Cerebral Blood Flow Measurements in Adults: A Review on the Effects of Dietary Factors and Exercise
Abstract
1. Introduction
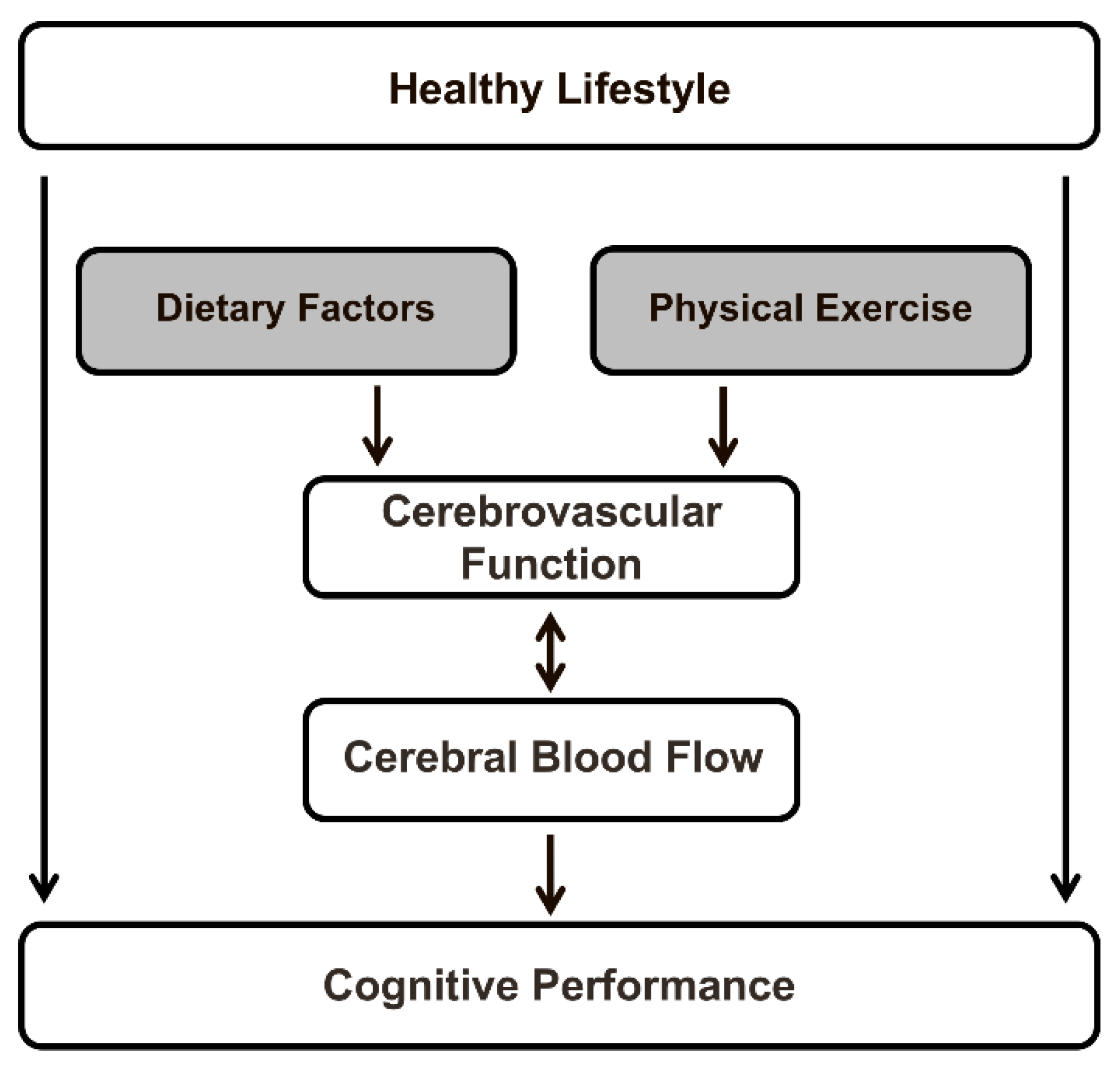
2. Cerebral Blood Flow and Cognitive Function
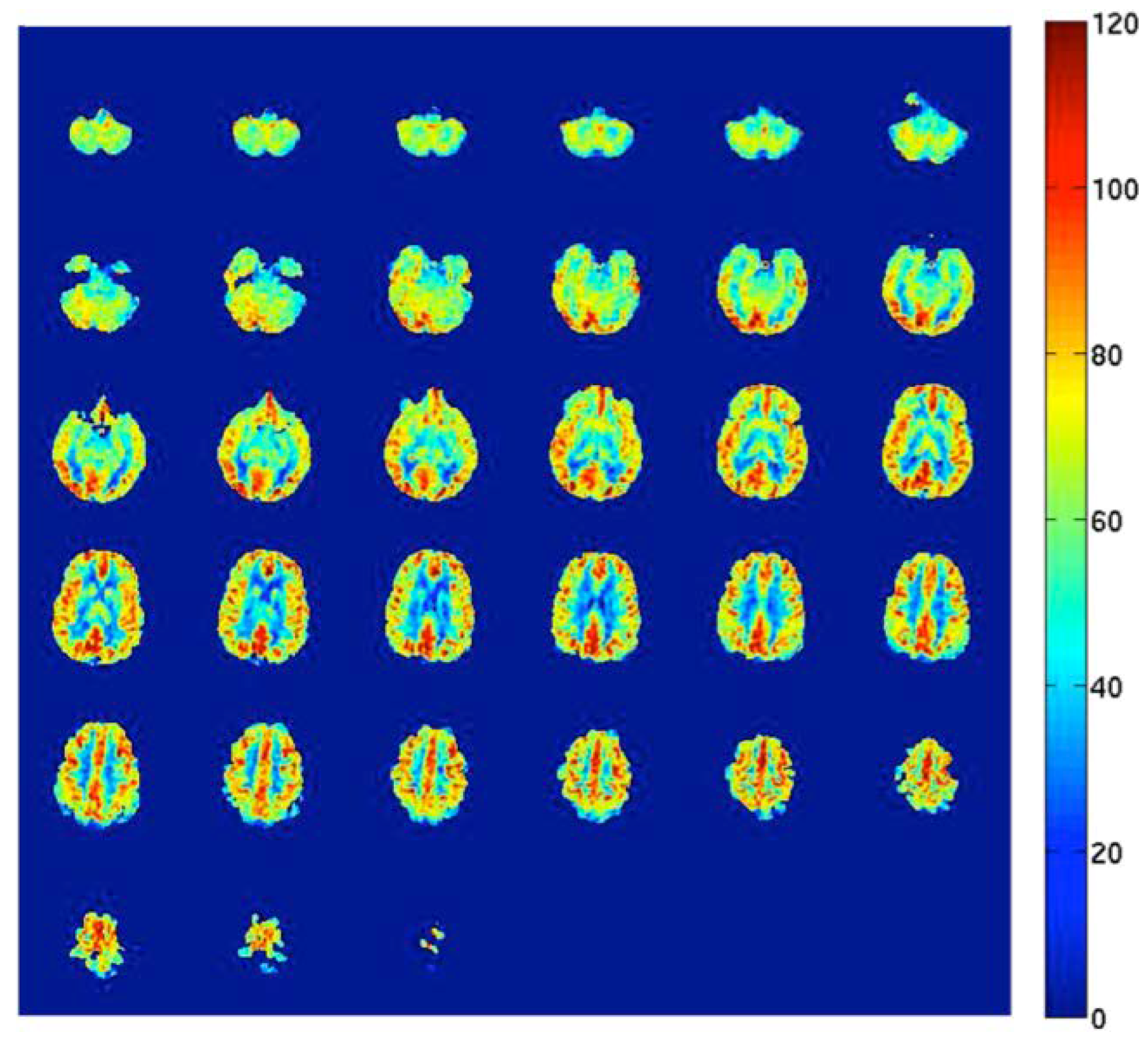
3. Measurements of Cerebral Blood Flow
3.1. Direct Methods
3.2. Indirect Methods
4. Dietary Factors and Cerebral Blood Flow
4.1. Dietary Nitrate
4.2. Polyphenols
4.3. Dietary Fatty Acids
4.4. Caffeine
4.5. Alcohol
5. Exercise and Cerebral Blood Flow
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs) Fact Sheet No. 404. February 2018. Available online: http://www.who.int/mediacentre/factsheets/fs404/en/ (accessed on 1 February 2018).
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Ladecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Martinez-Gonzalez, M.A. Mediterranean diet for primary prevention of cardiovascular disease. N. Engl. J. Med. 2013, 369, 676–677. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Quyyumi, A.A.; Hollenberg, N.K.; Jamerson, K.A. Surrogate markers for cardiovascular disease: Functional markers. Circulation 2004, 109, IV31–IV46. [Google Scholar] [CrossRef] [PubMed]
- Grove, T.; Taylor, S.; Dalack, G.; Ellingrod, V. Endothelial function, folate pharmacogenomics, and neurocognition in psychotic disorders. Schizophr. Res. 2015, 164, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, R.J.; Soiza, R.L. Evidence of endothelial dysfunction in the development of Alzheimer’s disease: Is Alzheimer’s a vascular disorder? Am. J. Cardiovasc. Dis. 2013, 3, 197–226. [Google Scholar] [PubMed]
- Liu, T.T.; Brown, G.G. Measurement of cerebral perfusion with arterial spin labeling: Part 1. Methods. J. Int. Neuropsychol. Soc. 2007, 13, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.G.; Clark, C.; Liu, T.T. Measurement of cerebral perfusion with arterial spin labeling: Part 2. Applications. J. Int. Neuropsychol. Soc. 2007, 13, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Pialoux, V.; Corbett, D.; Drogos, L.; Erickson, K.I.; Eskes, G.A.; Poulin, M.J. Promoting brain health through exercise and diet in older adults: A physiological perspective. J. Physiol. 2016, 594, 4485–4498. [Google Scholar] [CrossRef] [PubMed]
- Bentourkia, M.; Bol, A.; Ivanoiu, A.; Labar, D.; Sibomana, M.; Coppens, A.; Michel, C.; Cosnard, G.; De Volder, A.G. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: Effect of aging. J. Neurol. Sci. 2000, 181, 19–28. [Google Scholar] [CrossRef]
- Leenders, K.L.; Perani, D.; Lammertsma, A.A.; Heather, J.D.; Buckingham, P.; Healy, M.J.; Gibbs, J.M.; Wise, R.J.; Hatazawa, J.; Herold, S.; et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 1990, 113, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Parkes, L.M.; Rashid, W.; Chard, D.T.; Tofts, P.S. Normal cerebral perfusion measurements using arterial spin labeling: Reproducibility, stability, and age and gender effects. Magn. Reson. Med. 2004, 51, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Gordon, M.L.; Goldberg, T.E. Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer’s disease. Neurosci. Biobehav. Rev. 2017, 72, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Mak, H.K.; Chan, Q.; Zhang, Z.; Petersen, E.T.; Qiu, D.; Zhang, L.; Yau, K.K.; Chu, L.W.; Golay, X. Quantitative assessment of cerebral hemodynamic parameters by quasar arterial spin labeling in Alzheimer’s disease and cognitively normal elderly adults at 3-tesla. J. Alzheimers Dis. 2012, 31, 33–44. [Google Scholar] [PubMed]
- Bangen, K.J.; Nation, D.A.; Clark, L.R.; Harmell, A.L.; Wierenga, C.E.; Dev, S.I.; Delano-Wood, L.; Zlatar, Z.Z.; Salmon, D.P.; Liu, T.T.; et al. Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Front. Aging Neurosci. 2014, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Birdsill, A.C.; Carlsson, C.M.; Willette, A.A.; Okonkwo, O.C.; Johnson, S.C.; Xu, G.; Oh, J.M.; Gallagher, C.L.; Koscik, R.L.; Jonaitis, E.M.; et al. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity 2013, 21, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Crane, D.E.; Black, S.E.; Ganda, A.; Mikulis, D.J.; Nestor, S.M.; Donahue, M.J.; MacIntosh, B.J. Gray matter blood flow and volume are reduced in association with white matter hyperintensity lesion burden: A cross-sectional MRI study. Front. Aging Neurosci. 2015, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Gregg, N.M.; Kim, A.E.; Gurol, M.E.; Lopez, O.L.; Aizenstein, H.J.; Price, J.C.; Mathis, C.A.; James, J.A.; Snitz, B.E.; Cohen, A.D.; et al. Incidental cerebral microbleeds and cerebral blood flow in elderly individuals. JAMA Neurol. 2015, 72, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.A.; Chan, F.H.; Zheng, M.M.; Krassioukov, A.V.; Ainslie, P.N. Neurovascular coupling in humans: Physiology, methodological advances and clinical implications. J. Cereb. Blood Flow Metab. 2016, 36, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.H.; MacQueen, G.M. Cognitive dysfunction associated with metabolic syndrome. Obes. Rev. 2007, 8, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Proisy, M.; Mitra, S.; Uria-Avellana, C.; Sokolska, M.; Robertson, N.J.; Le Jeune, F.; Ferré, J.C. Brain perfusion imaging in neonates: An overview. AJNR Am. J. Neuroradiol. 2016, 37, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.P.; Jahanian, H.; Holdsworth, S.J.; Zaharchuk, G. Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: A systematic review. J. Cereb. Blood Flow Metab. 2016, 36, 842–861. [Google Scholar] [CrossRef] [PubMed]
- Aaslid, R.; Markwalder, T.M.; Nornes, H. Noninvasive transcranial doppler ultrasound recording of flow velocity in basal cerebral arteries. J. Neurosurg. 1982, 57, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, P.; Pekar, J.J.; Lu, H. Does acute caffeine ingestion alter brain metabolism in young adults? NeuroImage 2015, 110, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Steinbrink, J.; Villringer, A.; Kempf, F.; Haux, D.; Boden, S.; Obrig, H. Illuminating the BOLD signal: Combined fMRI-fNIRS studies. J. Magn. Reson. Imaging 2006, 24, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Davisson, R.L. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008, 7, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Mensink, R.P. Beetroot juice improves in overweight and slightly obese men postprandial endothelial function after consumption of a mixed meal. Atherosclerosis 2013, 231, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Presley, T.D.; Morgan, A.R.; Bechtold, E.; Clodfelter, W.; Dove, R.W.; Jennings, J.M.; Kraft, R.A.; King, S.B.; Laurienti, P.J.; Rejeski, W.J.; et al. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide 2011, 24, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Bond, V., Jr.; Curry, B.H.; Adams, R.G.; Asadi, M.S.; Millis, R.M.; Haddad, G.E. Effects of dietary nitrates on systemic and cerebrovascular hemodynamics. Cardiol. Res. Pract. 2013, 2013, 435629. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Thompson, K.G.; Blackwell, J.R.; Winyard, P.G.; Forster, J.; Jones, A.M.; Kennedy, D.O. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Physiol. Behav. 2015, 149, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Aguilera, C.M.; Gil, A. A systematic review of the efficacy of bioactive compounds in cardiovascular disease: Phenolic compounds. Nutrients 2015, 7, 5177–5216. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Reay, J.L.; Haskell, C.F.; Williamson, G.; Dew, T.P.; Kennedy, D.O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014, 112, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Reay, J.L.; Williamson, G.; Dew, T.; Zhang, W.; Kennedy, D.O. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br. J. Nutr. 2015, 114, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Nealon, R.S.; Scholey, A.; Howe, P.R. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.M.; Howe, P.R.; Wong, R.H. Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients 2017, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Haskell, C.F.; Forster, J.S.; Veasey, R.C.; Kennedy, D.O. Epigallocatechin gallate, cerebral blood flow parameters, cognitive performance and mood in healthy humans: A double-blind, placebo-controlled, crossover investigation. Hum. Psychopharmacol. 2012, 27, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.J.; Pal, D.; Moutsiana, C.; Field, D.T.; Williams, C.M.; Spencer, J.P.; Butler, L.T. The effect of flavanol-rich cocoa on cerebral perfusion in healthy older adults during conscious resting state: A placebo controlled, crossover, acute trial. Psychopharmacology 2015, 232, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Tagougui, S.; Heyman, E.; Meeusen, R. Acute cocoa flavanol improves cerebral oxygenation without enhancing executive function at rest or after exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006, 47, S215–S220. [Google Scholar] [CrossRef] [PubMed]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr. Dis. Treat. 2008, 4, 433–440. [Google Scholar] [PubMed]
- Bowtell, J.L.; Aboo-Bakkar, Z.; Conway, M.E.; Adlam, A.R.; Fulford, J. Enhanced task-related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl. Physiol. Nutr. Metab. 2017, 42, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Haast, R.A.; Kiliaan, A.J. Impact of fatty acids on brain circulation, structure and function. Prostaglandins Leukot. Essent. Fatty Acids 2015, 92, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Mensink, R.P. Effect of dietary fatty acid intake on cardiovascular disease—Chapter 7. In Functional Dietary Lipids, 1st ed.; Food Formulation, Consumer Issues and Innovation for Health; Woodhead Publishing: Cambridge, UK, 2016; pp. 177–191. [Google Scholar]
- Jackson, P.A.; Reay, J.L.; Scholey, A.B.; Kennedy, D.O. Docosahexaenoic acid-rich fish oil modulates the cerebral hemodynamic response to cognitive tasks in healthy young adults. Biol. Psychol. 2012, 89, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Umhau, J.C.; Zhou, W.; Carson, R.E.; Rapoport, S.I.; Polozova, A.; Demar, J.; Hussein, N.; Bhattacharjee, A.K.; Ma, K.; Esposito, G.; et al. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J. Lipid Res. 2009, 50, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Konagai, C.; Yanagimoto, K.; Hayamizu, K.; Han, L.; Tsuji, T.; Koga, Y. Effects of krill oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain function: A randomized controlled trial in healthy elderly volunteers. Clin. Interv. Aging 2013, 8, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Forster, J.S.; Bell, J.G.; Dick, J.R.; Younger, I.; Kennedy, D.O. DHA supplementation alone or in combination with other nutrients does not modulate cerebral hemodynamics or cognitive function in healthy older adults. Nutrients 2016, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Cameron, O.G.; Modell, J.G.; Hariharan, M. Caffeine and human cerebral blood flow: A positron emission tomography study. Life Sci. 1990, 47, 1141–1146. [Google Scholar] [CrossRef]
- Liu, T.T.; Behzadi, Y.; Restom, K.; Uludag, K.; Lu, K.; Buracas, G.T.; Dubowitz, D.J.; Buxton, R.B. Caffeine alters the temporal dynamics of the visual bold response. NeuroImage 2004, 23, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, D.; Rodricks, J.V.; Mariano, G.F.; Chowdhury, F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017, 89, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, R.; Greyling, A.; Draijer, R.; Corfield, D.R.; Parkes, L.M. The effect of black tea and caffeine on regional cerebral blood flow measured with arterial spin labeling. J. Cereb. Blood Flow Metab. 2013, 33, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.V.; Kaster, M.P.; Tome, A.R.; Agostinho, P.M.; Cunha, R.A. Adenosine receptors and brain diseases: Neuroprotection and neurodegeneration. Biochim. Biophys. Acta 2011, 1808, 1380–1399. [Google Scholar] [CrossRef] [PubMed]
- Perthen, J.E.; Lansing, A.E.; Liau, J.; Liu, T.T.; Buxton, R.B. Caffeine-induced uncoupling of cerebral blood flow and oxygen metabolism: A calibrated bold fMRI study. NeuroImage 2008, 40, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Merola, A.; Germuska, M.A.; Warnert, E.A.; Richmond, L.; Helme, D.; Khot, S.; Murphy, K.; Rogers, P.J.; Hall, J.E.; Wise, R.G. Mapping the pharmacological modulation of brain oxygen metabolism: The effects of caffeine on absolute CMRO2 measured using dual calibrated fMRI. NeuroImage 2017, 155, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Addicott, M.A.; Yang, L.L.; Peiffer, A.M.; Burnett, L.R.; Burdette, J.H.; Chen, M.Y.; Hayasaka, S.; Kraft, R.A.; Maldjian, J.A.; Laurienti, P.J. The effect of daily caffeine use on cerebral blood flow: How much caffeine can we tolerate? Hum. Brain Mapp. 2009, 30, 3102–3114. [Google Scholar] [CrossRef] [PubMed]
- Mulderink, T.A.; Gitelman, D.R.; Mesulam, M.M.; Parrish, T.B. On the use of caffeine as a contrast booster for BOLD fMRI studies. NeuroImage 2002, 15, 37–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bjork, J.M.; Gilman, J.M. The effects of acute alcohol administration on the human brain: Insights from neuroimaging. Neuropharmacology 2014, 84, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Wendt, P.E.; Wirsen, A.; Stenberg, G.; Risberg, J.; Ingvar, D.H. Acute effects of alcohol on regional cerebral blood flow in man. J. Stud. Alcohol 1993, 54, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.J.; Wilson, W.H. Regional cerebral blood flow changes associated with ethanol intoxication. Stroke 1986, 17, 1156–1159. [Google Scholar] [CrossRef] [PubMed]
- Newlin, D.B.; Golden, C.J.; Quaife, M.; Graber, B. Effect of alcohol ingestion on regional cerebral blood flow. Int. J. Neurosci. 1982, 17, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.A.; Speed, N.M.; Gross, M.D.; Lucey, M.R.; Bazakis, A.M.; Hariharan, M.; Beresford, T.P. Acute effects of alcohol administration on regional cerebral blood flow: The role of acetate. Alcohol Clin. Exp. Res. 1993, 17, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Mullani, N.; Gould, L.; Adler, S.S.; Guynn, R.W.; Overall, J.E.; Dewey, S. Effects of acute alcohol intoxication on cerebral blood flow measured with pet. Psychiatry Res. 1988, 24, 201–209. [Google Scholar] [CrossRef]
- Rickenbacher, E.; Greve, D.N.; Azma, S.; Pfeuffer, J.; Marinkovic, K. Effects of alcohol intoxication and gender on cerebral perfusion: An arterial spin labeling study. Alcohol 2011, 45, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Khalili-Mahani, N.; van Osch, M.J.; Baerends, E.; Soeter, R.P.; de Kam, M.; Zoethout, R.W.; Dahan, A.; van Buchem, M.A.; van Gerven, J.M.; Rombouts, S.A. Pseudocontinuous arterial spin labeling reveals dissociable effects of morphine and alcohol on regional cerebral blood flow. J. Cereb. Blood Flow Metab. 2011, 31, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, N.J.; Wierenga, C.E.; Hall, S.; Tapert, S.F.; Paulus, M.P.; Liu, T.T.; Smith, T.L.; Schuckit, M.A. Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcohol Clin. Exp. Res. 2011, 35, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Marxen, M.; Gan, G.; Schwarz, D.; Mennigen, E.; Pilhatsch, M.; Zimmermann, U.S.; Guenther, M.; Smolka, M.N. Acute effects of alcohol on brain perfusion monitored with arterial spin labeling magnetic resonance imaging in young adults. J. Cereb. Blood Flow Metab. 2014, 34, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Strang, N.M.; Claus, E.D.; Ramchandani, V.A.; Graff-Guerrero, A.; Boileau, I.; Hendershot, C.S. Dose-dependent effects of intravenous alcohol administration on cerebral blood flow in young adults. Psychopharmacology 2015, 232, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.; van Wageningen, H.; Grüner, H. Alcohol-induced changes in cerebral blood flow and cerebral blood volume in social drinkers. Alcohol Alcohol. 2013, 48, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Ogoh, S.; Ainslie, P.N. Cerebral blood flow during exercise: Mechanisms of regulation. J. Appl. Physiol. 2009, 107, 1370–1380. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.N. Exercise, cognitive function, and aging. Adv. Physiol. Educ. 2015, 39, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, P.N.; Cotter, J.D.; George, K.P.; Lucas, S.; Murrell, C.; Shave, R.; Thomas, K.N.; Williams, M.J.; Atkinson, G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J. Physiol. 2008, 586, 4005–4010. [Google Scholar] [CrossRef] [PubMed]
- Anazodo, U.C.; Shoemaker, J.K.; Suskin, N.; Ssali, T.; Wang, D.J.; St Lawrence, K.S. Impaired cerebrovascular function in coronary artery disease patients and recovery following cardiac rehabilitation. Front. Aging Neurosci. 2015, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.B.; Aslan, S.; Spence, J.S.; Defina, L.F.; Keebler, M.W.; Didehbani, N.; Lu, H. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front. Aging Neurosci. 2013, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Warnert, E.A.; Murphy, K.; Hall, J.E.; Wise, R.G. Noninvasive assessment of arterial compliance of human cerebral arteries with short inversion time arterial spin labeling. J. Cereb. Blood Flow Metab. 2015, 35, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Skatrud, J.B.; Morgan, B.; Chenuel, B.; Khayat, R.; Reichmuth, K.; Lin, J.; Dempsey, J.A. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J. Physiol. 2006, 577, 319–329. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | Study Design | Treatment | Dose | Duration | Study Population (n) | Effect on CBF 1 | Method | Effect on Cognition |
|---|---|---|---|---|---|---|---|---|
| Dietary Nitrate | ||||||||
| Presley, 2011 [29] | Randomized, controlled crossover trial | High-nitrate diet (2000 kcal/day) | 773 mg/day | 24 h | Older adults (14) | No effect on global CBF, but increased regional CBF in frontal lobe white matter | ASL MRI | (not evaluated) |
| Bond, 2013 [30] | Randomized, controlled crossover trial | Nitrate-rich beetroot juice (500 mL) | 750 mg | Acute | Healthy young women (12) | Increased MCA blood flow velocity during submaximal aerobic-based exercise | Doppler sonography | (not evaluated) |
| Wightman, 2015 [31] | Randomized, double-blind, placebo-controlled parallel trial | Nitrate-rich beetroot juice (450 mL) | 342 mg | Acute | Healthy young adults (40) | Modulated CBF in the prefrontal cortex during cognitive task performance | NIRS | Improved performance on one cognitive task |
| Polyphenols | ||||||||
| Kennedy, 2010 [33] | Randomized, double-blind, placebo-controlled crossover trial | trans-Resveratrol | 250 and 500 mg | Acute | Healthy young adults (22) | Increased CBF (dose-dependent) in the prefrontal cortex during cognitive task performance | NIRS | No effect on cognitive performance |
| Wightman, 2014 [34] | Randomized, double-blind, placebo-controlled crossover trial | trans-Resveratrol + piperine (20 mg) | 250 mg | Acute | Healthy young adults (23) | Increased CBF in the prefrontal cortex during cognitive task performance | NIRS | No effect on cognitive performance |
| Wightman, 2015 [35] | Randomized, double-blind, placebo-controlled parallel trial | trans-Resveratrol | 500 mg/day | 28 days | Healthy young adults (60) | Acutely increased CBF in the prefrontal cortex during cognitive task performance, but no chronic effects | NIRS | No effect on cognitive performance |
| Wong, 2016 [36] | Randomized, double-blind, placebo-controlled crossover trial | trans-Resveratrol | 75, 150 and 300 mg | Acute | Older type 2 diabetics (36) | Increased hypercapnia-induced MCA blood flow velocity response | Doppler sonography | (not evaluated) |
| Evans, 2017 [37] | Randomized, double-blind, placebo-controlled parallel trial | trans-Resveratrol | 150 mg/day | 14 weeks | Postmenopausal women (80) | Increased cognitive task/hypercapnia-induced MCA blood flow velocity response | Doppler sonography | Improved overall cognitive performance |
| Wightman, 2012 [38] | Randomized, double-blind, placebo-controlled crossover trial | Epigallocatechin gallate | 135 mg | Acute | Healthy young adults (27) | Reduced CBF in the prefrontal cortex during cognitive task performance | NIRS | No effect on cognitive performance |
| Lamport, 2015 [39] | Randomized, double-blind, placebo-controlled crossover trial | Cocoa flavanols | 494 mg | Acute | Older adults (18) | Increased regional CBF in the anterior cingulate cortex and central opercular cortex | ASL MRI | (not evaluated) |
| Decroix, 2016 [40] | Randomized, double-blind, placebo-controlled crossover trial | Cocoa flavanols | 903 mg | Acute | Healthy young men (12) | Increased CBF in the prefrontal cortex during cognitive task performance | NIRS | No effect on cognitive performance |
| Sorond, 2008 [42] | Randomized, double-blind, placebo-controlled parallel trial | Cocoa flavanols | 900 mg/day | 1 week | Older adults (21) | No effect on the hypercapnia-induced MCA blood flow velocity response | Doppler sonography | (not evaluated) |
| Bowtell, 2017 [43] | Randomized, double-blind, placebo-controlled parallel trial | Anthocyanin-rich blueberry concentrate | 387/day | 12 weeks | Older adults (26) | Increased regional CBF in parietal and occipital lobe gray matter | ASL MRI | Improved performance on one cognitive task |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joris, P.J.; Mensink, R.P.; Adam, T.C.; Liu, T.T. Cerebral Blood Flow Measurements in Adults: A Review on the Effects of Dietary Factors and Exercise. Nutrients 2018, 10, 530. https://doi.org/10.3390/nu10050530
Joris PJ, Mensink RP, Adam TC, Liu TT. Cerebral Blood Flow Measurements in Adults: A Review on the Effects of Dietary Factors and Exercise. Nutrients. 2018; 10(5):530. https://doi.org/10.3390/nu10050530
Chicago/Turabian StyleJoris, Peter J., Ronald P. Mensink, Tanja C. Adam, and Thomas T. Liu. 2018. "Cerebral Blood Flow Measurements in Adults: A Review on the Effects of Dietary Factors and Exercise" Nutrients 10, no. 5: 530. https://doi.org/10.3390/nu10050530
APA StyleJoris, P. J., Mensink, R. P., Adam, T. C., & Liu, T. T. (2018). Cerebral Blood Flow Measurements in Adults: A Review on the Effects of Dietary Factors and Exercise. Nutrients, 10(5), 530. https://doi.org/10.3390/nu10050530







