0.4 | 21.3 ± 0.4 | 0.12 | 0.89 |
| Body fat mass [kg] | 12.3 ± 1.2 | 12.2 ± 0.9 | 12.2 ± 1.1 | 0.04 | 0.97 |
| Plasma glucose [mmol/L] | 4.9 ± 0.1 | 4.8 ± 0.1 | 4.9 ± 0.1 | 1.23 | 0.31 |
| Plasma insulin [µU/mL] | 10.3 ± 0.7 | 9.8 ± 0.5 | 9.5 ± 0.7 | 2.33 | 0.11 |
| Plasma ghrelin [pg/mL] | 984.8 ± 94.1 | 925.7 ± 70.4 | 963.3 ± 94.1 | 0.90 | 0.41 |
| Hunger level [%] | 71.4 ± 5.1 | 73.3 ± 4.4 | 72.6 ± 4.7 | 0.12 | 0.89 |
| Thirst level [%] | 55.7 ± 5.5 | 67.1 ± 3.8 | 61.5 ± 5.1 | 2.38 | 0.11 |
| Satiety level [%] | 21.7 ± 4.2 | 23.6 ± 4.8 | 20.4 ± 3.4 | 0.25 | 0.78 |
| Taste cravings (1–9) | 5.4 ± 0.4 | 5.6 ± 0.5 | 5.8 ± 0.5 | 0.30 | 0.75 |
| Beverage Condition | |||
|---|---|---|---|
| WAT | SUC | NNS | |
| Total energy intake [kcal] | 942 ± 71 | 791 ± 62 a,b | 917 ± 70 |
| Energy intake from LF/NSW foods [kcal] | 142 ± 28 | 141 ± 29 | 167 ± 37 |
| Energy intake from LF/SW foods [kcal] | 77 ± 22 | 62 ± 15 | 78 ± 20 |
| Energy intake from HF/NSW foods [kcal] | 515 ± 74 | 428 ± 50 | 449 ± 56 |
| Energy intake from HF/SW foods [kcal] | 209 ± 36 | 161 ± 24 | 224 ± 35 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crézé, C.; Candal, L.; Cros, J.; Knebel, J.-F.; Seyssel, K.; Stefanoni, N.; Schneiter, P.; Murray, M.M.; Tappy, L.; Toepel, U. The Impact of Caloric and Non-Caloric Sweeteners on Food Intake and Brain Responses to Food: A Randomized Crossover Controlled Trial in Healthy Humans. Nutrients 2018, 10, 615. https://doi.org/10.3390/nu10050615
Crézé C, Candal L, Cros J, Knebel J-F, Seyssel K, Stefanoni N, Schneiter P, Murray MM, Tappy L, Toepel U. The Impact of Caloric and Non-Caloric Sweeteners on Food Intake and Brain Responses to Food: A Randomized Crossover Controlled Trial in Healthy Humans. Nutrients. 2018; 10(5):615. https://doi.org/10.3390/nu10050615
Chicago/Turabian StyleCrézé, Camille, Laura Candal, Jérémy Cros, Jean-François Knebel, Kevin Seyssel, Nathalie Stefanoni, Philippe Schneiter, Micah M. Murray, Luc Tappy, and Ulrike Toepel. 2018. "The Impact of Caloric and Non-Caloric Sweeteners on Food Intake and Brain Responses to Food: A Randomized Crossover Controlled Trial in Healthy Humans" Nutrients 10, no. 5: 615. https://doi.org/10.3390/nu10050615
APA StyleCrézé, C., Candal, L., Cros, J., Knebel, J.-F., Seyssel, K., Stefanoni, N., Schneiter, P., Murray, M. M., Tappy, L., & Toepel, U. (2018). The Impact of Caloric and Non-Caloric Sweeteners on Food Intake and Brain Responses to Food: A Randomized Crossover Controlled Trial in Healthy Humans. Nutrients, 10(5), 615. https://doi.org/10.3390/nu10050615





